Current Opinion on Usage of L-Carnitine in End-Stage Renal Disease Patients on Peritoneal Dialysis
Abstract
1. Introduction
2. L-Carnitine in Peritoneal Dialysis
2.1. Metabolism
2.2. Studies on Oral Supplementation of L-Carnitine
2.3. Studies on L-Carnitine Addition to PD Fluid
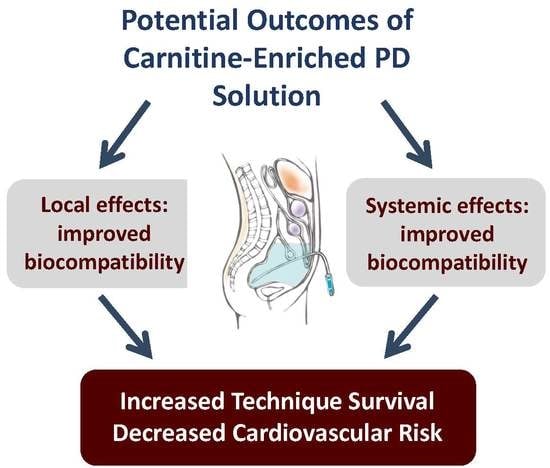
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Walker, R.C.; Howard, K. Cost effectiveness of dialysis modalities: A systemic review of economic evaluations. Appl. Health Econ. Health Policy 2019, 17, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Gokal, R.; Mallick, R.P. Peritoneal dialysis. Lancet 1999, 353, 823–828. [Google Scholar] [CrossRef]
- Davies, S.J. Peritoneal dialysis solutions. In Chronic kidney disease, dialysis, and transplantation: Companion to Brenner and Rector’s The Kidney, 2nd ed.; Pereira, B.J.G., Sayegh, M.H., Blake, P., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2004; pp. 534–552. [Google Scholar]
- Bartosova, M.; Schmitt, C.P. Biocompatible peritoneal dialysis: The target is still way off. Front. Physiol. 2019, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Phillips, L.; Griffiths, A.; Russell, L.H.; Naish, P.F.; Russell, G.I. What really happens to people on long-term peritoneal dialysis? Kidney Int. 1998, 54, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Burkart, J. Metabolic consequences of peritoneal dialysis. Semin. Dial. 2004, 17, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Krediet, R.T.; Balafa, O. Cardiovascular risk in the peritoneal dialysis patients. Nat. Rev. Nephrol. 2010, 6, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.K.; Lin, C.L.; Chen, H.C.; Lin, S.Y.; Chang, C.T.; Yen, T.H.; Sung, F.C. Risk of new-onset diabetes in end-stage renal disease patients undergoing dialysis: Analysis from registry data of Taiwan. Nephrol. Dial. Transplant. 2018, 33, 670–675. [Google Scholar] [CrossRef]
- Szeto, C.C.; Johnson, D.W. Low GDP solution and glucose-sparing strategies for peritoneal dialysis. Semin. Nephrol. 2017, 37, 30–42. [Google Scholar] [CrossRef]
- Guarnieri, G.; Situlin, R.; Biolo, G. Carnitine metabolism in uremia. Am. J. Kidney Dis. 2001, 38, S63–S67. [Google Scholar] [CrossRef]
- Eknoyan, G.; Latos, D.L.; Lindberg, J. Practice recommendations for the use of L-carnitine in dialysis-related carnitine disorder. National Kidney Foundation Carnitine Consensus Conference. Am. J. Kidney Dis. 2003, 41, 868–876. [Google Scholar] [CrossRef]
- Schröder, C.H. The management of anemia in pediatric peritoneal dialysis patients. Guidelines by an ad hoc European committee. Pediatr. Neprol. 2003, 18, 805–809. [Google Scholar] [CrossRef]
- Schröder, B.D. Debate forum: Levocarnitine therapy is rational and justified in selected dialysis patients. Blood Purif. 2006, 24, 128–139. [Google Scholar]
- Pliakogiannis, T.; Chatzidimitriou, C.; Evangeliou, A.; Bohles, H.J.; Kalaitzidis, K. Serum carnitine levels, lipid profile, and metabolic status of patients on continuous ambulatory peritoneal dialysis. Perit Dial. Int. 1993, 13, S440–S443. [Google Scholar] [PubMed]
- Constantin-Teodosiu, D.; Kirby, D.P.; Short, A.H.; Burdern, R.P.; Morgan, A.G.; Greenhaff, P.L. Free and esterified carnitine in continuous ambulatory peritoneal dialysis patients. Kidney Int. 1996, 49, 158–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Liberato, L.; Arduini, A.; Rossi, C.; Di Castelnuovo, A.; Posari, C.; Sacchetta, P.; Urbani, A.; Bonomini, M. L-Carnitine status in end-stage renal disease patients on automated peritonal dialysis. J. Nephrol. 2014, 27, 699–706. [Google Scholar] [CrossRef]
- Kosan, C.; Sever, L.; Arisoy, N.; Caliskan, S.; Kasapcopur, O. Carnitine supplementation improves apolipoprotein B levels in pediatric peritoneal dialysis patients. Pediatr. Nephrol. 2003, 18, 1184–1188. [Google Scholar] [CrossRef]
