Abstract
Novel anthranilic diamides with sulfilimidoyl and sulfoximidoyl functionalities were successfully prepared. Among newly-prepared organosulfur compounds, 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylthio)phenyl)-1H-pyrazole-5-carboxamide and (S,E)-3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-4-(S-methyl-N-(2,2,2-trifluoroacetyl)sulfinimidoyl)-6-(methylcarbamoyl)phenyl)-1H-pyrazole-5-carboxamide showed good levels of efficacy and a strong correlation between insecticidal activities and physical properties, respectively. In particular, available data indicated that the N-trifluoroacetyl sulfilimine moiety could be an appealing structural scaffold for the discovery of a new crop-protecting agent.
1. Introduction
After the discovery of sulfoxaflor by Dow AgroScience [1,2,3], compounds with sulfoximine moiety have received remarkable attention in crop protection. Consequently, a large number of studies have examined the chemistry and mode of action of sulfoxaflor and sulfoximine insecticides [4,5,6,7,8]. As shown in Figure 1, the interest in the sulfoximine moiety has led to the discovery of highly-active sulfilimine-containing insecticides 1–4 [9,10,11,12,13]. For patent applications, researchers at BASF prepared sulfilimine-based anthranilic diamides 4 and reported that it is highly active in insects which are resistant to ryanodine modulator insecticide [13]. Furthermore, various sulfoximine-containing anthranilamides 6 have been reported by researchers at Syngenta (Figure 1) [14,15]. In addition to crop-protection applications, many research groups reported that sulfoximine could be a bioisostere of sulfones and sulfonamides with enhanced absorption, distribution, metabolism, and excretion (ADME) properties. These suggests that introducing a sulfur–nitrogen bond could be a promising approach for the discovery of new biologically-active molecules [16,17,18,19,20,21,22]. For the preparation of sulfilimine- and sulfoximine-based compounds, synthetic methods and strategies have been widely investigated by many research groups [23,24,25,26,27]. In particular, Bolm et al. reported facile and practical synthetic approaches [28], which are applied in this study.
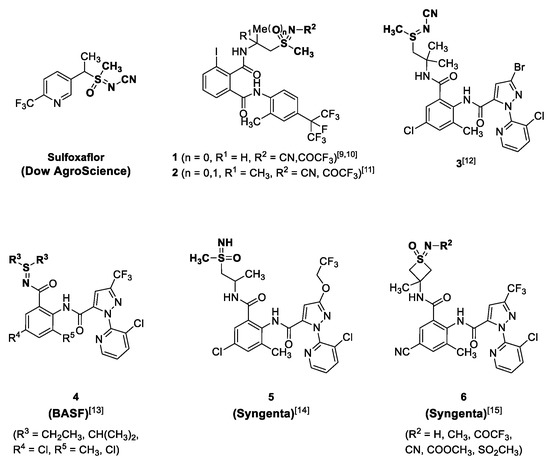
Figure 1.
Examples of alkyl sulfilimine- and sulfoximine-substituted insecticides [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15].
Based on previous research and interest in sulfilimine and sulfoximine functionalities, we began to identify these groups for the development of novel insecticides. Because alkyl sulfilimine- and sulfoximine-substituted diamides have so far been reported in the literature (Figure 1), a more focused exploration in our studies was sulfilimine and sulfoximine moieties directly substituted to the 4-position on the anthranilamide ring (Figure 2). We hypothesized that better insecticidal activity could be obtained by these small, lipophilic, electron-withdrawing substituents [1,29,30].
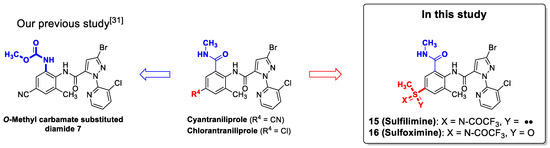
Figure 2.
Previously-developed anthranilic diamide [31] and newly-designed insecticides in this study.
2. Results
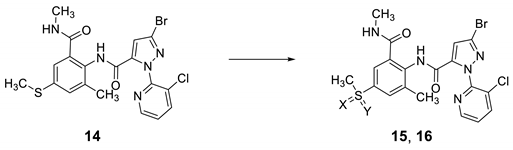
In our previous study on a novel anthranilic diamide insecticide 7, we showed that the replacement of N-methylcarbamoyl with O-methyl carbamate had a beneficial effect, resulting in high insecticidal activity and low toxicity (Figure 2) [31]. Encouraged by these results, we have initially investigated the synthesis of the sulfilimine- and sulfoximine-based diamide 15, 16 derivatives (Figure 2).
