Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck)
Abstract
1. Introduction
2. Results
2.1. Effects of Light Irradiation on the Total Soluble Solid, Titration Acid, and Citrus Color Index of Postharvest Sweet Oranges
2.2. Effect of Light Irradiation on the Soluble Sugar Content in Postharvest Sweet Oranges
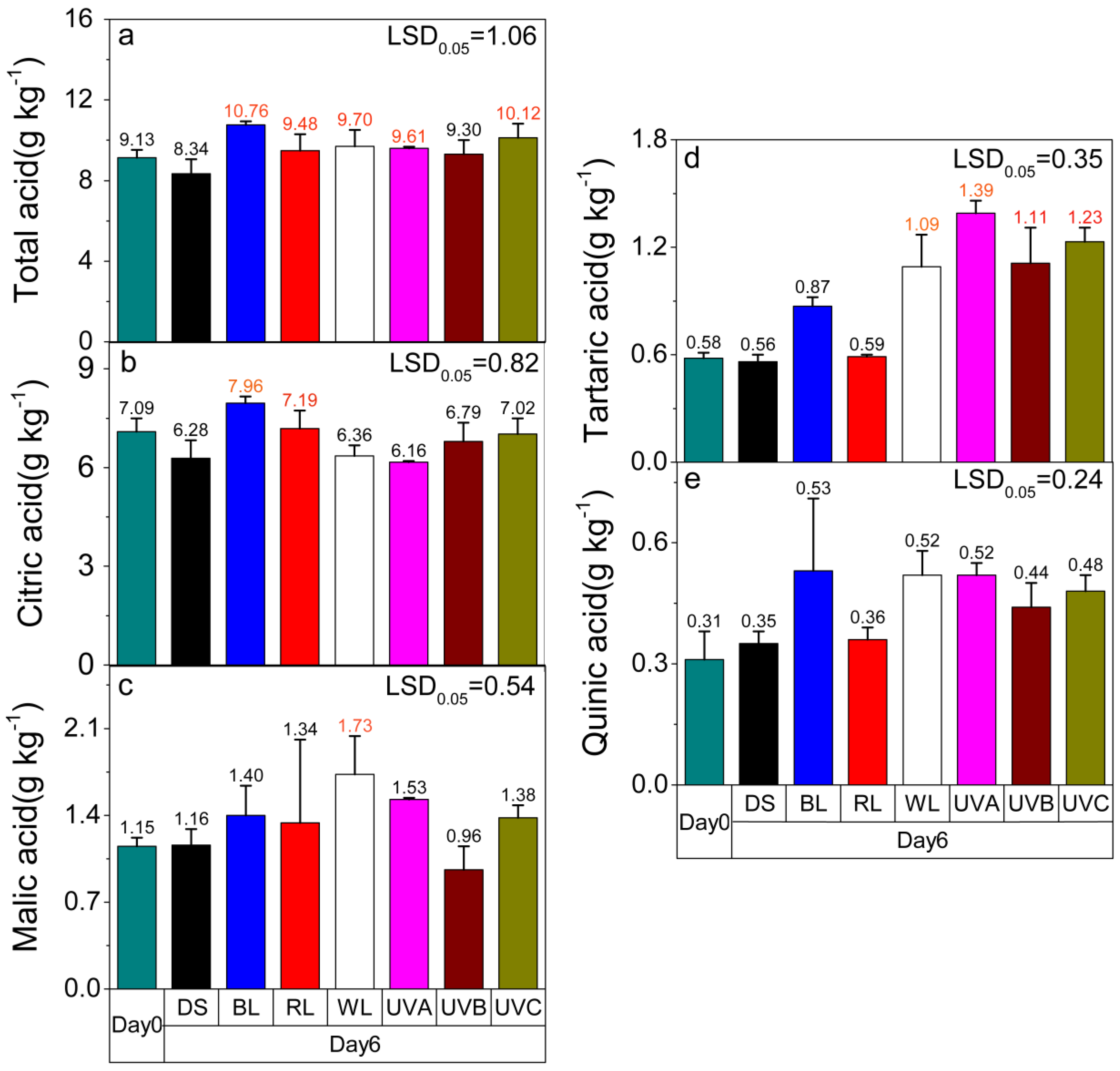
2.3. Effect of Light Irradiation on the Organic Acid Content in Postharvest Sweet Oranges
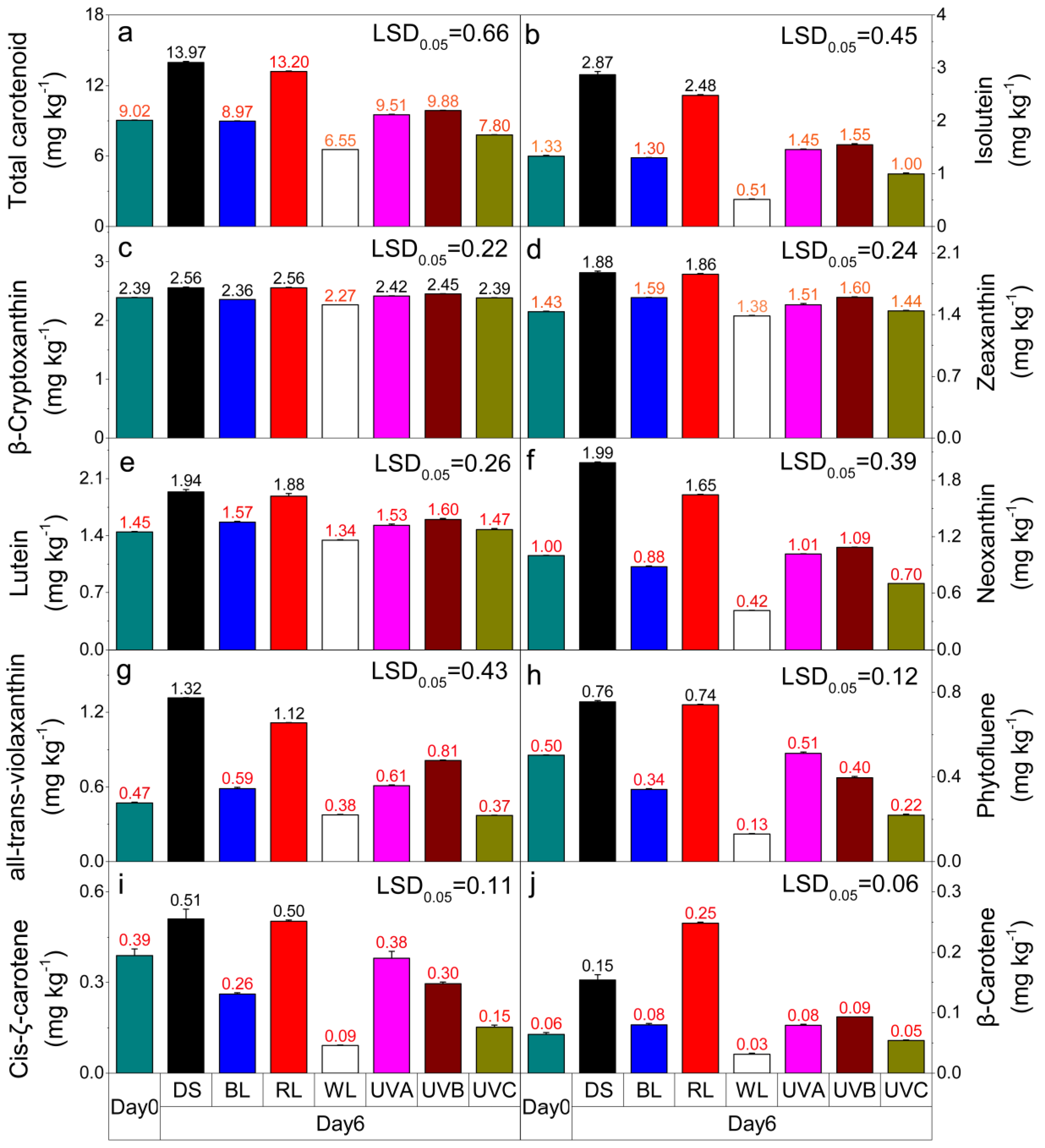
2.4. Effect of Light Irradiation on Carotenoid Content in Postharvest Sweet Oranges
2.5. Principal Component Analysis Based on Soluble Sugar, Organic Acid, and Carotenoid Content in Sweet Oranges Treated with Light Irradiation
3. Discussion
4. Materials and Methods
4.1. Fruit Materials
4.2. Postharvest Treatments
4.3. Determination of the Basic Ripening Parameters
4.4. Determination of the Soluble Sugar and Organic Acid Contents
4.5. Determination of Carotenoid Content
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food Sci. Nutr. 2018, 1–18. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nature Genet. 2012, 45, 59. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Cao, M.; Zheng, J.; Zhao, Y.; Zheng, Z.L. Gene coexpression network analysis of fruit transcriptomes uncovers a possible mechanistically distinct class of sugar/acid ratio-associated genes in sweet orange. BMC Plant Biol. 2017, 17, 186. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, K.; Zoratti, L.; Sarala, M.; Carvalho, E.; Hirsimaki, J.; Mentula, H.; Martens, S.; Haggman, H.; Jaakola, L. Carotenoid metabolism during bilberry (Vaccinium myrtillus L.) fruit development under different light conditions is regulated by biosynthesis and degradation. BMC Plant Biol. 2016, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Yungyuen, W.; Sato, Y.; Furuya, T.; Yahata, M.; Yamawaki, K.; Kato, M. Accumulation of carotenoids in a novel citrus cultivar ‘Seinannohikari’ during the fruit maturation. Plant Physiol. Biochem. 2018, 129, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Arbona, V.; Gómez-Cadenas, A. ABA accumulation in water-stressed Citrus roots does not rely on carotenoid content in this organ. Plant Sci. 2016, 252, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Pataro, G.; Sinik, M.; Capitoli, M.M.; Donsì, G.; Ferrari, G. The influence of post-harvest UV-C and pulsed light treatments on quality and antioxidant properties of tomato fruits during storage. Innov. Food Sci. Emerg. Technol. 2015, 30, 103–111. [Google Scholar] [CrossRef]
- Kokalj, D.; Zlatić, E.; Cigić, B.; Vidrih, R. Postharvest light-emitting diode irradiation of sweet cherries (Prunus avium L.) promotes accumulation of anthocyanins. Postharvest. Biol. Technol. 2019, 148, 192–199. [Google Scholar] [CrossRef]