- Aguilar-Kitsu, A.; Ibarra-Cazares, P.; Mendoza-Guevara, L.; Villasis-Keever, M.A.; Perez Andrade, M.E.; Castillo-Romero, L.; Morales-Nava, A.; Rodriguez-Leyva, F.; Sanchez-Barbosa, L. Frequency of low carnitine levels in children on dalysis. Adv. Perit. Dial. 2006, 22, 208–210. [Google Scholar]
- Verrina, E.; Caruso, U.; Calevo, M.G.; Emma, F.; Sorino, P.; De Palo, T.; Lavoratti, G.; Turrini Dertenois, L.; Cassanello, M.; Cerone, R.; et al. Effect of carnitine supplementation on lipid profile and anemia in children on chronic dialysis. Pediatr. Nephrol. 2007, 22, 727–733. [Google Scholar] [CrossRef]
- Wanner, C.; Forstner-Wanner, S.; Schaeffen, G.; Schollmeyer, P.; Horl, W.H. Serum free carnitine, carnitine esters and lipids in patients on peritoneal dialysis and hemodialysis. Am. J. Nephrol. 1986, 6, 206–211. [Google Scholar] [CrossRef]
- Warady, B.A.; Borum, P.; Stall, C.; Millspaugh, J.; Taggart, E.; Lum, G. Carnitine status of pediatric patients on continuous ambulatory peritoneal dialysis. Am. J. Nephrol. 1990, 10, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.B.; Azocar, M.; Molina, M.; Guerrero, J.L.; Ratner, R.; Cano, F. Total carnitine and acylated carnitine ratio: Relationship of free carnitine with lipid parameters in pediatric dialysis patients. Adv. Perit. Dial. 2006, 22, 130–135. [Google Scholar] [PubMed]
- Grzegorzewska, A.E.; Mariak, I.; Dobrowolska-Zachwieja, A. Continuous laboratory peritoneal dialysis [CAPD] adequacy influences serum free carnitine level. Int. Urol. Nephrol. 1999, 31, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Evans, A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. 2003, 41, S13–S26. [Google Scholar] [CrossRef]
- Ahmad, S. L-carnitine in dialysis patients. Semin Dial. 2001, 14, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, C.; Plikogiannis, T.; Evangeliou, A.; Tsalkidou, T.; Böhles, H.J.; Kalaitzidis, K. Evaluation of carnitine levels according to the peritoneal equilibration test in patients on continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 1993, 13, S444–S447. [Google Scholar]
- Lilien, M.R.; Duran, M.; Quak, J.M.; Frankhuisen, J.J.; Schröder, C.H. Oral L-carnitine does not decrease erythropoietin requirement in pediatric dialysis. Pediatr. Nephrol. 2000, 15, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Sotirakopoulos, N.; Athanasiou, G.; Tsitsios, T.; Mavromatidis, K. The influence of l-carnitine supplementation on hematocrit and hemoglobin levels in patients with end stage renal failure on CAPD. Ren Fail. 2002, 24, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, P.; Bon, G.B.; Cazzolato, G.; Quinci, G.B. Are apoliproteins better discriminators then lipids for atherosclerosis? Lancet 1979, 1, 901–903. [Google Scholar] [CrossRef]
- Brunzell, J.D.; Sniderman, A.D.; Albers, J.J.; Kwiterovich, P.O. Jr. Apoproteins B and A-I and coronary artery disease in humans. Arteriosclerosis 1984, 4, 79–83. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Austin, M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studfies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Del Vecchio, L.; Sirolli, V.; Locatelli, F. New treatment approaches for the anemia of CKD. Am. J. Kidney Dis. 2016, 67, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Zammit, V.; Pusey, C.D.; De Vecchi, A.; Arduini, A. Pharmacologcal use of L-carnitine in uremic anemia: Has its full potential been exploited? Pharmacol. Res. 2011, 63, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, M.; de Oliveira, A.A.; Milne, F.J. Physical properties of the red blood cells in chronic renal failure. Nephron 1991, 59, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nikolaos, S.; George, A.; Telemachos, T.; Maria, S.; Yannis, M.; Konstantinos, M. Effect of L-carnitine supplementation on red blood cells deformability in hemodialysis patients. Ren. Fail. 2000, 22, 73–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, A.M.; Fornasini, G. Pharmacokinetics of L-carnitine. Clin. Pharmacokinet. 2003, 42, 941–967. [Google Scholar] [CrossRef] [PubMed]