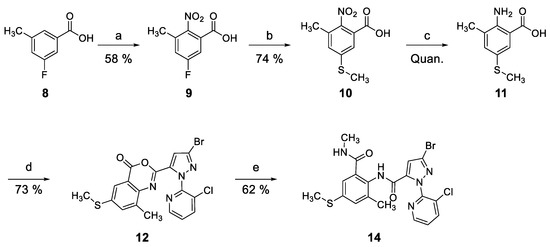
As shown in Scheme 1, the targeted compound 14 was successfully prepared using commercially-available 3-fluoro-5-methylbenzoic acid 8. Regio-selective nitration of 8 provided 2-nitrobenzoic acid 9 in good yield [29,32]. The nitrated product 9 was readily converted to thiomethylated aniline 11 by reaction sequence previously used [31,33,34]. Then, 2-amino-3-methyl-5-(methylthio)benzoic acid 11 was coupled with 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid 13 [12,14,33,34] to give the desired benzoxazinone 12 in good yield [13]. After ring opening reaction of 12 with methylamine [12,14,29,30,34], 4-methylthio anthranilamide 14 was readily obtained.
Scheme 1.
Reagents and conditions: (a) KNO3, H2SO4, RT, 1 h; (b) aqueous NaSMe (21 wt%), 150 °C, 18 h; (c) Na2S2O4, THF/H2O (3:2), 60 °C, 0.5 h; (d) 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid 13, MeSO2Cl, pyridine, CH3CN, RT, 13 h; (e) CH3NH2 (2.0 M in THF), THF, 60 °C, 5 h.
Next, the sulfur imination and oxidation of diamides 14 was explored (Table 1). Rhodium-catalyzed imination of secondary amide-bearing sulfide 14 and sulfoxide 15b also provided the desired sulfilimine 15a and sulfoximine 16a, respectively (entries 1 and 2, Table 1) [28]. It is worth noting that this metal-catalyzed imination of sulfide 14 led to a mixture of sulfilimine 15a and sulfoxide 15b (entry 1, Table 1). For the 4-sulfone group, an oxidation method using MonoPeroxyPhthalate hexahydrate (MMPP·6H2O) easily provided the desired compound 16b (entry 3, Table 1).

Table 1.
Synthesis of aryl organosulfur analogs of diamide insecticides
For the practical test, newly-prepared compounds 14–16 were evaluated for their insecticidal activities against the third instar larvae of Spodoptera litura according to the reported leaf-dip method [35].
In addition to sulfilimine 15a and sulfoximine 16a, all other synthetic compounds, that is, sulfide 14, sulfoxide 15b, and sulfone 16b, were also tested for their larvicidal activities (Table 2). Among them, sulfide 14 and N-trifluoroacetyl sulfilimine 15a showed good activities with high inhibition of feeding behaviors (eating area—14 and 15a: 5–10%, ref.: 0–5%, Table 2) (for images, please see the Supplementary Materials). Highly sensitive and functional group specific insecticidal activities were observed.

Table 2.
Insecticidal activities of sulfide- and N-trifluoroacetyl sulfilimine-based diamide 14 and 15a against the third instar larvae of Spodoptera litura.
Because we believed that the physicochemical properties of newly-prepared diamides play important roles in their insecticidal activities [14,36], the studies were extended to investigate the bioavailability of organosulfur-based crop-protecting agents 14, 15a, 15b, 16a, and 16b in terms of plant systemic properties [14] and membrane permeability [36]. As references, their properties of chlorantraniliprole and cyantraniliprole were also measured. Equilibrium solubility, log P, and parallel artificial membrane permeability assay (PAMPA) values are reported in Figure 3 [37].
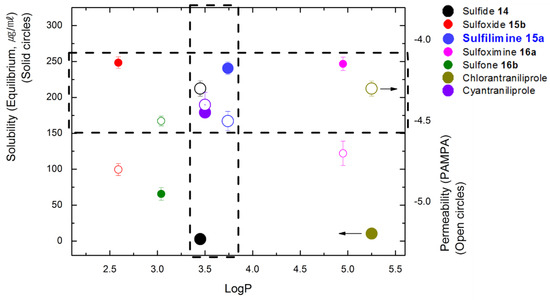
Figure 3.
Physical properties of organosulfur-substituted anthranilic diamides 14–16 [38,39,40]: Open and solid circles represent permeability (parallel artificial permeability assay, PAMPA) and solubility (equilibrium), respectively; horizontal dash box describes similar permeability (PAMPA) with chlorantraniliprole (dark yellow) and cyantraniliprole (violet); vertical dash box represents similar log P with cyantraniliprole (violet).