- Huang, J.Y.; Xu, F.; Zhou, W. Effect of LED irradiation on the ripening and nutritional quality of postharvest banana fruit. J. Sci. Food Agric. 2018, 98, 5486–5493. [Google Scholar] [CrossRef]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Haggman, H.; Jaakola, L. On the developmental and environmental regulation of secondary metabolism in Vaccinium spp. Berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Yamawaki, K.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Ohta, S.; Kato, M. Effect of blue LED light intensity on carotenoid accumulation in citrus juice sacs. J. Plant Physiol. 2015, 188, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Schumann, T.; Paul, S.; Melzer, M.; Dormann, P.; Jahns, P. Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress. Front. Plant Sci. 2017, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Slominski, A.T. UVB Activates hypothalamic-pituitary-adrenal Axis in C57BL/6 mice. J. Investig. Dermatol. 2015, 135, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Dou, J.; Liu, W. Comparative transcriptome analysis reveals key genes potentially related to soluble sugar and organic acid accumulation in watermelon. PLoS ONE 2018, 13, e0190096. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.; Kammona, S.; Jaswir, I.; Jamal, P.; Hatta, F.A.M. Effect of abiotic stress on carotenoids accumulation in orange sweet potato callus under light and dark conditions. Int. Food Res. J. 2017, 24, S481–S487. [Google Scholar]
- Castle, W.S.; Baldwin, J.C.; Muraro, R.P. Rootstocks and the Performance and economic returns of ‘Hamlin’ sweet orange trees. Hortscience 2010, 45, 875–881. [Google Scholar] [CrossRef]
- Fidelibus, M.W.; Koch, K.E.; Davies, F.S. Gibberellic acid alters sucrose, hexoses, and their gradients in peel tissues during color break delay in ‘Hamlin’ orange. J. Am. Soc. Hortic. Sci. 2008, 133, 760–767. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Hu, G.; Liu, H.; Zhu, Y.; Hernandez, M.; Koutchma, T.; Shao, S. Suppression of the formation of furan by antioxidants during UV-C light treatment of sugar solutions and apple cider. Food Chem. 2018, 269, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Interdonato, R.; Rosa, M.; Nieva, C.B.; Juan, A.G.; Hilal, M.; Prado, F.E. Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environ. Exp. Bot. 2011, 70, 204–211. [Google Scholar] [CrossRef]
- Mohd-Hanif, H.; Shamsudin, R.; Adzahan, N.M. UVC dosage effects on the physico-chemical properties of lime (Citrus aurantifolia) juice. Food Sci. Biotechnol. 2016, 25, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Onik, J.C.; Xie, Y.; Duan, Y.; Hu, X.; Wang, Z.; Lin, Q. UV-C treatment promotes quality of early ripening apple fruit by regulating malate metabolizing genes during postharvest storage. PLoS ONE 2019, 14, e0215472. [Google Scholar] [CrossRef] [PubMed]
