The Role of Thickness Control and Interface Modification in Assembling Efficient Planar Perovskite Solar Cells
Abstract
1. Introduction
2. Results and Discussion
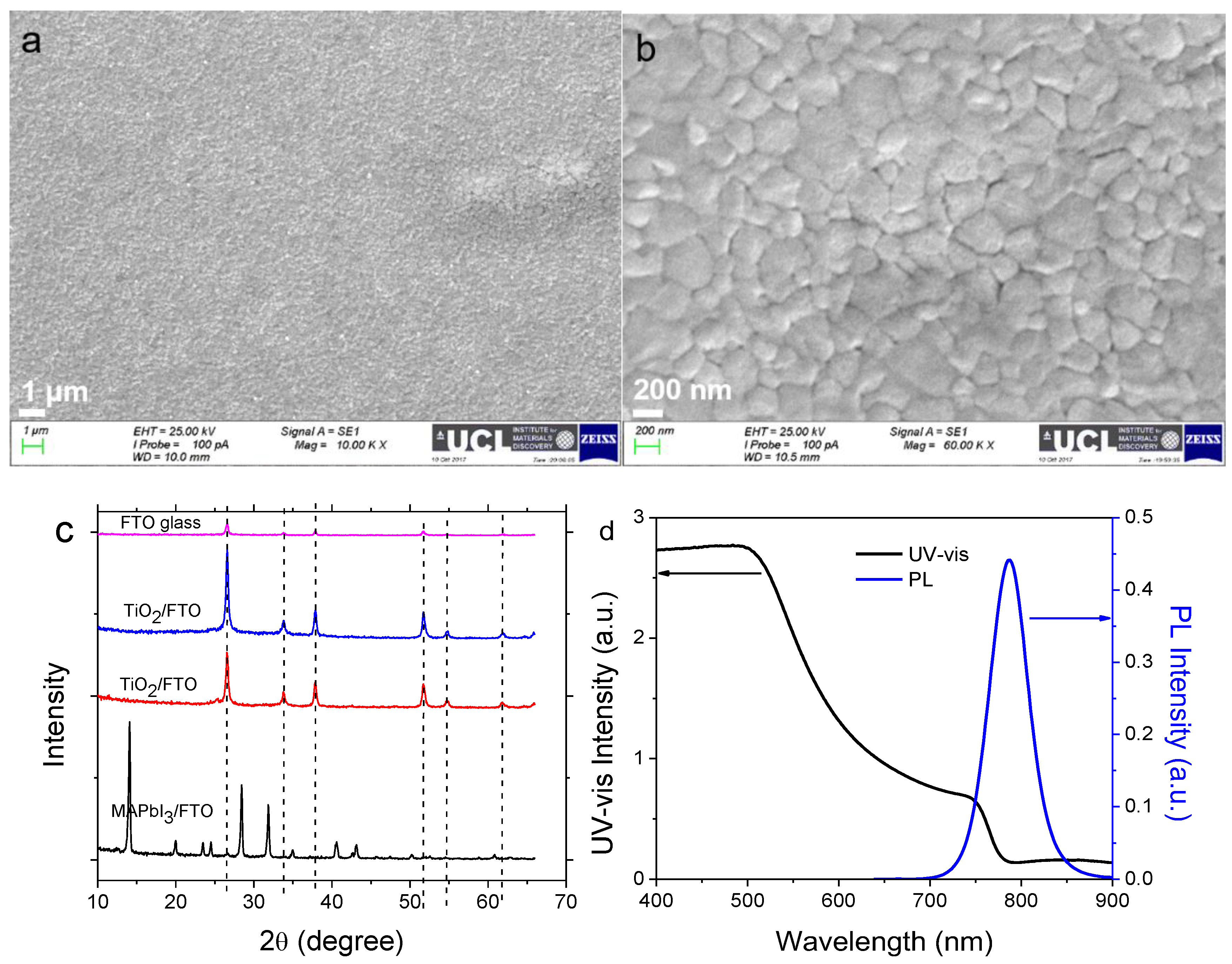
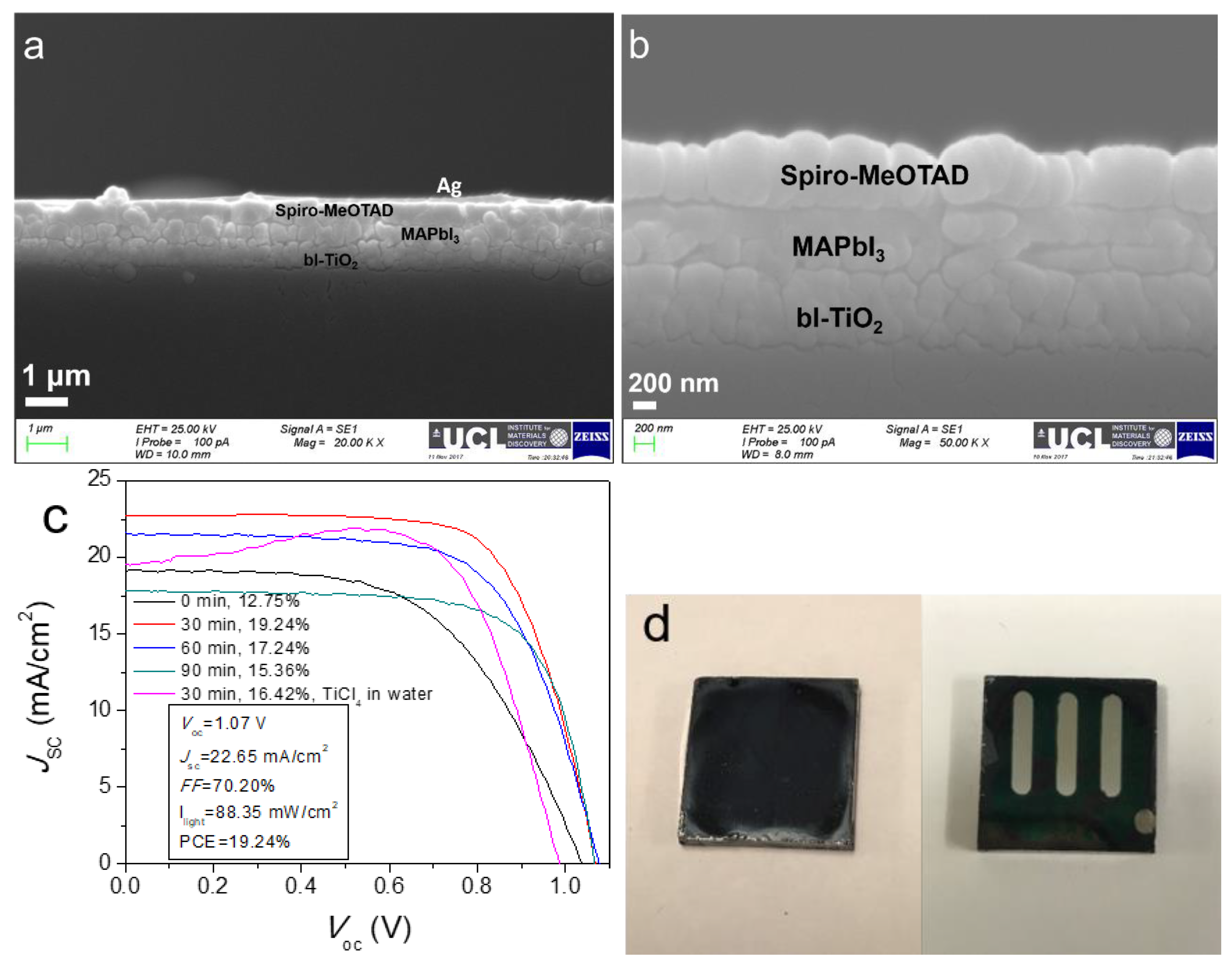
2.1. Characterisation of Perovskite Film
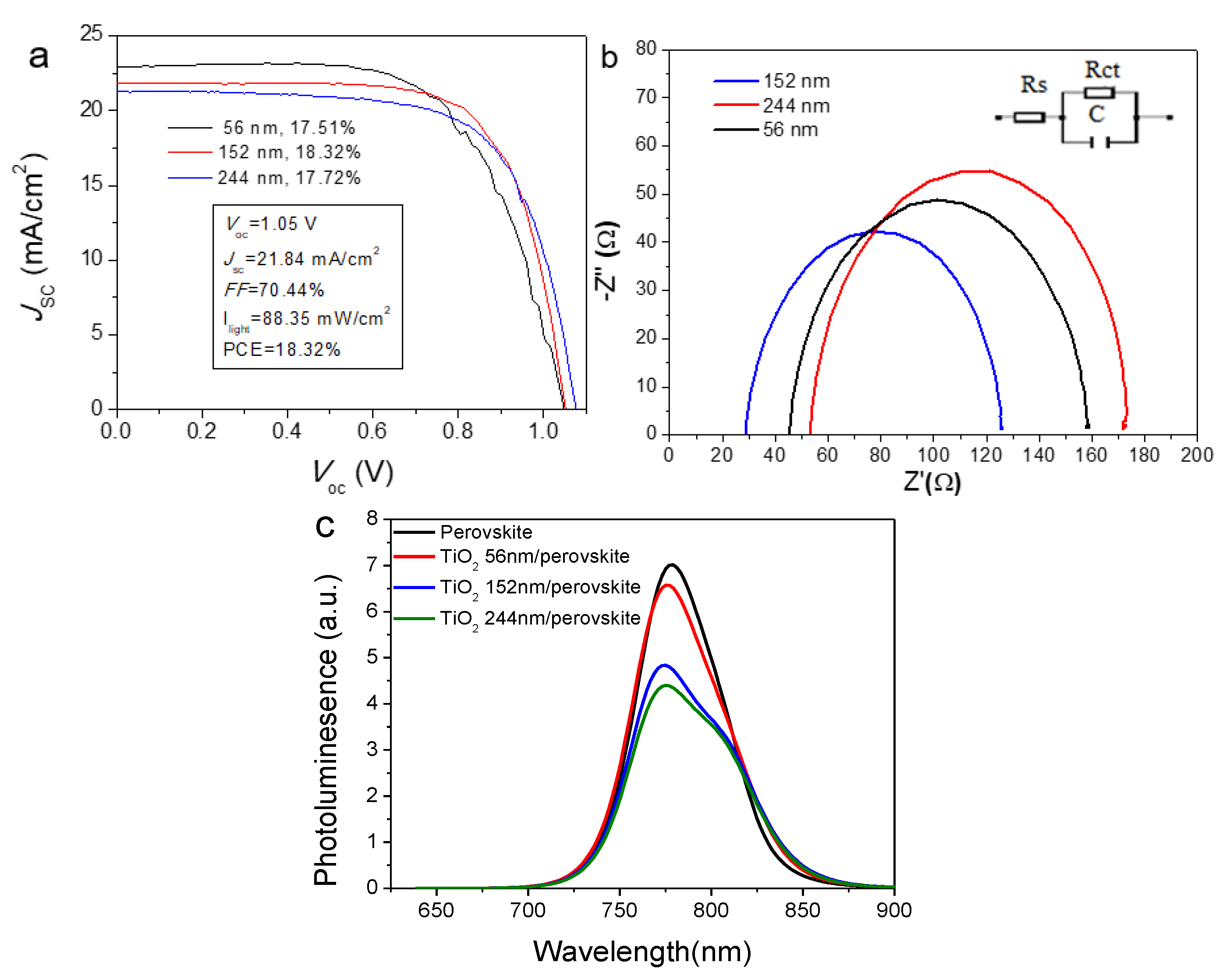
2.2. Thickness Influence of Electron Transport Layer TiO2 on Device Performance
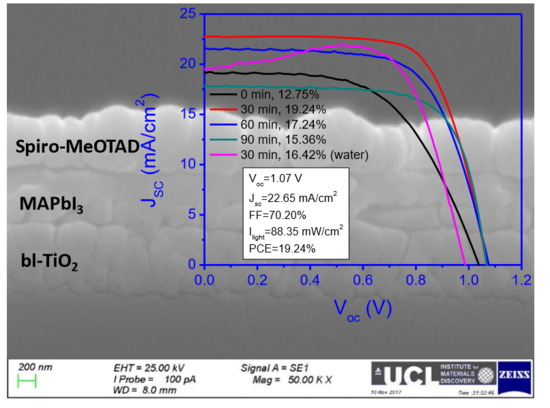
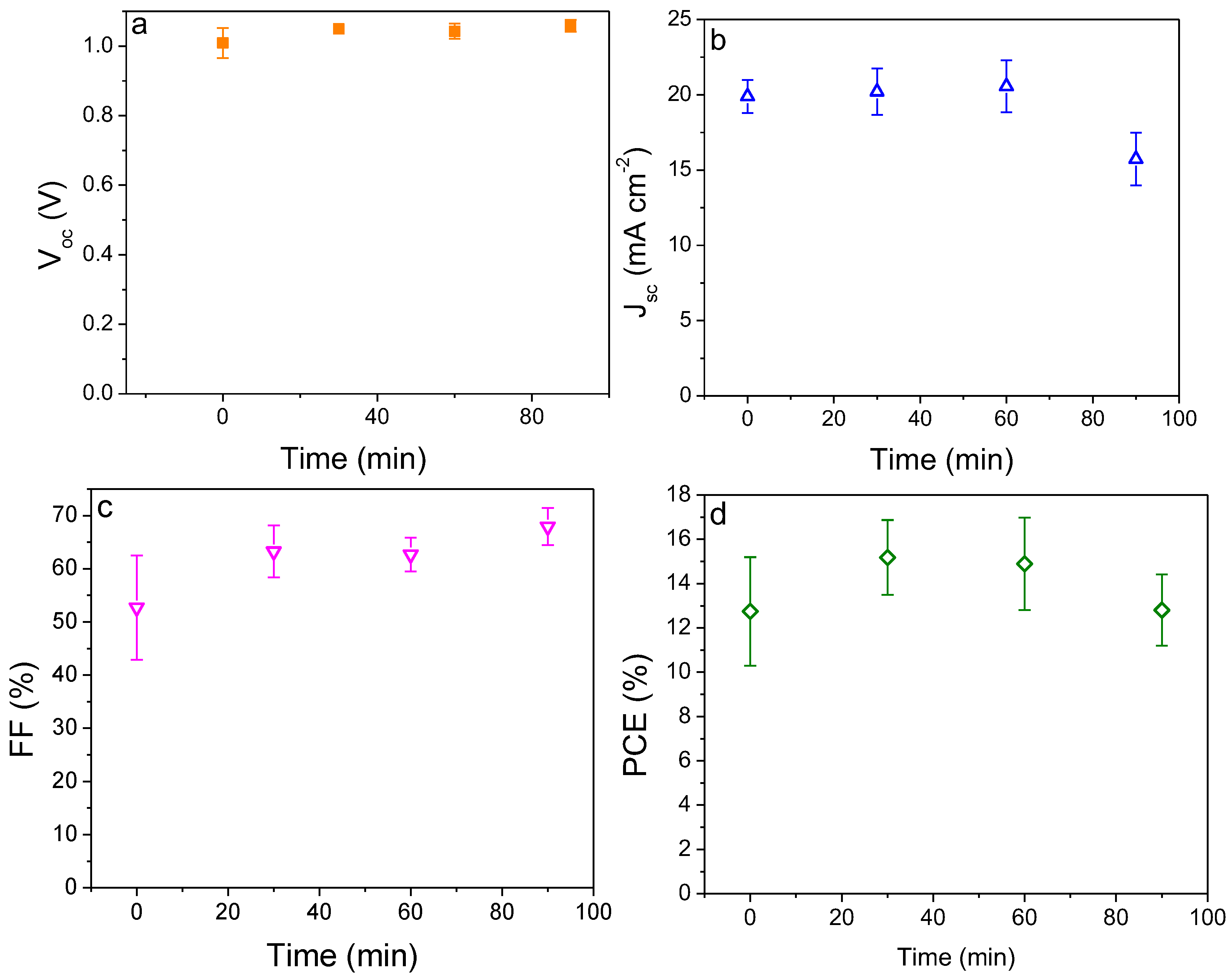
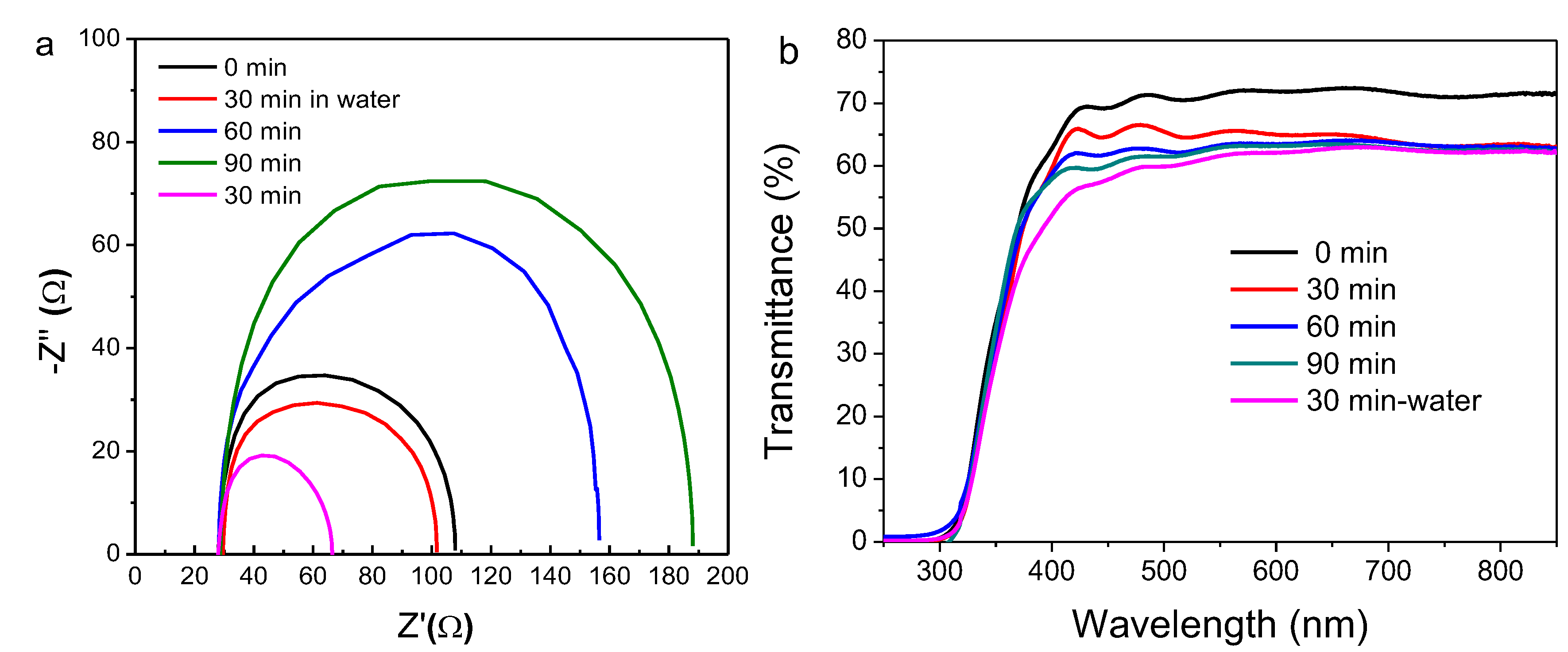
2.3. Influence of Treatment Time of Titanium Tetrachloride on Device Performance
3. Experimental
3.1. Chemicals
3.2. Device Fabrication
3.3. Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, L.F.; Zhou, P.; Li, J.; Mo, Y.P.; Huang, W.C.; Xiao, J.Y.; Li, W.; Ku, Z.L.; Zhong, J.; Peng, Y.; et al. Suppressed hysteresis and enhanced performance of triple cation perovskite solar cell with chlorine incorporation. J. Mater. Chem. C 2018, 6, 13157–13161. [Google Scholar] [CrossRef]
- Shi, C.C.; Wang, M.Y.; Zhang, K.L.; Cheng, Y.H.; Zhang, X.J. Semi-analytical model for rigid and erosive long rods penetration into sand with consideration of compressibility. Int. J. Impact Eng. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Shintate, K.; Sekine, H. Numerical simulation of hypervelocity impacts of a projectile on laminated composite plate targets by means of improved SPH method. Compos. Part A: Appl. Sci. Manuf. 2004, 35, 683–692. [Google Scholar] [CrossRef]