Although poor water solubility was observed, sulfide 14 displayed relatively low log P and high permeability value (black circle, Figure 3). According to the solubility and log P data, sulfoxide 15b is believed to have hydrophilic properties (red circle, Figure 3) [41]. In the case of N-trifluoroacetyl sulfilimine 15a and sulfoximine 16a, it seems that sulfilimine 15a is more bioavailable than sulfoximine 16a, especially considering its log P value (blue circle vs pink circle, Figure 3). In physicochemical property tests on organosulfur-based anthranilic diamides, it has been proven that similar values (log P and permeability) of sulfide 14 and N-trifluoroacetyl sulfilimine 15a to cyantraniliprole have resulted in promising insecticidal activities. It is worth noting that sulfilimine 15a has highly competitive water solubility and permeability to cyantraniliprole, which should aid in plant uptake and translocation [29].
3. Material and Methods
General Information
Analytical thin layer chromatography (TLC) was performed on Kieselgel 60 F254 glass plates precoated with a 0.2 mm thickness of silica gel. The TLC plates were visualized by shortwave (254 nm), potassium permanganate, or ceric ammonium molybdate stain. Flash chromatography was carried out with Kieselgel 60 (230−400 mesh) silica gel. Melting points: Barnstead/Electrothermal 9300, measurements were performed in open glass capillaries. NMR spectra: Bruker AV 300MHz (1H-NMR: 300 MHz, 13C-NMR: 75 MHz), AV 500MHz (1H-NMR: 500 NHz, 13C-NMR: 125 MHz), AV2 500MHz (19F-NMR: 470 MHz), the spectra were recorded in CDCl3 and DMSO-d6 using TMS as internal standard and are reported in ppm. 1H-NMR data are reported as: (s = singlet, d = doublet, t = triplet, q = quartet, br = broad singlet, qui = quintet, oct = octet, m = multiplet; coupling constant(s) in Hz; integration, proton assignment). High resolution mass spectra (HRMS): JEOL JMS-700. All solvents were purified using a column filter solvent purification system before use unless otherwise indicated. Reagents were purchased and used without further purification.
5-Fluoro-3-methyl-2-nitrobenzoic Acid (9)
To a solution of 3-fluoro-5-methylbenzoic acid (8, 100 mg, 0.6488 mmol) in H2SO4 (0.8 mL) was added potassium nitrate (72.16 mg, 0.7137 mmol) at 0 °C. After stirring at room temperature for 1 h, the resulting solid was washed with H2O to give 5-fluoro-3-methyl-2-nitrobenzoic acid (9, 75.3 mg, 58%) as a white solid. Analytical data: lit [33].
3-Methyl-5-(methylthio)-2-nitrobenzoic Acid (10)
To a solution of 5-fluoro-3-methyl-2-nitrobenzoic acid (9, 387.2 mg, 1.95 mmol) was added an aqueous sodium thiomethoxide (21%, 6.5 mL, 19.5 mmol) at 0 °C. After stirring at 150 °C for 18 h, the reaction mixture was extracted with EtOAc. The organic layer was dried over anhydrous MgSO4, filtered and evaporated. The resulting solid was washed with H2O to give 3-methyl-5-(methylthio)-2-nitrobenzoic acid (10, 325.5 mg, 74%). mp. 178 °C; 1H-NMR (300 MHz, DMSO) δ 7.54 (s, 1H), 7.41 (s, 1H), 2.54 (s, 3H), 2.23 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 165.4, 147.2, 141.5, 130.2, 129.4, 128.8, 124.4, 16.5, 14.3.
2-Amino-3-methyl-5-(methylthio)benzoic acid (11)
To a solution of 3-methyl-5-(methylthio)-2-nitrobenzoic acid (17, 42.9 mg, 0.1888 mmol) in THF (3 mL) was added sodium hydrosulfite (75%, 219 mg, 0.9440 mmol) in H2O (2 mL) at 0 °C. After stirring at 60 °C for 0.5 h, the reaction mixture was extracted with EtOAc. The organic layer was dried over anhydrous MgSO4, filtered, and evaporated. The resulting solid was washed with H2O to give 2-amino-3-methyl-5-(methylthio)benzoic acid (11, quantitative). mp. 172 °C; 1H-NMR (300 MHz, DMSO) δ 7.60 (d, J = 2.2 Hz, 1H), 7.23 (d, J = 1.6 Hz, 1H), 3.34 (br, 2H), 2.36 (s, 3H), 2.10 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 169.4, 148.6, 135.9, 129.8, 124.5, 120.4, 109.7, 17.9, 17.3.
