Atroxlysin-III, A Metalloproteinase from the Venom of the Peruvian Pit Viper Snake Bothrops atrox (Jergón) Induces Glycoprotein VI Shedding and Impairs Platelet Function
Abstract
1. Introduction
2. Results
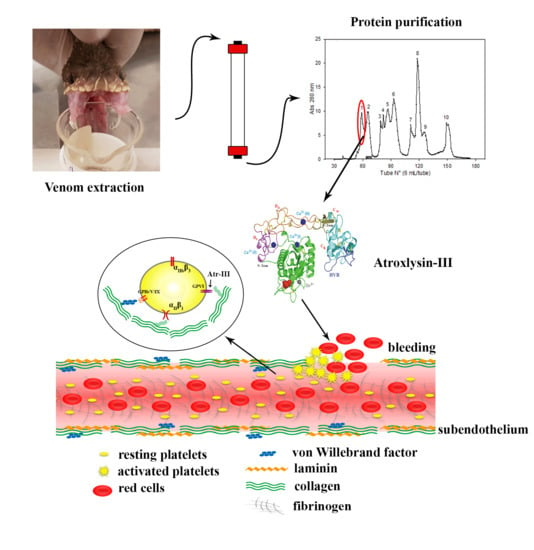
2.1. Purification of Atroxlysin-III
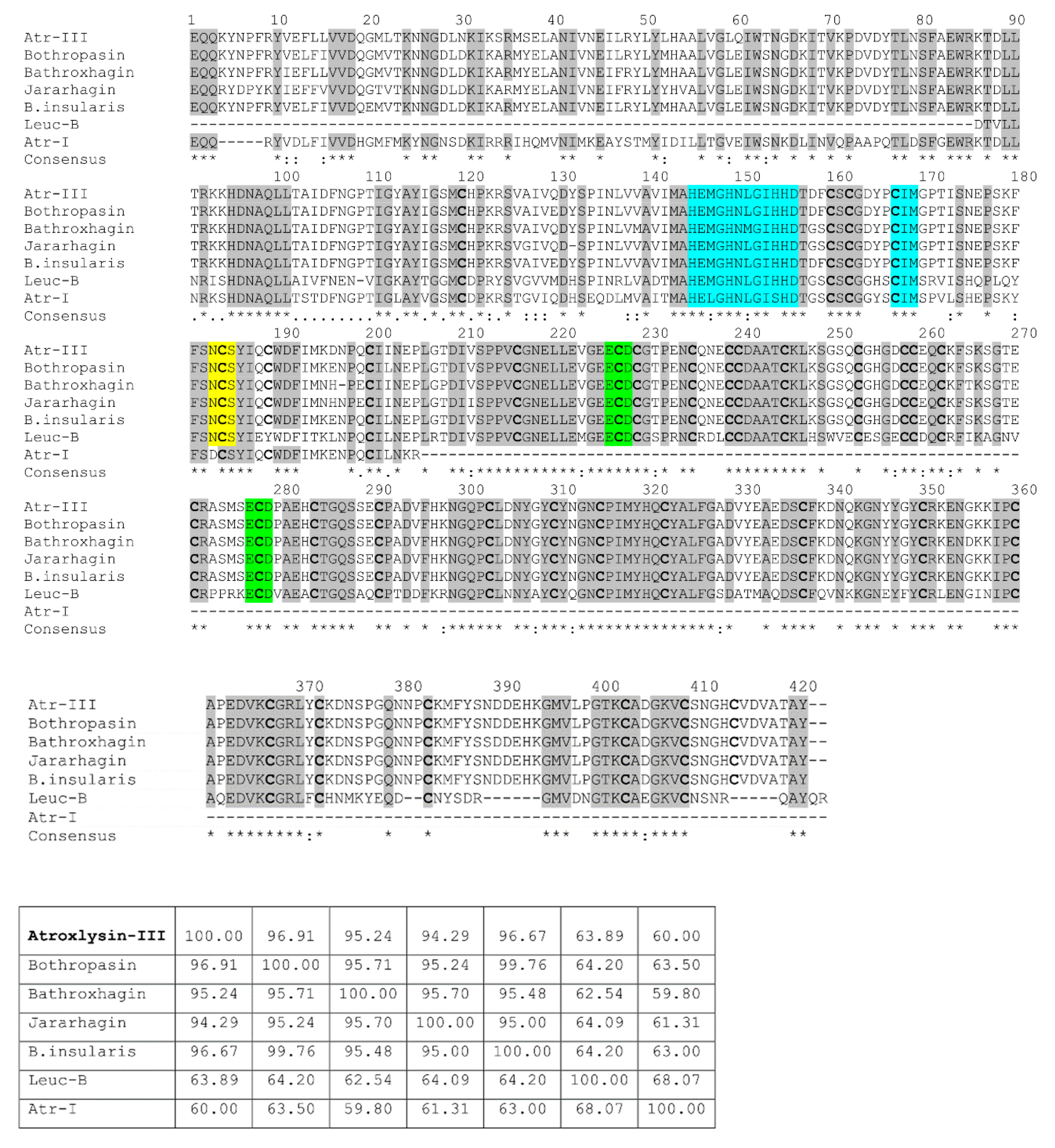
2.2. Nucleotide Sequencing of Atroxlysin-III cDNA
2.3. Three-dimensional Model and Phylogenetic Study
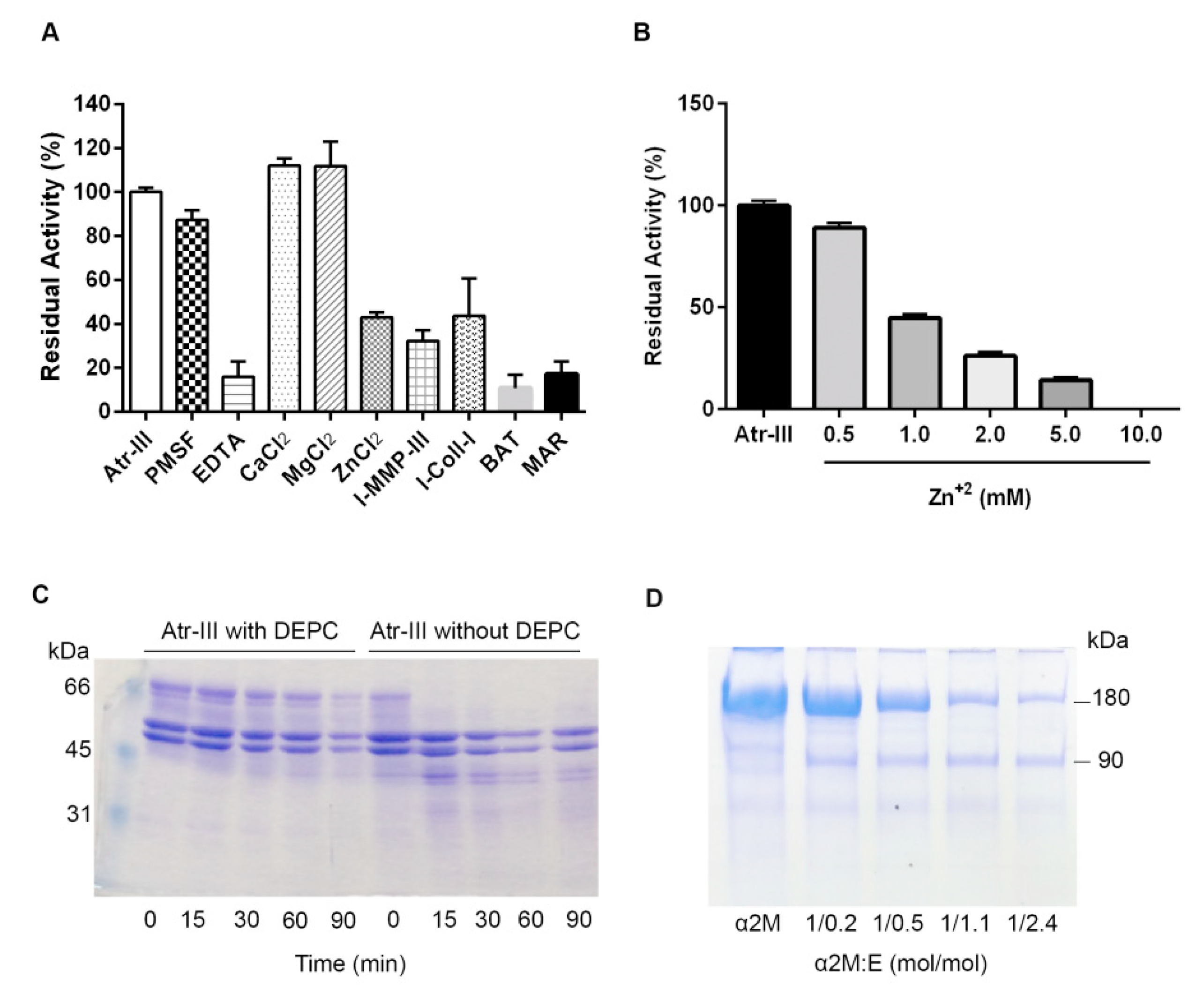
2.4. Biochemical Features of Atr-III
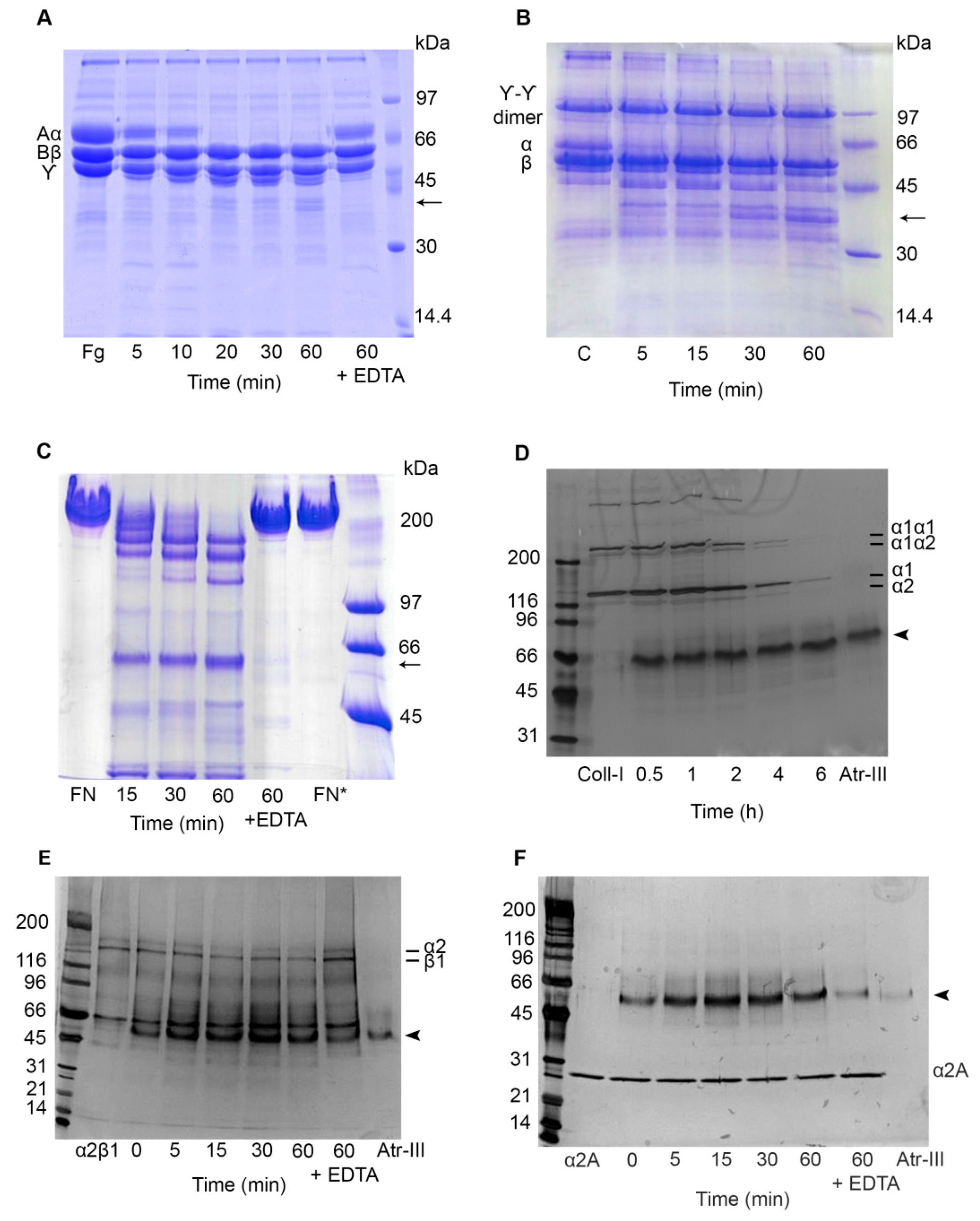
2.5. Effect of Atr-III on Plasma and ECM Components
2.6. Atr-III also Targets Platelet Receptors
3. Discussion
4. Materials and Methods
4.1. Purification of Atroxlysin III
4.2. MALDI-TOF Mass Spectrometry
4.3. Glycosylation Studies
4.4. Enzymatic Features of Atr-III
4.5. Modification of Histidine Residues in Atr-III
4.6. Degradation of Plasma and ECM Proteins by Atr-III
4.7. Effect of Atr-III on α2β1 Integrin, on its Recombinant α2A-Domain (rα2A-Domain) and on Recombinant von Willebrand Factor A1-domain (rWF-A1 Domain)
4.8. Synthesis and Sequencing of cDNA
4.9. Multiple Alignment and Phylogenetic Tree Construction
4.10. Modeling of Atr-III Three-dimensional Structure
4.11. Platelet Aggregation Assay
4.12. Western Blot Assays
4.13. Platelet Adhesion Assays Using xCELLigence Technology
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Calvete, J.J. Venomics: Integrative venom proteomics and beyond. Biochem. J. 2017, 474, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; King, G.F. Venomics as a drug discovery platform. Expert Rev. Proteom. 2009, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Marsh, N. Practical applications of snake venom toxins in hemostasis. Toxicon 2005, 45, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S.; Swenson, S.D. Application of snake toxins in biomedicine. In Venom Genomics and Proteomics, Toxinology; Gopalakrishnakone, P., Calvete, J.J., Eds.; Springer: Dordrecht, Germany, 2016; pp. 393–423. [Google Scholar]