- Tein, I. Carnitine transport: Pathophysiology and metabolism of known molecular defects. J. Inherit. Metab. Dis. 2003, 26, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Bonomini, M.; Savica, V.; Amato, A.; Zammit, V. Carnitine in metabolic disease: Potential for pharmacological intervention. Pharmacol. Ther. 2008, 120, 149–156. [Google Scholar] [CrossRef]
- De Vecchi, A.; Patrosso, C.; Novembrino, C.; Finazzi, S.; Colucci, P.; De Franceschi, M.; Fasano, M.A.; Bamonti-Catena, F. Folate supplementation in peritoneal dialysis patients with normal erythrocyte folate. Effect on plasma homocysteine. Nephron 2001, 89, 297–302. [Google Scholar] [CrossRef]
- Zammit, V.A.; Rona, R.R.; Bonomini, M.; Arduini, A. Carnitine, mitochondrial function and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1353–1362. [Google Scholar] [CrossRef]
- Brass, E.P.; Adler, S.; Sietsema, K.E.; Hiatt, V.R.; Orlando, A.M.; Amato, A. Intravenous L-carnitine increases plasma carnitine, reduces fatigue, and may preserve exercise capacity in hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 1018–1028. [Google Scholar] [CrossRef]
- Bonomini, M.; Di Liberato, L.; Del Rosso, G.; Stingone, A.; Marinangeli, G.; Consoli, A.; Bertoli, S.; De Vecchi, A.; Bosi, E.; Russo, R.; et al. Effect of an L-carnitine-containing peritoneal dialysate on insulin sensitivity in patients treated with CAPD: A 4-month prospective, multicenter randomized trial. Am. J. Kidney Dis. 2013, 62, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.J. Glucotoxicity in peritoneal dialysis – Solutions for the solution! Adv. Chronic Kidney Dis. 2007, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Dousdampanis, P.; Musso, C.; Trigka, K. Icodextrin and peritoneal dialysis: Advantages and new applications. Int. Urol. Nephrol. 2018, 50, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Johnson, D.W.; Badve, S.; Craig, J.C.; Strippoli, G.F.; Wiggins, K.J. Impact of icodextrin on clinical outcomes in peritoneal dialysis: A systematic review of randomized controlled trials. Nephrol Dial. Transplant. 2013, 28, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Culleton, B.F.; Ariza, A.; Do, J.Y.; Johnson, D.W.; Sanabria, M.; Shockley, T.R.; Story, K.; Vatazin, A.; Verrelli, M.; et al. Randomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patients. J. Am. Soc. Nephrol. 2013, 24, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Li, F.K.; Chan, L.Y.; Woo, J.C.; Ho, S.K.; Lo, W.K.; Lai, K.N.; Chan, T.M. A 3-year, prospective, randomized, controlled study on amino acid dialysate in patients on CAPD. Am. J. Kidney Dis. 2003, 42, 173–183. [Google Scholar] [CrossRef]
- Jones, M.; Hagen, T.; Boyle, C.A.; Vonesh, E.; Hamburger, R.; Charytan, C.; Sandroni, S.; Bernard, D.; Piraino, B.; Schreiber, M.; et al. Treatment of malnutrition with 1,1% amino acid peritoneal dialysis solution: Results of a multicenter outpatient study. Am. J. Kidney Dis. 1998, 32, 761–769. [Google Scholar] [CrossRef]
- Johnson, D.W.; Agar, J.; Collins, J.; Disney, A.; Harris, D.C.; Ibels, L.; Irish, A.; Saltissi, D.; Suranyi, M. Recommendations for the use of icodextrin in peritoneal dialysis patients. Nephrology [Carlton] 2003, 8, 1–7. [Google Scholar] [CrossRef]
- Budavari, S.K.; Smith, K.A.; Heckelman, P. (Eds.) The Merck Index: An Encyclopedia of Drugs, Chemical, and Biologicals, 12th ed.; Merck & CO: Whitehouse Station, NJ, USA, 1996; pp. 302–303. [Google Scholar]
- Di Paolo, N.; Sacchi, G. Atlas of peritoneal histology. Perit. Dial. Int. 2000, 20, S5–S96. [Google Scholar]