2-(3-Bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-6-(methylthio)-4H-benzo[d][1,3]oxazin-4-one (12)
To a solution of 3-bromo-1-(pyridin-2-yl)-1H-pyrazole-5-carboxylic acid (13, 432.5 mg, 1.4298 mmol) in dried acetonitrile (1 mL) was added pyridine (0.23 mL, 2.8596 mmol) and methanesulfonyl chloride (0.16 mL, 2.1447 mmol) at 0 °C. After stirring at 0 °C for 30 min, a solution of 2-amino-3-methyl-5-(methylthio)benzoic acid (11, 282.67 mg, 1.4298 mmol) in dried acetonitrile and pyridine (0.35 mL, 4.2894 mmol) were added at 0 °C. After stirring at room temperature for 13 h, the reaction mixture was extracted with EtOAc (200 mL). The organic layer was dried over anhydrous MgSO4, filtered, and evaporated. The resulting crude residue was purified by column chromatography on silica gel (EtOAc/n-Hexane, 1:1) to give 2-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-6-(methylthio)-4H-benzo[d][1,3]oxazin-4-one (12, 481.4 mg, 73%). 1H-NMR (300 MHz, DMSO) δ 8.62 (dd, J = 1.4 Hz, J = 4.7 Hz, 1H), 8.34 (dd, J = 1.4 Hz, J = 8.1 Hz, 1H), 7.76 (dd, J = 4.7 Hz, J = 8.1 Hz, 1H), 7.64 (d, J = 2.1 Hz, 1H), 7.57 (d, J = 1.5 Hz, 1H), 7.47 (s, 1H), 2.54 (s, 3H), 1.71 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 157.7, 148.6, 147.7, 145.5, 140.5, 140.3, 139.9, 136.3, 135.8, 134.3, 128.5, 127.9, 127.3, 120.5, 117.8, 112.5, 15.8, 14.3.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylthio)phenyl)-1H-pyrazole-5-carboxamide (14)
To a solution of 2-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-6-(methylthio)-4H-benzo[d][1,3]oxazin-4-one (12, 400 mg, 0.8626 mmol) in THF (1.5 mL) was added methylamine solution (2.0 M in THF, 1.4 mL, 2.8465 mmol) at 0 °C. The mixture was stirred at 0 °C for 5 h, the mixture was extracted with EtOAc (200 mL). The organic layer was dried over anhydrous Na2SO4, filtered and evaporated. The resulting crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH, 20:1) to give 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylthio)phenyl)-1H-pyrazole-5-carboxamide (14, 263.4 mg, 62%). mp. 247 °C; LC/MS: Rt = 2.82 mins, m/z (ES-) = 494 (M + H for C19H17BrClN5O2S); IR (KBr): ν 1654, 1633, 1540, 1535, 1459, 1344, 1311, 1024, 958, 878, 861, 801, 794, 761 cm−1; 1H-NMR (300 MHz, CDCl3) δ 10.00 (s, 1H), 8.45 (s, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.36 (s, 1H), 7.17 (s, 1H), 7.05 (s, 2H), 6.26 (s, 1H), 2.92 (d, J = 3.1 Hz, 3H), 2.43 (s, 3H), 2.14 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 167.0, 155.5, 148.3, 147.0, 139.5, 139.1, 136.8, 136.6, 134.6, 129.3, 128.6, 127.7, 126.7, 126.5, 122.5, 110.4, 26.0, 17.7, 14.5; HRMS (EI) calcd for C19H17BrClN5O3S 494.9975, found 494.9949.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-4-(S-methyl-N-(2,2,2-trifluoroacetyl)sulfinimidoyl)-6-(methylcarbamoyl)phenyl)-1H-pyrazole-5-carboxamide (15a) and 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylsulfinyl)phenyl)-1H-pyrazole-5-carboxamide (15b)
To a solution of 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylthio)phenyl)-1H-pyrazole-5-carboxamide (14, 50 mg, 0.1014 mmol) in dried CH2Cl2 (1 mL) was added trifluoroacetamide (22.92 mg, 0.2028 mmol), magnesium oxide (16.35 mg, 0.4056 mmol), Rhodium(II) acetate (2.2 mg, 0.005 mmol), and iodobenzene diacetate (49 mg, 0.1521 mmol) at 0 °C. After stirring at room temperature for 13 h, the reaction mixture was extracted with CH2Cl2 (100 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated. The resulting crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH, 20:1) to give 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-4-(S-methyl-N-(2,2,2-trifluoroacetyl)sulfinimidoyl)-6-(methylcarbamoyl)phenyl)-1H-pyrazole-5-carboxamide (15a, 17.9 mg, 29%) and 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylsulfinyl)phenyl)-1H-pyrazole-5-carboxamide (15b, 11.3 mg, 22%). 