- Li, Q.H.; Li, H.Y.; Shen, H.B.; Wang, F.F.; Zhao, F.; Li, F.; Zhang, X.G.; Li, D.Y.; Jin, X.; Sun, W.F. Solid Ligand-Assisted Storage of Air-Stable Formamidinium Lead Halide Quantum Dots via Restraining the Highly Dynamic Surface toward Brightly Luminescent Light-Emitting Diodes. ACS Photonics 2017, 4, 2504–2512. [Google Scholar] [CrossRef]
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.J.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef]
- Sun, H.X.; Tian, W.; Cao, F.R.; Xiong, J.; Li, L. Ultrahigh-Performance Self-Powered Flexible Double-Twisted Fibrous Broadband Perovskite Photodetector. Adv. Mater. 2018, 30, 1706986. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Yoshita, M.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (Version 53). Prog. Photovolt. 2019, 27, 3–12. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Il Seol, S. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Zhou, H.P.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.R.; You, J.B.; Liu, Y.S.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- You, J.B.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.; Chang, W.H.; Hong, Z.R.; Chen, H.J.; Zhou, H.P.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–82. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, X.; Zhang, K.J.; Pang, S.P.; Zhou, X.H.; Xu, H.X.; Dong, S.M.; Han, P.X.; Zhang, Z.Y.; Zhang, C.J.; et al. Transition-metal nitride nanoparticles embedded in N-doped reduced graphene oxide: Superior synergistic electrocatalytic materials for the counter electrodes of dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 3340–3346. [Google Scholar] [CrossRef]