- Panjai, L.; Noga, G.; Fiebig, A.; Hunsche, M. Effects of continuous red light and short daily UV exposure during postharvest on carotenoid concentration and antioxidant capacity in stored tomatoes. Sci. Hortic. 2017, 226, 97–103. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Quan, J.; Zheng, Q.; Xi, W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016, 205, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, H.; Baldazzi, V.; van Leeuwen, C.; Bertin, N.; Gautier, H.; Wu, B.; Duchêne, E.; Gomès, E.; Delrot, S.; et al. Inter-species comparative analysis of components of soluble sugar concentration in fleshy fruits. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Shi, L.; Cao, S.; Shao, J.; Chen, W.; Yang, Z.; Zheng, Y. Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest. Biol. Technol. 2016, 111, 197–204. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Ai, Z.; Pang, L.; Li, Y.; Zhu, M. Light exposure during storage preserving soluble sugar and L-ascorbic acid content of minimally processed romaine lettuce (Lactuca sativa L. var. longifolia). Food Chem. 2013, 136, 273–278. [Google Scholar] [CrossRef]
- Shi, C.Y.; Guo, L.X.; Kamran, H.M.; Sadka, A.; Liu, Y.Z. Recent advances in the regulation of citric acid metabolism in citrus fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Zhu, K.; Yang, W.; Ye, J.; Chai, L.; Xu, Q.; Deng, X. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. Plant Physiol. 2018, 176, 2657–2676. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Tuan, P.A.; Thwe, A.A.; Kim, J.K.; Kim, Y.B.; Lee, S.; Park, S.U. Molecular characterisation and the light–dark regulation of carotenoid biosynthesis in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). Food Chem. 2013, 141, 3803–3812. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Dai, Z.; Liu, C.; Xiao, Y.; Wei, Q.; Song, J.; Li, D. Effect of UV-B radiation and a supplement of CaCl2 on carotenoid biosynthesis in germinated corn kernels. Food Chem. 2019, 278, 509–514. [Google Scholar] [CrossRef]
- Castagna, A.; Chiavaro, E.; Dall’Asta, C.; Rinaldi, M.; Galaverna, G.; Ranieri, A. Effect of postharvest UV-B irradiation on nutraceutical quality and physical properties of tomato fruits. Food Chem. 2013, 137, 151–158. [Google Scholar] [CrossRef]
- Bravo, S.; García-Alonso, J.; Martín-Pozuelo, G.; Gómez, V.; García-Valverde, V.; Navarro-González, I.; Periago, M.J. Effects of postharvest UV-C treatment on carotenoids and phenolic compounds of vine-ripe tomatoes. Int. J. Food Sci. Technol. 2013, 48, 1744–1749. [Google Scholar] [CrossRef]