- Gaggiotti, E.; Arduini, A.; Bonomini, M.; Valentini, G.; Sacchi, G.; Sansoni, E.; Salvo, D.; Di Paolo, N. Prevention of peritoneal sclerosis: A new proposal to substitute glucose with carnitine dialysis solution (biocompatibility testing in vitro and in rabbits). Int. J. Artif. Organs 2005, 28, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Pandolfi, A.; Di Liberato, L.; Di Silvestre, S.; Cnops, Y.; Di Tomo, P.; D’arezzo, M.; Monaco, M.P.; Giardinelli, A.; Di Pietro, N.; et al. L-carnitine is an osmotic agent suitable for peritoneal dialysis. Kidney Int. 2011, 80, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, A.P.; Nordin, M.K.; Kjellstrand, P.T.; Boberg, U.C. Toxicity of peritoneal dialysis fluids on cultured fibroblasts, L-929. Kidney Int. 1991, 40, 77–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bazzato, G.; Lucatello, S.; Landini, S. Intraperitoneal L-carnitine administration: Successful therapeutic approach in patients on double-bag system CAPD. Presented at the II International Symposium on Peritoneal Dialysis, Berlin, Germany, June 1981. [Google Scholar]
- Kopple, J.D.; Qing, D.P. Effect of L-carnitine on nitrogen balance in CAPD patients. Presented at the 32nd Annual Meeting of the American Society of Nephrology, Miami Beach, FL, USA, November 1999. [Google Scholar]
- Yeung, C.H.; Anapolski, M.; Setiawan, I.; Lang, F.; Cooper, T.G. Effects of putative epididymal osmolytes on sperm volume regulation of fertile and infertile c-ros transgenic mice. J. Androl. 2004, 25, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Corrales, R.M.; Luo, L.; Chang, E.Y.; Pflugfelder, S.C. Effects of osmoprotectans on hyperosmolar stress in cultured human corneal epithelial cells. Cornea 2008, 27, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Rippe, B. A three-pore model of peritoneal transport. Perit. Dial. Int. 1993, 13, S35–S38. [Google Scholar] [PubMed]
- Devuyst, O.; Ni, J.; Verbavatz, J.M. Aquaporin-1 in the peritoneal membrane: Implications for peritoneal dialysis and endothelial cell function. Biol. Cell 2005, 97, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Bene, J.; Hadzsiev, K.; Melegh, B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr. Diabetes 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Lopaschuk, G.D.; Arduini, A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis 2013, 231, 456–461. [Google Scholar] [CrossRef]
- Veeravalli, S.; Karu, K.; Scott, F.; Fennema, D.; Phillips, I.R.; Shephard, E.A. Effect of flavin-containing monooxygenase genotype, mouse strain, and gender on trimethylamine N-oxide production, plasma cholesterol concentration, and an index of atherosclerosis. Drug Metab. Dispos. 2018, 46, 20–25. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. L-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Mayo Clin. Proc. 2013, 88, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Günal, A.I.; Celiker, H.; Dönder, E.; Günal, S.Y. The effect of L-carnitine on insulin resistance in hemodialysed patients with chronic renal failure. J. Nephrol. 1999, 12, 38–40. [Google Scholar]
- Biolo, G.; Stulle, M.; Bianco, F.; Mengozzi, G.; Barazzoni, R.; Vasile, A.; Panzetta, G.; Guarnieri, G. Insulin action on glucose and protein metabolism during L-carnitine supplementation in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2008, 23, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G.; Zanetti, M.; Vinci, P.; Cattin, M.R.; Barazzoni, R. Insulin resistance in chronic uremia. J. Ren. Nutr. 2009, 19, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.M.; Lai, K.N. The importance of residual renal function in dialysis patients. Kidney Int. 2006, 69, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Matthys, E.; Dolkart, R.; Lameire, N. Potential hazards of glycerol dialysate in diabetic CAPD patients. Perit Dial. Bull. 1987, 7, 16–19. [Google Scholar]
- Bonomini, M. Carnitine. Presented at the 52nd European Renal Association-European Dialysis and Transplant Association Congress, London, UK, May 2015. [Google Scholar]
- Bonomini, M.; Di Silvestre, S.; Di Tomo, P.; Di Pietro, N.; Mandatori, D.; Di Liberato, L.; Sirolli, V.; Chiarelli, F.; Indiveri, C.; Pandolfi, A.; et al. Effect of peritoneal dialysis fluid containing osmo-metabolic agents on human endothelial cells. Drug Design Dev. Ther. 2016, 10, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1053–E1061. [Google Scholar] [CrossRef]