15a: mp. 199–200 °C; IR (KBr): ν 1635, 1535, 1465, 1413, 1354, 1302, 1266, 1183, 1145, 962, 801 cm−1; 1H-NMR (500 MHz, CDCl3) δ 10.70 (s, 1H), 8.43 (d, J = 4.6 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.72 (s, 1H), 7.65 (s, 1H), 7.37 (dd, J = 4.7 Hz, J = 8.0 Hz, 1H), 7.14 (s, 1H), 6.93 (d, J = 4.7 Hz, 1H), 2.94 (d, J = 4.7 Hz, 3H), 2.86 (s, 3H), 2.24 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.0, 164.6 (q, CF3CO, J = 33.4 Hz), 155.4, 148.2, 147.0, 139.2, 139.1, 138.2, 136.6, 135.5, 131.2, 130.3, 127.7, 126.8, 126.5, 124.6, 116.8 (q, CF3, J = 289.8 Hz), 110.8, 32.8, 26.0, 17.9; 19F NMR (470 MHz, CDCl3) δ -73.28 ppm; HRMS (EI) calcd for C21H17BrClF3N6O3S 603.9907, found 603.9898. 15b: mp. 155 °C; IR (KBr): ν 1654, 1636, 1533, 1459, 1355, 1344, 1297, 1241, 1041, 1024, 959, 797 cm−1; 1H NMR (500 MHz, CDCl3) δ 10.64 (s, 1H), 8.46 (dd, J = 1.5 Hz, J = 4.7 Hz, 1H), 7.85 (dd, J = 1.5 Hz, J = 8.0 Hz, 1H), 7.71 (d, J = 1.7 Hz, 1H), 7.38 (dd, J = 4.7 Hz, J = 8.0 Hz, 2H), 7.09 (s, 1H), 6.70 (d, J = 4.7 Hz, 1H), 2.97 (d, J = 4.8 Hz, 3H), 2.67 (s, 3H), 2.27 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 168.3, 155.7, 149.0, 146.9, 143.0, 139.0, 138.9, 137.7, 136.8, 130.2, 129.0, 128.3, 128.3, 125.8, 119.6, 111.0, 44.0, 26.9, 19.6; HRMS (EI) calcd for C19H17BrClN5O3S 508.9924, found 508.9893.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-4-(S-methyl-N-(2,2,2-trifluoroacetyl)sulfonimidoyl)-6-(methylcarbamoyl)phenyl)-1H-pyrazole-5-carboxamide (16a)
To a solution of 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylsulfinyl)phenyl)-1H-pyrazole-5-carboxamide (15b, 30 mg, 0.0587 mmol) in dried CH2Cl2 (1 mL) was added trifluoroacetamide (13.28 mg, 0.1175 mmol), magnesium oxide (9.46 mg, 0.2348 mmol), Rhodium(II) acetate (1.3 mg, 0.003 mmol), and iodobenzene diacetate (28.36 mg, 0.0881 mmol) at 0 °C. After stirring at room temperature for 12 h, the reaction mixture was extracted with CH2Cl2 (×2). The organic layer was dried over anhydrous Na2SO4, filtered and evaporated. The resulting crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH, 20:1) to give 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-4-(S-methyl-N-(2,2,2-trifluoroacetyl)sulfonimidoyl)-6-(methylcarbamoyl)phenyl)-1H-pyrazole-5-carboxamide (16a, 15.7 mg, 43%). mp. 213.3 °C; IR (KBr): ν 1678, 1640, 1545, 1468, 1411, 1367, 1315, 1239, 1177, 1151, 1111, 994, 963, 838 cm−1; 1H-NMR (300 MHz, CDCl3) δ 10.79 (s, 1H), 8.46 (dd, J = 1.5 Hz, J = 4.7 Hz, 1H), 7.93 (d, J = 2.0 Hz, 1H), 7.87 (dd, J = 1.5 Hz, J = 8.0 Hz, 1H), 7.81 (s, 1H), 7.39 (dd, J = 4.7 Hz, J = 8.0 Hz, 1H), 7.09 (s, 1H), 6.50 (s, 1H), 3.41 (s, 3H), 3.01 (d, J = 4.9 Hz, 3H), 2.32 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 167.5, 164.1 (CF3CO), 155.5, 148.8, 146.9, 140.5, 139.1, 138.8, 138.4, 133.4, 131.4, 130.1, 129.1, 128.4, 125.9, 123.5, 115.8 (q, CF3, J = 288.5 Hz), 111.3, 44.2, 27.1, 19.9; 19F NMR (471 MHz, CDCl3) δ -76.90 (s); HRMS (EI) calcd for C21H17BrClF3N6O4S 619.9856, found 619.9852.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylsulfonyl)phenyl)-1H-pyrazole-5-carboxamide (16b)
To a solution of 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylthio)phenyl)-1H-pyrazole-5-carboxamide (14, 30 mg, 0.0609 mmol) in MeOH/CH2Cl2 (1:5, 1 mL) was added magnesium bis(monoperoxyphthalate) hexahydrate (80%, 75.25 mg, 0.1217 mmol) at 0 °C. After stirring at room temperature for 18 h, the reaction mixture was extracted with EtOAc (×2). The organic layer was dried over anhydrous Na2SO4, filtered and evaporated. The resulting crude residue was purified by column chromatography on silica gel (EtOAc/n-Hexane, 3:1) to give 3-bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-6-(methylcarbamoyl)-4-(methylsulfonyl)phenyl)-1H-pyrazole-5-carboxamide (16b, 29.9 mg, 93%). mp. 122 °C; IR (KBr): ν 1662, 1543, 1464, 1411, 1342, 1307, 1141, 962, 796, 764 cm−1; 1H-NMR (300 MHz, DMSO) δ 10.54 (br, 1H), 8.50 (dd, J = 1.3 Hz, J = 4.7 Hz, 1H), 8.17 (dd, J = 1.3 Hz, J = 8.1 Hz, 1H), 7.91 (s, 1H), 7.82 (s, 1H), 7.61 (dd, J = 4.7 Hz, J = 8.1 Hz, 1H), 7.41 (s, 1H), 3.22 (s, 3H), 2.69 (d, J = 4.5 Hz, 3H), 2.26 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 166.2, 155.5, 148.3, 147.2, 139.4, 139.2, 138.5, 137.8, 137.4, 134.6, 130.1, 127.9, 126.9, 126.7, 124.5, 110.9, 43.5, 26.2, 18.1; HRMS (EI) calcd for C19H17BrClN5O4S 524.9873, found 524.9844.
4. Conclusions
In summary, novel anthranilic diamides, in which organosulfur groups were substituted at the 4-position on the phenyl ring, were prepared and tested for their insecticidal activities and physical properties. For preparation of the target molecules, we expanded reported sulfur imination procedures [28] to amide groups containing sulfide 14. Our results concerning the relationship between insecticidal activities and physical properties showed that a better bioavailability profile (relatively low log P and high permeability) results in efficacy of sulfide 14 and sulfilimine 15a (Table 2 and Figure 3). Due to its higher water solubility, N-trifluoroacetyl sulfilimine 15a could be considered the most promising candidate for discovery of a new diamide insecticide. Notably, these studies have demonstrated that changing the substituents on sulfur atoms could lead to compounds with the desired physicochemical property profiles. Among organosulfur groups, N-trifluoroacetyl sulfilimine motif brings about desired properties such as solubility, lipophilicity, and permeability.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/19/3451/s1, Figure S1: 1H, 13C, and 19F NMR of compounds 9–16, Figure S2: pH-metric log P of compounds 14–16, Table S1: Larvicidal activity depend on time, Table S2: Picture of eating area.
Author Contributions
Methodology, H.J.L., W.H.L. and S.J.P.; investigation, H.J.L. and S.J.P.; analysis: H.J.L., W.H.L. and S.J.P.; writing—original draft preparation, S.J.P.; writing—review and editing, H.J.L. and S.J.P.
Funding
This work was fully supported by KRICT/ Kyung Nong Co. Ltd. co-research project (TS171-09R, TS181-10R, TS191-06R, and KK1932-30). This research was funded by Ministry of Trade, Industry and Energy, Republic of Korea, grant number 10052734 and 10077494.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Zhy, Y.; Loso, M.R.; Watson, G.B.; Sparks, T.C.; Rogers, R.B.; Huang, J.X.; Gerwick, B.C.; Babcock, J.M.; Kelley, D.; Hegde, V.B.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2010, 59, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.B.; Loso, M.R.; Babcock, J.M.; Hasler, J.M.; Letherer, T.J.; Young, C.D.; Zhu, Y.; Casida, J.E.; Sparks, T.C. Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem. Mol. Biol. 2011, 41, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Loso, M.R.; Nugent, B.M.; Huang, J.X.; Rogers, R.B. Multi-substituted Pyridyl Sulfoximines and Their Use as Insecticides. WO/2008/057129 A1, 15 May 2018. [Google Scholar]
- Sparks, T.C.; DeBoer, G.J.; Wang, N.X.; Hasler, J.M.; Loso, M.R.; Watson, G.B. Differential metabolism of sulfoximine and neonicotinoid insecticides by Drosophila melanogaster monooxygenase CYP6G1. Pestic. Biochem. Physiol. 2012, 103, 159–165. [Google Scholar]
- Longhurst, C.; Babcock, J.M.; Denholm, I.; Gorman, K.; Thomas, J.D.; Sparks, T.C. Cross-resistance relationships of the sulfoximine insecticide sulfoxaflor with neonicotinoids and other insecticides in the whiteflies Bemisia tabaci and Trialeurodes vaporariorum. Pest Manag. Sci. 2013, 69, 809–813. [Google Scholar]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nugent, B.M.; Buysse, A.M.; Loso, M.R.; Babcock, J.M.; Johnson, T.C.; Oliver, M.R.; Martin, T.P.; Ober, M.S.; Breaux, N.; Robinson, A.; et al. Expanding the structure–activity relationship of sulfoxaflor: the synthesis and biological activity of N-heterocyclic sulfoximines. Pest Manag. Sci. 2015, 71, 928–936. [Google Scholar]