- Liu, L.H.; Zabaras, D.; Bennett, L.E.; Aguas, P.; Woonton, B.W. Effects of UV-C, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chem. 2009, 115, 495–500. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Postharvest. Biol. Technol. 2015, 99, 99–104. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of blue and red LED light irradiation on β-cryptoxanthin accumulation in the flavedo of citrus fruits. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Ma, L.; Yi, X.; Ruan, J. Metabolomic analysis using ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS) uncovers the effects of light intensity and temperature under shading treatments on the metabolites in tea. PLoS ONE 2014, 9, e112572. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Stanojević, L.; Cvetković, D.; Fallik, E. Effects of the modification of light intensity by color shade nets on yield and quality of tomato fruits. Sci. Hortic. 2012, 139, 90–95. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, M.; Wu, R.; Niu, Q.; Teng, Y.; Zhang, D. Postharvest pigmentation in red Chinese sand pears (Pyrus pyrifolia Nakai) in response to optimum light and temperature. Postharvest. Biol. Technol. 2014, 91, 64–71. [Google Scholar] [CrossRef]
- Liu, S.; Hu, L.; Jiang, D.; Xi, W. Effect of post-harvest LED and UV lightirradiation on the accumulation of flavonoids and limonoids in the segments of Newhall navel oranges (Citrus sinensis Osbeck). Molecules 2019, 24, 1755. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Su, T.; Yu, S.; Wang, J.; Zhang, F.; Yu, Y.; Zhang, D.; Zhao, X.; Wang, W. Loss of function of the carotenoid isomerase gene BrCRTISO confers orange color to the inner leaves of Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol. Biol. Rep. 2015, 33, 648–659. [Google Scholar] [CrossRef]
- Liu, Y.; Roof, S.; Ye, Z.; Barry, C.; van Tuinen, A.; Vrebalov, J.; Bowler, C.; Giovannoni, J. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc. Natl. Acad. Sci. USA 2004, 101, 9897–9902. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. https://doi.org/10.3390/molecules24193440
Hu L, Yang C, Zhang L, Feng J, Xi W. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules. 2019; 24(19):3440. https://doi.org/10.3390/molecules24193440
Chicago/Turabian StyleHu, Linping, Can Yang, Lina Zhang, Jing Feng, and Wanpeng Xi. 2019. "Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck)" Molecules 24, no. 19: 3440. https://doi.org/10.3390/molecules24193440
APA StyleHu, L., Yang, C., Zhang, L., Feng, J., & Xi, W. (2019). Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules, 24(19), 3440. https://doi.org/10.3390/molecules24193440