- Makinen, K.K. Can the pentitol-hexitol theory explain the clinical observations made with xylitol? Med. Hypotheses 2000, 54, 603–613. [Google Scholar] [CrossRef]
- Wang, Y.M.; van Eys, J. Nutritional significance of fructose and sugar alcohols. Annu. Rev. Nutr. 1981, 1, 437–475. [Google Scholar] [CrossRef] [PubMed]
- Bazzato, G.; Coli, U.; Landini, S.; Fracasso, A.; Morachiello, P.; Righetto, F.; Scanferla, F.; Onesti, G. Xylitol as osmotic agent in CAPD: An alternative to glucose for uremic diabetic patients? Trans. Am. Soc. Artif Intern. Organs 1982, 28, 280–286. [Google Scholar] [PubMed]
- Seo, E.Y.; An, S.H.; Cho, J.H.; Suh, H.S.; Park, S.H.; Gwak, H.; Kim, Y.L.; Ha, H. Effect of biocompatible peritoneal dialysis solution on residual renal function: A systematic review of randomized controlled trials. Perit Dial. Int. 2014, 34, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Mujais, S. Glucose sparing in peritoneal dialysis: Implications and metrics. Kidney Int. 2006, 70, S104–S109. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Patient Population | L-Carnitine Treatment | Duration of Treatment | Results |
|---|---|---|---|---|---|
| Warady et al. [22] | Randomized, no placebo-controlled | 6 CAPD (pediatric) | 100 mg/kg/day | 2 months | No change in triglycerides |
| Kosan et al. [18] | Open-label, no control group | 20 CAPD (pediatric) | 50 mg/kg/day, two divided doses | 30 days | Decreased apolipoprotein B |
| Verrina et al. [20] | Open-label, no control group | 13 CAPD (pediatric) | 20 mg/kg/day, two divided doses | 3 months | Positive carnitine balance, no effects on hematological or lipid parameters |
| Lilien et al. [28] | Open-label, no control group | 4 CAPD (pediatric) | 20 mg/kg/day, two divided doses | 26 weeks | No change in rHuEPO 1 requirement |
| Sotirakopoulos et al. [29] | Open-label, no control group | 12 CAPD (adult) | 2 g/day | 3 months | Increased hematocrit and hemoglobin levels; decreased rHuEPO dose |
| Study | Study Design | Patient Population | L-Carnitine Treatment | Duration of Treatment | Results |
|---|---|---|---|---|---|
| Bazzato et al. [56] | Open-label, no control group | 6 CAPD | 2 g/day in the PD solution for nocturnal exchange | 2 months | Improved lipid pattern in 6 patients |
| Kopple and Qing [57] | Open-label, no control group | 5 CAPD | 20 mg/kg/day, in the first daily PD solution | 14 days | Improved nitrogen balance |
| Bonomini et al. [54] | Open-label, no control group | 4 CAPD | 5 g in the PD solution for nocturnal exchange | 5 days | Increased net peritoneal nocturnal ultrafiltration |
| Bonomini et al. [43] | Randomized, single-blind, control group | 27 CAPD (standard solution, n = 12; experimental solution, n = 15) | 2 g in the PD solution for diurnal exchanges | 4 months | Increased insulin sensitivity, maintenance of urine output |
| Di Liberato et al. [17] | Open-label, no control group | 5 APD | 5 g in the first solution bag of night exchanges | 5 days | Stable laboratory, metabolic and dialytic parameters |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomini, M.; Di Liberato, L.; Zammit, V.; Arduini, A. Current Opinion on Usage of L-Carnitine in End-Stage Renal Disease Patients on Peritoneal Dialysis. Molecules 2019, 24, 3449. https://doi.org/10.3390/molecules24193449
Bonomini M, Di Liberato L, Zammit V, Arduini A. Current Opinion on Usage of L-Carnitine in End-Stage Renal Disease Patients on Peritoneal Dialysis. Molecules. 2019; 24(19):3449. https://doi.org/10.3390/molecules24193449
Chicago/Turabian StyleBonomini, Mario, Lorenzo Di Liberato, Victor Zammit, and Arduino Arduini. 2019. "Current Opinion on Usage of L-Carnitine in End-Stage Renal Disease Patients on Peritoneal Dialysis" Molecules 24, no. 19: 3449. https://doi.org/10.3390/molecules24193449
APA StyleBonomini, M., Di Liberato, L., Zammit, V., & Arduini, A. (2019). Current Opinion on Usage of L-Carnitine in End-Stage Renal Disease Patients on Peritoneal Dialysis. Molecules, 24(19), 3449. https://doi.org/10.3390/molecules24193449