- Arndt, K.E.; Bland, D.C.; Irvine, N.M.; Powers, S.L.; Martin, T.P.; McConnell, J.R.; Podhorez, D.E.; Renga, J.M.; Ross, R.; Roth, G.A.; et al. Development of a Scalable Process for the Crop Protection Agent Isoclast. Org. Process. Res. Dev. 2015, 19, 454–462. [Google Scholar] [CrossRef]
- Zhou, S.; Jia, Z.; Xiong, L.; Yan, T.; Yang, N.; Wu, G.; Song, H.; Li, Z. Chiral Dicarboxamide Scaffolds Containing a Sulfiliminyl Moiety as Potential Ryanodine Receptor Activators. J. Agric. Food Chem. 2014, 62, 6269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gu, Y.; Liu, M.; Wu, C.; Zhou, S.; Zhao, Y.; Jia, Z.; Wang, B.; Xiong, L.; Yang, N.; et al. Insecticidal Activities of Chiral N-Trifluoroacetyl Sulfilimines as Potential Ryanodine Receptor Modulators. J. Agric. Food Chem. 2014, 62, 11054. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, T.; Li, Y.; Jia, Z.; Wang, B.; Zhao, Y.; Qiao, Y.; Xiong, L.; Li, Y.; Li, Z. Novel phthalamides containing sulfiliminyl moieties and derivatives as potential ryanodine receptor modulators. Org. Biomol. Chem. 2014, 12, 6643. [Google Scholar] [CrossRef]
- Hua, X.; Mao, W.; Fan, Z.; Ji, X.; Li, F.; Zong, G.; Song, H.; Li, J.; Zhou, L.; Zhou, L.; et al. Novel Anthranilic Diamide Insecticides: Design, Synthesis, and Insecticidal Evaluation. Aust. J. Chem. 2014, 67, 1491. [Google Scholar] [CrossRef]
- Koerber, K.; Wach, J.; Kaiser, F.; Pohlman, M.; Deshmukh, P.; Culbertson, D.L.; Rogers, W.D.; Gunjima, K.; David, M.; Braun, F.J.; et al. Method of Controlling Ryanodine-Modulator Insecticide Resistant Insects. WO/2014/053406 A1, 10 April 2014. [Google Scholar]
- Gnamm, C.; Jeanguenat, A.; Dutton, A.C.; Grimm, C.; Kloer, D.P. Novel diamide insecticides: Sulfoximines, sulfonimidamides and other new sulfonimidoyl derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Muehlebach, M.; Jeanguenat, A.; Hall, R.G. Novel Insecticides. WO/2007/080131 A2, 19 July 2007. [Google Scholar]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, N.; Garden, H.; Evertsson, E.; Bratt, E.; Lepistö, M.; Johannesson, P.; Svensson, P.H. Synthesis and Functionalization of Cyclic Sulfonimidamides: A Novel Chiral Heterocyclic Carboxylic Acid Bioisostere. ACS Med. Chem. Lett. 2012, 3, 574. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Huryn, D.M.; Smith, A.B., III. Carboxylic Acid (Bio)Isosteres in Drug Design. Chem. Med. Chem. 2013, 8, 385–395. [Google Scholar]
- Nishimura, N.; Norman, M.H.; Liu, L.; Yang, K.C.; Ashton, K.S.; Bartberger, M.D.; Chmait, S.; Chen, J.; Cupples, R.; Fotsch, C. Small Molecule Disruptors of the Glucokinase–Glucokinase Regulatory Protein Interaction: 3. Structure–Activity Relationships within the Aryl Carbinol Region of the N-Arylsulfonamido-N′-arylpiperazine Series. J. Med. Chem. 2014, 57, 3094–3116. [Google Scholar] [CrossRef] [PubMed]
- Borhade, S.R.; Svensson, R.; Brandt, P.; Artursson, P.; Arvidsson, P.I.; Sandstroem, A. Preclinical Characterization of Acyl Sulfonimidamides: Potential Carboxylic Acid Bioisosteres with Tunable Properties. Chem. Med. Chem. 2015, 10, 455–460. [Google Scholar] [CrossRef]
- Luecking, U. For review focusing on medicinal chemistry of sulfoximines. Sulfoximines: A Neglected Opportunity in Medicinal Chemistry. Angew. Chem. Int. Ed. 2013, 52, 9399. [Google Scholar] [CrossRef]
- Seong, J.P. Sulfilimine- and Sulfoximine-Based Bioactives: Syntheses, COX Inhibition, and Anticancer Activity. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2013. [Google Scholar]