- Calvete, J.J.; Borges, A.; Segura, A.; Flores-Dias, M.; Alape-Giron, A.; Gutierrez, J.M.; Diez, N.; De Souza, L.; Kiriokos, D.; Sanchez, E.; et al. Snake venomics and antivenomics of Bothrops colombiensis, a medically important pitviper of the Bothrops atrox-asper complex endemic to Venezuela: Contributing to its taxonomy and snake bite management. J. Proteom. 2009, 72, 227–240. [Google Scholar] [CrossRef]
- Kohlhoff, M.; Borges, M.H.; Yarleque, A.; Cabezas, C.; Richardson, M.; Sanchez, E.F. Exploring the proteomes of the venoms of the Peruvian pit vipers Bothrops atrox, B. barnetti and B. pictus. J. Proteom. 2012, 75, 2181–2195. [Google Scholar] [CrossRef]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta 2012, 1824, 164–176. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M. Insights into speculations about sanke venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef]
- Takeda, S. ADAM and ADAMTS family proteins and snake venom metalloproteinases: A structural overview. Toxins 2016, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Schneider, F.S.; Yarleque, A.; Borges, M.; Richardson, M.; Figueiredo, S.G.; Evangelista, K.; Eble, J.A. The novel metalloproteinase atroxlysin-I from Peruvian Bothrops atrox (Jergón) snake venom acts both on ECM and platelets. Arch. Biochem. Biophys. 2010, 496, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Richardson, M.; Gremski, L.H.; Veiga, S.S.; Yarleque, A.; Niland, S.; Lima, A.M.; Estevao-Costa, M.I.; Eble, J.A. A novel fibrinolytic metalloproteinase, barnettlysin-I from Bothrops barnetti (barnett’s pitviper) snake venom with anti-platelet properties. Biochim. Biophys. Acta. 2016, 1860, 542–556. [Google Scholar] [CrossRef] [PubMed]
