Separation of Five Flavonoids from Aerial Parts of Salvia Miltiorrhiza Bunge Using HSCCC and Their Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
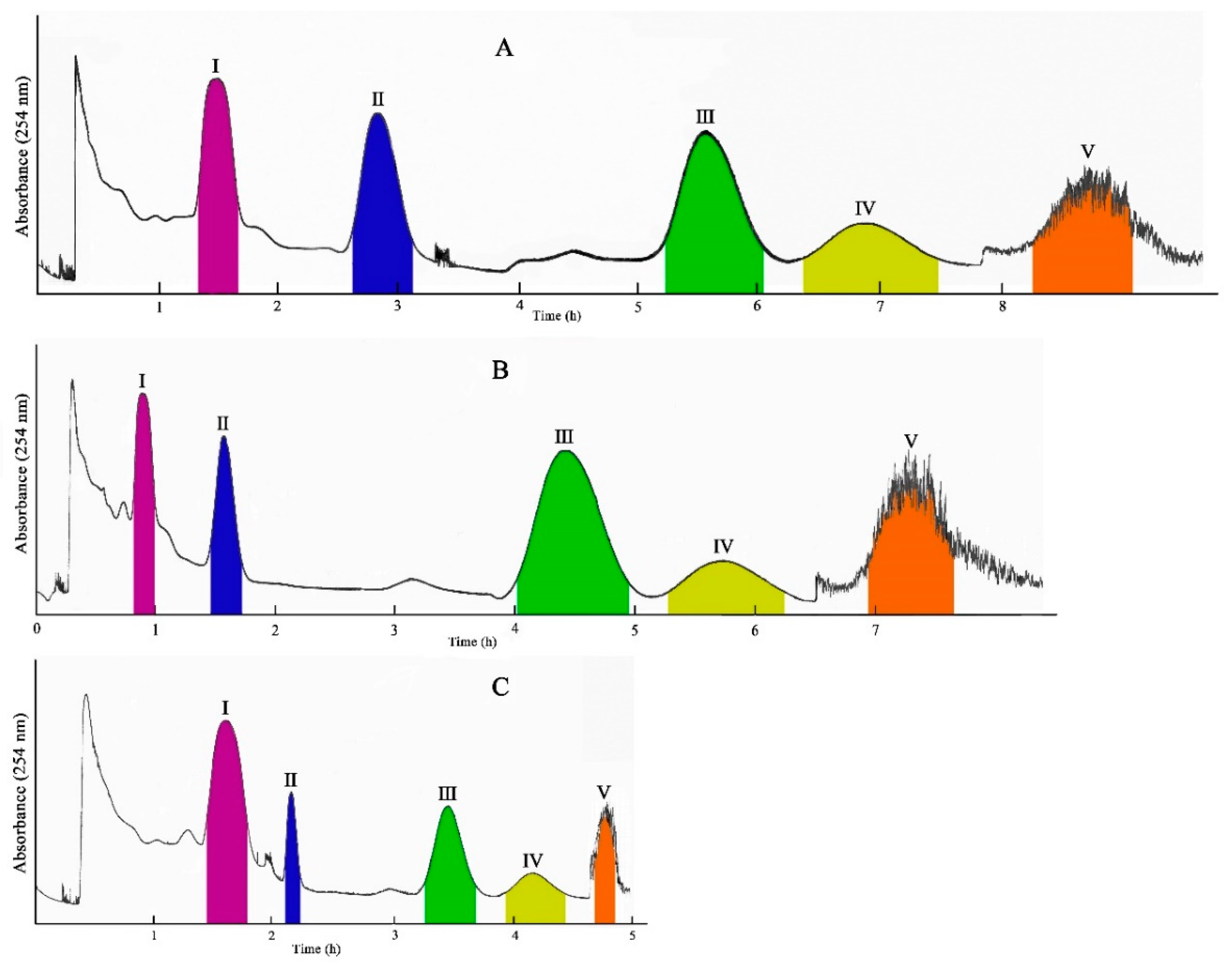
2.1. Selection of the HSCCC Solvent Systems
2.2. Selection of the Optimum HSCCC Experimental Conditions
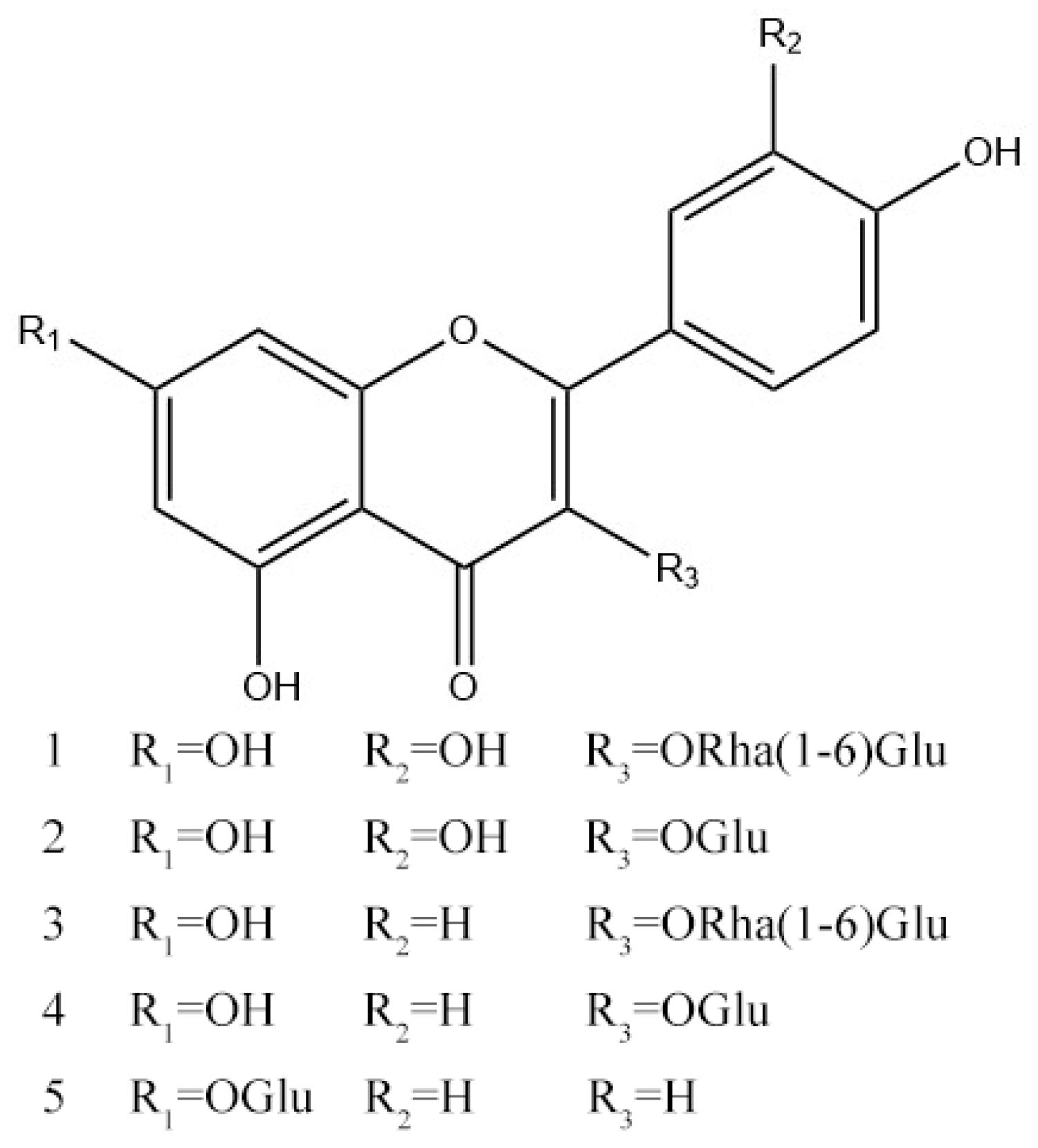
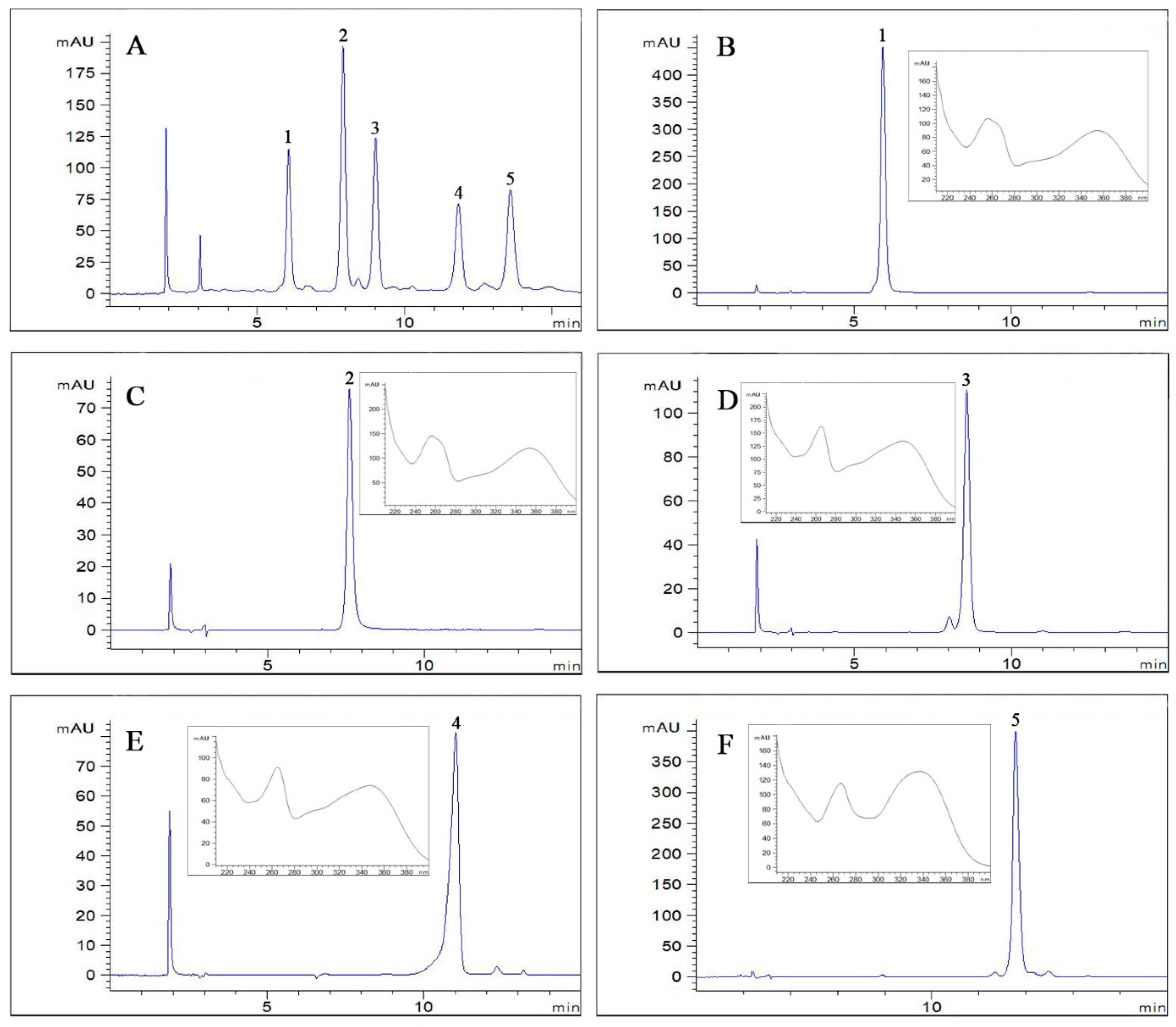
2.3. Identification of Compounds
2.4. Antioxidant Activity
3. Materials and Methods
3.1. Reagents and Materials
3.2. Apparatus
3.3. Preparation of Crude Extract
3.4. Selection of Solvent System
3.5. Preparation of the Solvent System and Sample Solutions
3.6. Optimization of HSCCC Separation Procedure
3.7. HPLC Analysis
3.8. Structural Identification
3.9. Evaluation of Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, C.-Y.; Ming, Q.-L.; Rahman, K.; Han, T.; Qin, L.-P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wang, Z. Antioxidant activities of leaf extract of Salvia miltiorrhiza Bunge and related phenolic constituents. Food Chem. Toxicol. 2010, 48, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Xu, L.; Chen, L. In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid β-induced cytotoxicity, and tanshinones act as acetylcholinesterase inhibitors. Env. Toxicol. Pharm. 2011, 31, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.F.Y.; Yeung, J.H.K.; Cheung, J.H.Y.; Or, P.M.Y. Pharmacological evidence for calcium channel inhibition by Danshen (Salvia miltiorrhiza) on rat isolated femoral artery. J. Cardiovasc. Pharm. 