- Behner, T.; Orphal, D.L.; Hohler, V.; Anderson, C.E.; Mason, R.L.; Templeton, D.W. Hypervelocity penetration of gold rods into SiC-N for impact velocities from 2.0 to 6.2 km/s. Int. J. Impact Eng. 2006, 33, 68–79. [Google Scholar] [CrossRef]
- Li, G.R.; Song, J.; Pan, G.L.; Gao, X.P. Highly Pt-like electrocatalytic activity of transition metal nitrides for dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 1680–1683. [Google Scholar] [CrossRef]
- Yun, S.N.; Zhang, H.; Pu, H.H.; Chen, J.H.; Hagfeldt, A.; Ma, T.L. Metal Oxide/Carbide/Carbon Nanocomposites: In Situ Synthesis, Characterization, Calculation, and their Application as an Efficient Counter Electrode Catalyst for Dye-Sensitized Solar Cells. Adv. Energy Mater. 2013, 3, 1407–1412. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Li, M.; Lei, Y.H.; Sheng, N.; Ohtsuka, T. Preparation of low-platinum-content platinum-nickel, platinum-cobalt binary alloy and platinum-nickel-cobalt ternary alloy catalysts for oxygen reduction reaction in polymer electrolyte fuel cells. J. Power Sources 2015, 294, 420–429. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Han, G.Y. Efficient hydrothermal-processed platinum nickel bimetallic nano-catalysts for use in dye-sensitized solar cells. J. Power Sources 2015, 294, 8–15. [Google Scholar] [CrossRef]