- You, W.-K.; Jang, Y.-J.; Chung, K.-H. Functional roles of two distinct domains of halysase, a snake venom metalloprotease, to inhibit human platelet aggregation. Biochem. Biophys. Res. Comm. 2006, 339, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Gibeler, N.; Zigrino, P. A disintegrin and metalloprotease (ADAM): Historical overview of their functions. Toxins 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, D.; Scully, M.F.; Kakkar, V.V. Snake venom metalloproteinase containing a disintegrin-like domain, its structure-activity relationships at interacting with integrins. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2005, 3, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Tanjoni, I.; Evangelista, K.; Della-Casa, M.S.; Butera, D.; Magalhães, G.S.; Baldo, C.; Clisa, P.B.; Fernandes, I.; Eble, J.; Moura da Silva, A.M. Different regions of the class P-III snake venom metalloproteinase jararhagin are involved in binding to α2β1 integrin and collagen. Toxicon 2010, 55, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E. Proteolytic processing of platelet receptors. Res. Pract. Thromb. Haemost. 2017, 2, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Andrews, R.K. Platelet receptor expression and shedding: Glycoprotein Ib-IX-V and glycoprotein VI. Transfus. Med. Rev. 2014, 28, 56–60. [Google Scholar] [CrossRef]
- Ruggeri, C.M. Platelets in atherothrombosis. Nat. Med. 2002, 8, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Shih, C.-H.; Huang, T.-F. Primary structure and antiplatelet mechanism of snake venom metalloproteinase, acurhagin, from Agkistrodon acutus venom. Biochimie 2005, 87, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S. Platelets as targets of snake venom metalloproteinases. Toxicon 2005, 45, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Wijeyewickrema, L.C.; Gardiner, V.; Moroi, M.; Berndt, M.C.; Andrews, R.K. Snake venom metalloproteinases, crotarhagin and alborhagin, induce ectodomain shedding of platelet collagen receptor, glycoprotein VI. Thromb. Haemost. 2007, 98, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, V.; Cid, P.; Sanz, L.; La Torre, P.D.; Angulo, Y.; Lomonte, B.; Gutierrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J. Proteom. 