2006, 47, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.N.; An, C.N.; Pu, X.P. Protocatechuic acid inhibits neurotoxicity induced by MPTP in vivo. Neurosci. Lett. 2010, 474, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.K.; Ho, M.T.W.; Lin, H.Q.; Lau, K.F.; Rudd, J.A.; Chung, R.C.K.; Wan, D.C.C. Cryptotanshinone, an acetylcholinesterase inhibitor from Salvia miltiorrhiza, ameliorates scopolamine-induced amnesia in Morris water maze task. Planta. Med. 2010, 76, 228–234. [Google Scholar] [CrossRef]
- Kang, D.G.; Oh, H.; Sohn, E.J.; Hur, T.Y.; Lee, K.C.; Kim, K.J.; Lee, H.S. Lithospermic acid B isolated from Salvia miltiorrhiza ameliorates ischemia/reperfusion induced renal injury in rats. Life Sci. 2004, 75, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wu, C.H.; Luo, M.H.; Huang, Y.J.; Wang, C.N.; Shiao, M.S.; Huang, Y.T. In vitro protective effects of salvianolic acid B on primary hepatocytes and hepatic stellate cells. J. Ethnopharmacol. 2006, 105, 215–222. [Google Scholar] [CrossRef]
- Wu, Z.; Wen, T.; Tan, Y.; Liu, Y.; Ren, F.; Wu, H. Effects of salvianolic acid A on oxidative stress and liver injury induced by carbon tetrachloride in rats. Basic Clin. Pharmacol. 2007, 100, 115–120. [Google Scholar] [CrossRef]
- Tian, X.; Xue, W.; Ding, X. Application of Danshen injection on early stage of renal transplantation. Chin. J. Integr. Med. 2005, 25, 404–407. [Google Scholar]
- Zhang, Q.; Chang, Z.; Yang, J.; Wang, Q. Antiatherogenic property of triterpenoids-enriched extract from the aerial parts of Salvia miltiorrhiza. Phytother. Res. 2008, 22, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Wang, S.Q. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-α (TNF-α)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J. Mol. Cell Cardiol. 2006, 41, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z. Chemical composition, antimicrobial and antioxidant activities of the essential oil in leaves of Salvia miltiorrhiza Bunge. J. Essent. Oil Res. 2009, 21, 476–480. [Google Scholar] [CrossRef]
- Qian, L.; Zong, S.L.; Jun, R.W.; Xu, W.H. Essential oil composition of Salvia miltiorrhiza flower. Food Chem. 2009, 113, 592–594. [Google Scholar]
- Chen, H.; Zhang, Q.; Wang, X.; Yang, J.; Wang, Q. Qualitative analysis and simultaneous quantification of phenolic compounds in the aerial parts of Salvia miltiorrhiza by HPLC-DAD and ESI/MSn. Phytochem. Anal. 2011, 22, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.D.; Su, S.L.; Zeng, H.T.; Li, Y.; Zhu, Z.; Guo, J.; Zhu, Y.; Guo, S.; Li, Y.; Qian, D.; et al. Protective effects of Salvia miltiorrhiza on adenine-induced by regulating the metabolic profiling and modulating the NADPH oxidase/ROS/ERK and TGF-β/Smad signaling pathways. J. Ethnopharmacol. 2018, 212, 153–165. [Google Scholar] [CrossRef]
- Gu, J.F.; Su, S.L.; Guo, J.M.; Zhu, Y.; Zhao, M.; Duan, J.A. The aerial parts of Salvia miltiorrhiza Bge. strengthen intestinal barrier and modulate gut microbiota imbalance in streptozocin-induced diabetic mice. J. Funct. Foods. 2017, 36, 362–374. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Eguchi, T.; Kakinuma, K.; Ikekawa, N.; Sahai, M.; Gupta, Y.K. Squamocin, a new cytotoxic bis-tetrahydrofuran containing acetogenin from Annona squamosal. Chem.Pharm. Bull. 1988, 36, 4802–4806. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Fisher, D. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J. Chromatogr. A. 2009, 1216, 740–753. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, J.; Tan, N. The Chemical Constituents of Parakmeria yunnanensis. Acta. Botanica. Yunnanica. 2001, 23, 352–356. [Google Scholar]
- Zhang, Q.; Zhou, Q.; Huo, C.; Wang, Y.; Wu, Y.; Zhang, M.; L, L.; Jin, S.; Shi, Q.; Gu, Y. Phenolic Components of the Aerial Parts of Achillea alpina. Chem. Nat. Compd. 2019, 55, 337–339. [Google Scholar] [CrossRef]
- Leong, C.N.A.; Tako, M.; Hanashiro, I.; Tamaki, H. Antioxidant flavonoid glycosides from the leaves of Ficus pumila L. Food Chem. 2008, 109, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Si, C.L.; Du, Z.G.; Fan, S.; An, L.L. Isolation and Structure Elucidation of a New Flavonol Glycoside from Sophora japonica. Chem. Nat. Compd. 2016, 52, 794–797. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |
| Solvent systems | Ratio (v/v) | K | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| ethyl acetate/n-butanol/water | 4:1:5 | 0.67 | 5.61 | 1.92 | 52.31 | 18.64 |
| n-butanol/water | 1:1 | 0.42 | 0.67 | 0.61 | 0.75 | 0.85 |
| tert-butyl methyl ether/n-butanol/acetonitrile/water | 2:2:1:5 | 1.83 | 7.25 | 3.70 | 15.92 | 10.06 |
| tert-butyl methyl ether/n-butanol/acetonitrile/water | 2.5:1.5:1:5 | 1.44 | 7.68 | 2.95 | 12.70 | 8.11 |
| tert-butyl methyl ether/n-butanol/acetonitrile/water | 3:1:1:5 | 0.62 | 4.68 | 1.51 | 9.53 | 5.78 |
| tert-butyl methyl ether/n-butanol/acetonitrile/water | 3.5:0.5:1:5 | 0.11 | 2.22 | 0.57 | 4.39 | 2.53 |