- Tang, Q.W.; Zhang, H.H.; Meng, Y.Y.; He, B.L.; Yu, L.M. Dissolution Engineering of Platinum Alloy Counter Electrodes in Dye-Sensitized Solar Cells. Angew. Chem.-Int. Edit. 2015, 54, 11448–11452. [Google Scholar] [CrossRef]
- Oregan, B.; Gratzel, M. A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Duan, J.L.; Tang, Q.W.; Zhang, H.H.; Meng, Y.Y.; Yu, L.M.; Yang, P.Z. Counter electrode electrocatalysts from one-dimensional coaxial alloy nanowires for efficient dye-sensitized solar cells. J. Power Sources 2016, 302, 361–368. [Google Scholar] [CrossRef]
- Makuta, S.; Liu, M.N.; Endo, M.; Nishimura, H.; Wakamiya, A.; Tachibana, Y. Photo-excitation intensity dependent electron and hole injections from lead iodide perovskite to nanocrystalline TiO2 and spiro-OMeTAD. Chem. Commun. 2016, 52, 673–676. [Google Scholar] [CrossRef]
- Liu, M.N.; Endo, M.; Shimazaki, A.; Wakamiya, A.; Tachibana, Y. Excitation Wavelength Dependent Interfacial Charge Transfer Dynamics in a CH3NH3PbI3 Perovskite Film. J. Photopolym Sci. Technol. 2018, 31, 633–642. [Google Scholar] [CrossRef]
- Li, X.M.; Wu, Y.; Zhang, S.L.; Cai, B.; Gu, Y.; Song, J.Z.; Zeng, H.B. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Di Credico, B.; Levi, M.; Turni, S. An efficient method for the output of new self-repairing materials through a reactive isocyanate encapsulation. Eur. Polym. J. 2013, 49, 2467–2476. [Google Scholar] [CrossRef]
- Feng, W.; Patel, S.H.; Young, M.Y.; Zunino, J.L.; Xanthos, M. Smart polymeric coatings—Recent advances. Adv. Polym. Tech. 2007, 26, 1–13. [Google Scholar] [CrossRef]