2009, 73, 57–78. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Perez, A.; Borges, A.; Vargas, A.M.; Lomonte, B.; Angulo, Y.; Gutierrez, J.M.; Chalkidis, H.M.; Mourão, R.H.V.; et al. Snake population venomics and antivenomics of Bothrops atrox: Paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteom. 2011, 74, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snakebites in Central and South America: Epidemiology, clinical features and clinical management. In The Venomous Reptiles of the Western Hemisphere; Campbell, J.A., Lamar, W.W., Eds.; Comstock Publishing Associates: London, UK, 2004; pp. 709–762. [Google Scholar]
- Zavaleta, A. Mordedura de serpiente (Ofidismo): Un problema de salud en el Perú. Rev. Med. Hered. 2004, 15, 61–63. [Google Scholar] [CrossRef]
- Paine, M.J.I.; Desmond, H.P.; Theakston, R.D.G.; Crampton, J.M. Purification, cloning and molecular characterization of a high molecular weight hemorrhagic metalloproteinase, jararhagin from Bothrops jararaca venom. J. Biol. Chem. 1992, 267, 22869–22876. [Google Scholar]
- Assakura, M.T.; Silva, C.A.; Mendele, R.; Camargo, A.C.M.; Serrano, S.M.T. Molecular cloning and expression, of structural domains of bothropasin, a P-III metalloproteinase from the venom of Bothrops jararaca. Toxicon 2003, 41, 217–227. [Google Scholar] [CrossRef]
- Freitas-de-Sousa, L.A.; Amazonas, D.R.; Souza, L.F.; San’tAnna, S.S.; Nishiyama, M.Y.; Serrano, S.M.T.; Junqueira-de Azevedo, I.L.; Chalkidis, H.M.; Moura-da-Silva, A.M. Comparisons of venoms from wild and long-term captive Bothrops atrox snakes and characterization of batroxrhagin, the predominant class P-III metalloproteinase from the venom of this species. Biochimie 2015, 118, 60–70. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.; Ho, P.L. A survey of gene expression and diversity in the venom glands of the pitviper snake Bothrops insularis throught the generation of expressed sequence tags (ESTs). Gene 2002, 299, 279–291. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Gabriel, L.M.; Gontijo, S.; Gremski, L.H.; Veiga, S.S.; Evangelista, K.; Eble, J.A.; Richardson, M. Structural and functional characterization of a P-III metalloproteinase, leucurolysin-B, from Bothrops leucurus venom. Arch. Biochem. Biophys. 2007, 468, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Araki, S.; Mori, H.; Takeda, S. Crystal structures of catrocolastatin/VAP2B reveal a dynamic, modular architecture of ADAM/adamalysin/reprolysin family proteins. FEBS Lett. 2007, 581, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Wallnoefer, H.C.; Lingott, T.; Gutierrez, J.M.; Merfort, I.; Liedl, K.R. Backbone flexibility controls the activity and specificity of a protein-protein interface: Specificity in snake venom metalloproteases. J. Am. Chem. Soc. 2010, 132, 10330–10337. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Huang, T.-F. Purification and characterization of a novel metalloproteinase, acurhagin, from Agkistrodon acutus venom. Thromb. Haemost. 2002, 87, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Wang, D.; Shannon, J.D.; Pinto, A.F.M.; Polanowska-Grabowska, R.K. Interaction of the cysteine-rich domain of snake venom metalloproteinases with the A1 domain of von Willebrand factor promotes site-specific proteolysis of von Willebrand factor and inhibition of von Willebrand factor-mediated platelet aggregation. FEBS J. 2007, 274, 3611–3621. [Google Scholar] [CrossRef]
- Herrera, C.; Voisin, M.B.; Escalante, T.; Rucavado, A.; Nourshargh, S.; Gutierrez, J.M. Effects of PI and PIII snake venom haemorrhagic metalloproteinases on the microvasculature: A confocal microscopy study on the mouse cremaster muscle. PLoS ONE 2016, 11, e0168643. [Google Scholar] [CrossRef] [PubMed]
- Bernardoni, J.L.; Sousa, L.F.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.T.; Zingali, R.B.; Moura-da-Silva, A.M. Functional variability of snake venom metalloproteinases: Adaptive advantages in targeting different prey and implications for human envenomation. PLoS ONE 2014, 9, e109651. [Google Scholar] [CrossRef]