- Reggelin, M.; Zur, C. Sulfoximines: Structures, Properties and Synthetic Applications. Synthesis 2000, 2000, 1–64. [Google Scholar] [CrossRef]
- Okamura, H.; Bolm, C. Sulfoximines: Synthesis and Catalytic Applications. Chem. Lett. 2004, 33, 482. [Google Scholar] [CrossRef]
- Bolm, C. Asymmetric Synthesis with Chemical and Bilolgical Methods; Enders, D., Jaeger, K.-E., Eds.; Wiley/VCH: Weinheim, Germany, 2007; p. 149. [Google Scholar]
- Worch, C.; Mayer, A.C.; Bolm, C. Organosulfur Chemsitry in Asymmetric Synthesis; Toru, T., Bolm, C., Eds.; Wiley/VCH: Weinheim, Germany, 2008; p. 209. [Google Scholar]
- Bizet, V.; Hendriks, C.M.M.; Bolm, C. Sulfur imidations: access to sulfimides and sulfoximines. Chem. Soc. Rev. 2015, 44, 3378. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Bolm, C. Rhodium-Catalyzed Imination of Sulfoxides and Sulfides: Efficient Preparation of N-Unsubstituted Sulfoximines and Sulfilimines. Org. Lett. 2004, 6, 1305. [Google Scholar] [CrossRef] [PubMed]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Cordova, D.; Flexner, L.; Bellin, C.A.; Dubas, C.M.; Smith, B.K.; Hughes, K.A.; et al. Rynaxypyr™: A new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 2007, 17, 6274–6279. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.A.; Cordova, D.; Annan, I.B.; Barry, J.D.; Benner, E.A.; Currie, M.J.; Pahutski, T.F. Discovery of cyantraniliprole, a potent and selective anthranilic diamide ryanodine receptor activator with cross-spectrum insecticidal activity. Bioorg. Med. Chem. Lett. 2013, 23, 6341. [Google Scholar]
- Chang, S.Y.; Heo, J.N.; Lee, H.; Lim, H.J.; Kim, B.T.; Kim, J.K.; Kim, J. Diaminoaryl Derivatives Substituted by Carbamate and Pesticidal Composition Containing Same. WO2013/168967 A1, 14 November 2013. [Google Scholar]
- Zhang, J.; Xu, J.; Wang, B.; Li, Y.; Xiong, L.; Li, Y.; Ma, Y.; Li, Z. Synthesis and Insecticidal Activities of Novel Anthranilic Diamides Containing Acylthiourea and Acylurea. J. Agric. Food Chem. 2012, 60, 7565. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, A.; Basdevant, B.; Wanie, V.; Legault, C.Y. Catalytic Enantioselective α-Tosyloxylation of Ketones Using Iodoaryloxazoline Catalysts: Insights on the Stereoinduction Process. J. Org. Chem. 2012, 77, 11283. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Ontoria, O.J.M.; Scarpelli, R.; Schultz-Fademrecht, C. Amide Substituted Indazoles as Poly(ADP-ribose)Polymerase(PARP) Inhibitors. WO2008/084261 A1, 17 July 2008. [Google Scholar]
- According to the literature 3a, 3b, and 3d methods, the insecticidal assays were performed by Kyung Nong Co. Ltd., Korea (http://www.knco.co.kr/company/en_aboutus/). In detail, please see the Supplementary materials.
- Akamatsu, M. Importance of Physicochemical Properties for the Design of New Pesticides. J. Agric. Food Chem. 2011, 59, 2909. [Google Scholar] [CrossRef] [PubMed]
- The physicochemical measurements for determinations of equilibrium solubility, log P, and PAMPA permeability were performed by Drug Discovery Platform Technology Team, KRICT, Korea (https://english.krict.re.kr/eng/main).
- Avdeef, A.; Berger, C.M.; Brownell, C. pH-Metric Solubility. 2. Correlation between the acid-base titration and the saturation shake-flask solubility-pH methods. Pharm. Res. 2000, 17, 85–89. [Google Scholar] [CrossRef] [PubMed]
- For LogP, Using ACD/Labs T3 method (pH—metric), for graphs; please see the Supplementary materials.
- Avdeef, A.; Artursson, P.; Neuhoff, S.; Lazarova, L.; Gråsjö, J.; Tavelin, S. Caco-2 permeability of weakly basic drugs predicted with the double-sink PAMPA pKaflux Method. Eur. J. Pharm. Sci. 2005, 24, 333–349. [Google Scholar] [CrossRef]
- Kerns, E.H.; Di, L. Drug-Like Properties: Concepts, Structure Design and Methods; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).