- Song, J.Z.; Li, J.H.; Li, X.M.; Xu, L.M.; Dong, Y.H.; Zeng, H.B. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Zou, S.H.; Liu, Y.S.; Li, J.H.; Liu, C.P.; Feng, R.; Jiang, F.L.; Li, Y.X.; Song, J.Z.; Zeng, H.B.; Hong, M.C.; et al. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Astaraie, F.R. Carbonaceous materials as substitutes for conventional dye-sensitized solar cell counter electrodes. J. Mater. Chem. A 2015, 3, 20849–20862. [Google Scholar] [CrossRef]
- Hu, Z.L.; Xia, K.; Zhang, J.; Hu, Z.Y.; Zhu, Y.J. Highly transparent ultrathin metal sulfide films as efficient counter electrodes for bifacial dye-sensitized solar cells. Electrochim. Acta 2015, 170, 39–47. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Gratzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Fang, Y.J.; Bi, C.; Wang, D.; Huang, J.S. The Functions of Fullerenes in Hybrid Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 782–794. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Liu, M.; Endo, M.; Shimazaki, A.; Wakamiya, A.; Tachibana, Y. Identifying an Optimum Perovskite Solar Cell Structure by Kinetic Analysis: Planar, Mesoporous Based, or Extremely Thin Absorber Structure. ACS Appl. Energy Mater. 2018, 1, 3722–3732. [Google Scholar] [CrossRef]
- He, B.; Tang, Q.; Luo, J.; Li, Q.; Chen, X.; Cai, H. Rapid charge-transfer in polypyrrole–single wall carbon nanotube complex counter electrodes: Improved photovoltaic performances of dye-sensitized solar cells. J. Power Sources 2014, 256, 170–177. [Google Scholar] [CrossRef]