- Wang, W.-J. Purification and functional characterization of AAVI, a novel P-III metalloproteinase, from Formosan Agkistrodon acutus venom. Biochimie 2007, 89, 105–115. [Google Scholar] [CrossRef]
- De Meyer, S.F.; Vanhoorelbeke, K.; Broos, K.; Salles, I.I.; Deckmyn, H. Antiplatelet drugs. Br. J. Haematol. 2008, 142, 515–528. [Google Scholar] [CrossRef]
- Montague, S.J.; Andrews, R.K.; Gardiner, E.E. Mechanisms of receptor shedding in platelets. Blood 2018, 132, 2535–2545. [Google Scholar] [CrossRef]
- Oliveira, A.K.; Leme, A.F.P.; Asega, A.F.; Camargo, A.C.M.; Fox, J.W.; Serrano, M.T.S. New insights into the structural elements involved in the skin haemorrhage induced by snake venom metalloproteinases. Thromb. Haemost. 2010, 104, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Santos, C.I.; Magalhaes, A.; Diniz, C.R.; Figueiredo, S.; Gilroy, J.; Richardson, M. Isolation of a proteinase with plasminogen-activating activity from Lachesis muta muta (bushmaster) snake venom. Arch. Biochem. Biophys. 2000, 378, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977, 47, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Beermann, B.; Hinz, H.-J. α2β1 integrin is not recognized by rhodocythin but is the specific high affinity target of rodochetin, an RGD-independent disintegrin and potent inhibitor of cell adhesion to collagen. J. Biol. Chem. 2001, 216, 12274–122784. [Google Scholar] [CrossRef] [PubMed]
- Vivas-Ruiz, D.E.; Sandoval, G.A.; Mendoza, J.; Inga, R.R.; Gontijo, S.; Richardon, M.; Eble, J.A.; Yarleque, A.; Sanchez, E.F. Coagulant thrombin-like enzyme (barnettobin) from Bothrops barnetti venom: Molecular sequence analysis of its cDNA and biochemical properties. Biochimie 2013, 95, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionay Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1997, 234, 779–815. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Macarthur, M.W.; Moss, D.S.; Thorton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993, 6, 283–291. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