| tert-butyl methyl ether/n-butanol/acetonitrile/water | 3:1:1:20 | 0.59 | 5.11 | 1.42 | 12.65 | 6.35 |
| Flow Rate (mL/min) | Retention volume (L) | Ratio (Retention volume/Total volume, %) |
|---|---|---|
| 2 | 243 | 81.0 |
| 4 | 225 | 75.0 |
| 6 | 215 | 71.7 |
| 8 | 205 | 68.3 |
| 10 | 200 | 66.7 |
| 12 | 193 | 64.3 |
| 14 | 188 | 62.7 |
| Samples | DPPH (IC50, μg/mL) a |
|---|---|
| Crude flavonoid extract | 34.2 |
| 1 | 16.7 |
| 2 | 17.1 |
| 3 | 136.6 |
| 4 | 159.5 |
| 5 | 177.1 |
| l-Ascorbic acid b | 6.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Qi, Y.; Liu, W.; Li, J.; Wang, D.; Fang, L.; Zhang, Y. Separation of Five Flavonoids from Aerial Parts of Salvia Miltiorrhiza Bunge Using HSCCC and Their Antioxidant Activities. Molecules 2019, 24, 3448. https://doi.org/10.3390/molecules24193448
Yang F, Qi Y, Liu W, Li J, Wang D, Fang L, Zhang Y. Separation of Five Flavonoids from Aerial Parts of Salvia Miltiorrhiza Bunge Using HSCCC and Their Antioxidant Activities. Molecules. 2019; 24(19):3448. https://doi.org/10.3390/molecules24193448
Chicago/Turabian StyleYang, Fan, Yingxue Qi, Wei Liu, Jia Li, Daijie Wang, Lei Fang, and Yongqing Zhang. 2019. "Separation of Five Flavonoids from Aerial Parts of Salvia Miltiorrhiza Bunge Using HSCCC and Their Antioxidant Activities" Molecules 24, no. 19: 3448. https://doi.org/10.3390/molecules24193448
APA StyleYang, F., Qi, Y., Liu, W., Li, J., Wang, D., Fang, L., & Zhang, Y. (2019). Separation of Five Flavonoids from Aerial Parts of Salvia Miltiorrhiza Bunge Using HSCCC and Their Antioxidant Activities. Molecules, 24(19), 3448. https://doi.org/10.3390/molecules24193448