- Dimitrov, D.B.; Koprinarova, J.; Pazov, J.; Angelov, C. Conductivity of micro-porous magnetron-sputtered thin TiO2 films. Vacuum 2000, 58, 344–350. [Google Scholar] [CrossRef]
- Gu, G.R.; He, Z.; Tao, Y.C.; Lia, Y.A.; Li, J.J.; Yin, H.; Lia, W.Q.; Zhao, Y.N. Conductivity of nanometer TiO2 thin films by magnetron sputtering. Vacuum 2003, 70, 17–20. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yang, H.; Zhang, H. Molecular Dynamics Simulation on the Orientation of Alkane Mixture on Graphene. Chem. J. Chin. Univ.-Chin. 2018, 39, 1729–1733. [Google Scholar]
- Sorkin, V.; Sha, Z.D.; Branicio, P.S.; Pei, Q.X.; Zhang, Y.W. Atomistic Molecular Dynamics Study of Structural and Thermomechanical Properties of Zdol Lubricants on Hydrogenated Diamond-Like Carbon. IEEE Trans. Magn. 2013, 49, 5227–5235. [Google Scholar] [CrossRef]
- Zaminpayma, E. Molecular Dynamics Simulation of Mechanical Properties and Interaction Energy of Polythiophene/Polyethylene/Poly(p-phenylenevinylene) and CNTs Composites. Polym. Compos. 2014, 35, 2261–2268. [Google Scholar] [CrossRef]
- Lee, S.W.; Ahn, K.S.; Zhu, K.; Neale, N.R.; Frank, A.J. Effects of TiCl4 Treatment of Nanoporous TiO2 Films on Morphology, Light Harvesting, and Charge-Carrier Dynamics in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 21285–21290. [Google Scholar] [CrossRef]