| Proteolytic (U/mg) 1 | Hemorrhagic (U/mg) 2 | Fibrinolytic (U/µg) 3 Bovine Fibrin | |
|---|---|---|---|
| Crude venom | 1.45 ± 0.39 | 68.9 ± 2.26 | 7.33 ± 0.65 |
| Atr-III | 27.8 ± 2.47 | 4000 ± 1.54 | 8.43 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, L.S.; Estevão-Costa, M.I.; Alvarenga, V.G.; Vivas-Ruiz, D.E.; Yarleque, A.; Lima, A.M.; Cavaco, A.; Eble, J.A.; Sanchez, E.F. Atroxlysin-III, A Metalloproteinase from the Venom of the Peruvian Pit Viper Snake Bothrops atrox (Jergón) Induces Glycoprotein VI Shedding and Impairs Platelet Function. Molecules 2019, 24, 3489. https://doi.org/10.3390/molecules24193489
Oliveira LS, Estevão-Costa MI, Alvarenga VG, Vivas-Ruiz DE, Yarleque A, Lima AM, Cavaco A, Eble JA, Sanchez EF. Atroxlysin-III, A Metalloproteinase from the Venom of the Peruvian Pit Viper Snake Bothrops atrox (Jergón) Induces Glycoprotein VI Shedding and Impairs Platelet Function. Molecules. 2019; 24(19):3489. https://doi.org/10.3390/molecules24193489
Chicago/Turabian StyleOliveira, Luciana S., Maria Inácia Estevão-Costa, Valéria G. Alvarenga, Dan E. Vivas-Ruiz, Armando Yarleque, Augusto Martins Lima, Ana Cavaco, Johannes A. Eble, and Eladio F. Sanchez. 2019. "Atroxlysin-III, A Metalloproteinase from the Venom of the Peruvian Pit Viper Snake Bothrops atrox (Jergón) Induces Glycoprotein VI Shedding and Impairs Platelet Function" Molecules 24, no. 19: 3489. https://doi.org/10.3390/molecules24193489
APA StyleOliveira, L. S., Estevão-Costa, M. I., Alvarenga, V. G., Vivas-Ruiz, D. E., Yarleque, A., Lima, A. M., Cavaco, A., Eble, J. A., & Sanchez, E. F. (2019). Atroxlysin-III, A Metalloproteinase from the Venom of the Peruvian Pit Viper Snake Bothrops atrox (Jergón) Induces Glycoprotein VI Shedding and Impairs Platelet Function. Molecules, 24(19), 3489. https://doi.org/10.3390/molecules24193489