- Boppella, R.; Basak, P.; Manorama, S.V. Viable Method for the Synthesis of Biphasic TiO2 Nanocrystals with Tunable Phase Composition and Enabled Visible-Light Photocatalytic Performance. ACS Appl. Mater. Interfaces 2012, 4, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Zhang, L.Z.; Deng, K.J.; Chen, X.Y.; Zou, Z.G. Low temperature synthesis and photocatalytic activity of rutile TiO2 nanorod superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Jaramillo-Quintero, O.A.; Sanchez, R.S.; Rincon, M.; Mora-Sero, I. Bright Visible-Infrared Light Emitting Diodes Based on Hybrid Halide Perovskite with Spiro-OMeTAD as a Hole-Injecting Layer. J. Phys. Chem. Lett. 2015, 6, 1883–1890. [Google Scholar] [CrossRef]
- Werner, J.; Geisskihler, J.; Dabirian, A.; Nicolay, S.; Morales-Masis, M.; De Wolf, S.; Niesen, B.; Ballif, C. Parasitic Absorption Reduction in Metal Oxide-Based Transparent Electrodes: Application in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 17260–17267. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; McIlroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
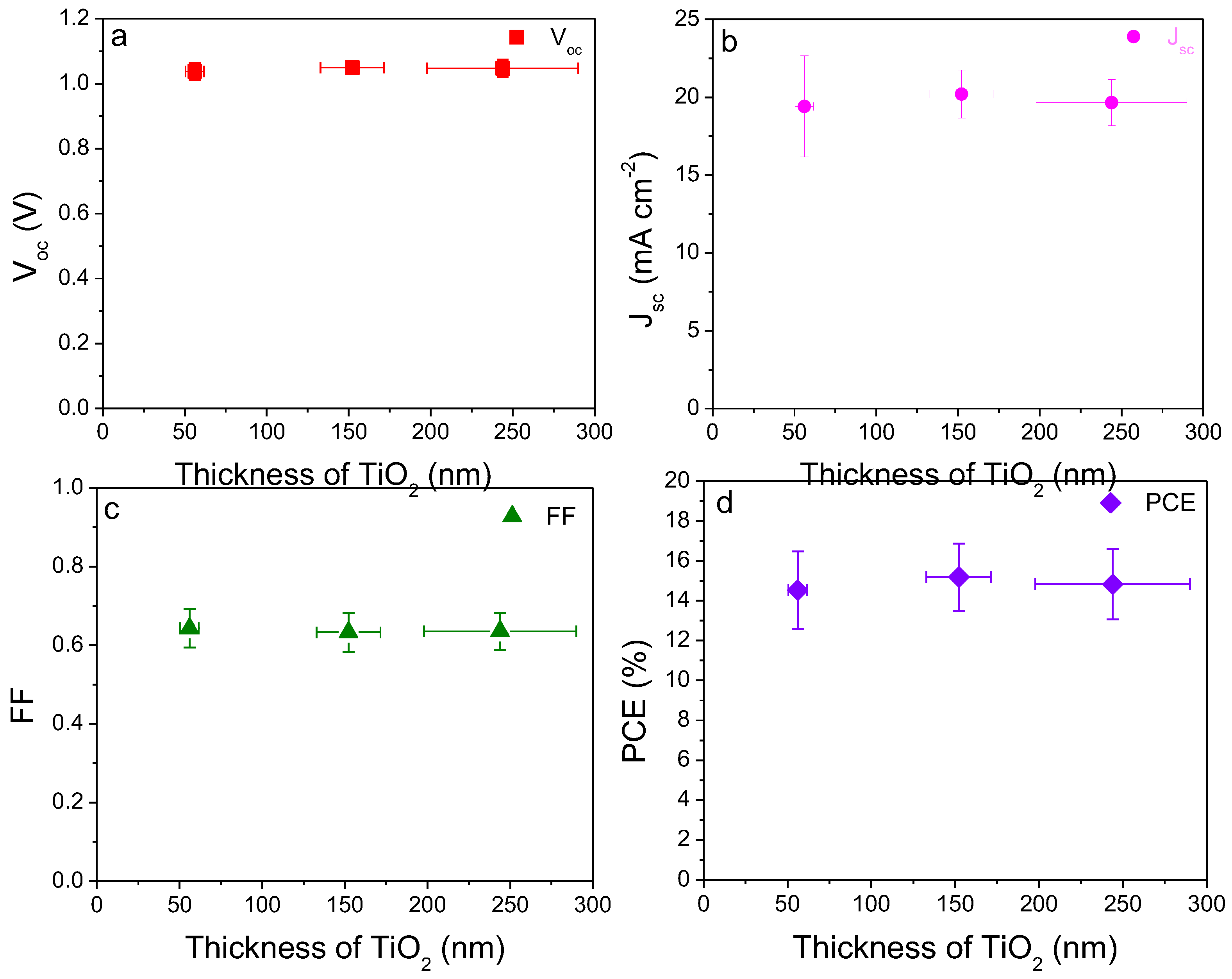
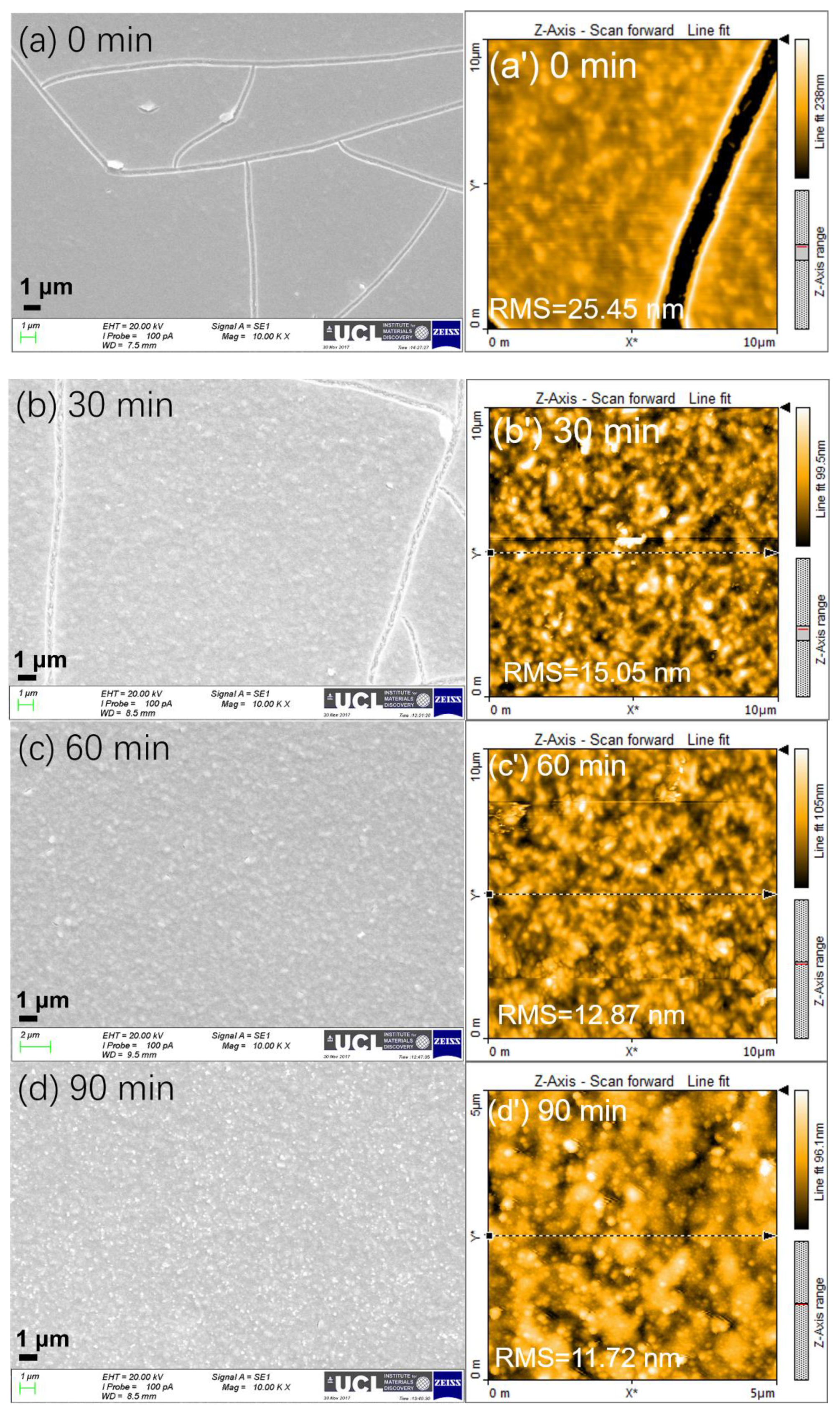
| Thickness a (nm) | Voc(V) | Jsc (mA cm−2) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|
| #1 | 56.10 ± 5.60 | 1.04 ± 0.03 | 19.42 ± 3.25 | 64.25 ± 4.86 | 14.53 ± 1.94 |
| Champion | 1.05 | 22.95 | 64.33 | 17.51 | |
| #2 | 152.20 ± 19.40 | 1.05 ± 0.01 | 20.21 ± 1.54 | 63.24 ± 4.90 | 15.18 ± 1.69 |
| Champion | 1.05 | 21.84 | 70.44 | 18.32 | |
| #3 | 244.0 ± 46.10 | 1.05 ± 0.03 | 19.66 ± 1.48 | 63.54 ± 4.75 | 14.82 ± 1.76 |
| Champion | 1.07 | 21.26 | 68.56 | 17.72 |
| Time (min) | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|
| #1 | 0 | 1.03 ± 0.01 a | 19.39 ± 0.70 | 51.74 ± 3.71 | 11.70 ± 1.00 |
| Champion | 1.04 | 19.14 | 56.70 | 12.75 | |
| #2 | 30 | 1.06 ± 0.01 | 20.19 ± 1.56 | 63.67 ± 3.16 | 15.28 ± 1.60 |
| Champion | 1.07 | 22.65 | 70.20 | 19.24 | |
| #3 | 60 | 1.06 ± 0.02 | 19.82 ± 2.28 | 63.00 ± 5.00 | 14.89 ± 2.08 |
| Champion | 1.08 | 21.54 | 65.71 | 17.24 | |
| #4 | 90 | 1.06 ± 0.02 | 15.73 ± 1.74 | 67.92 ± 3.48 | 12.80 ± 1.61 |
| Champion | 1.07 | 17.80 | 71.40 | 15.36 | |
| #5b | 30 | 0.98 ± 0.01 | 16.26 ± 2.54 | 69.64 ± 5.18 | 12.55 ± 1.89 |
| Champion | 0.99 | 19.48 | 75.42 | 16.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Choy, K.-L.; Wang, M. The Role of Thickness Control and Interface Modification in Assembling Efficient Planar Perovskite Solar Cells. Molecules 2019, 24, 3466. https://doi.org/10.3390/molecules24193466
Sun W, Choy K-L, Wang M. The Role of Thickness Control and Interface Modification in Assembling Efficient Planar Perovskite Solar Cells. Molecules. 2019; 24(19):3466. https://doi.org/10.3390/molecules24193466
Chicago/Turabian StyleSun, Weifu, Kwang-Leong Choy, and Mingqing Wang. 2019. "The Role of Thickness Control and Interface Modification in Assembling Efficient Planar Perovskite Solar Cells" Molecules 24, no. 19: 3466. https://doi.org/10.3390/molecules24193466
APA StyleSun, W., Choy, K.-L., & Wang, M. (2019). The Role of Thickness Control and Interface Modification in Assembling Efficient Planar Perovskite Solar Cells. Molecules, 24(19), 3466. https://doi.org/10.3390/molecules24193466