Drug Repurposing as an Antitumor Agent: Disulfiram-Mediated Carbonic Anhydrase 12 and Anion Exchanger 2 Modulation to Inhibit Cancer Cell Migration
Abstract
1. Introduction
2. Results
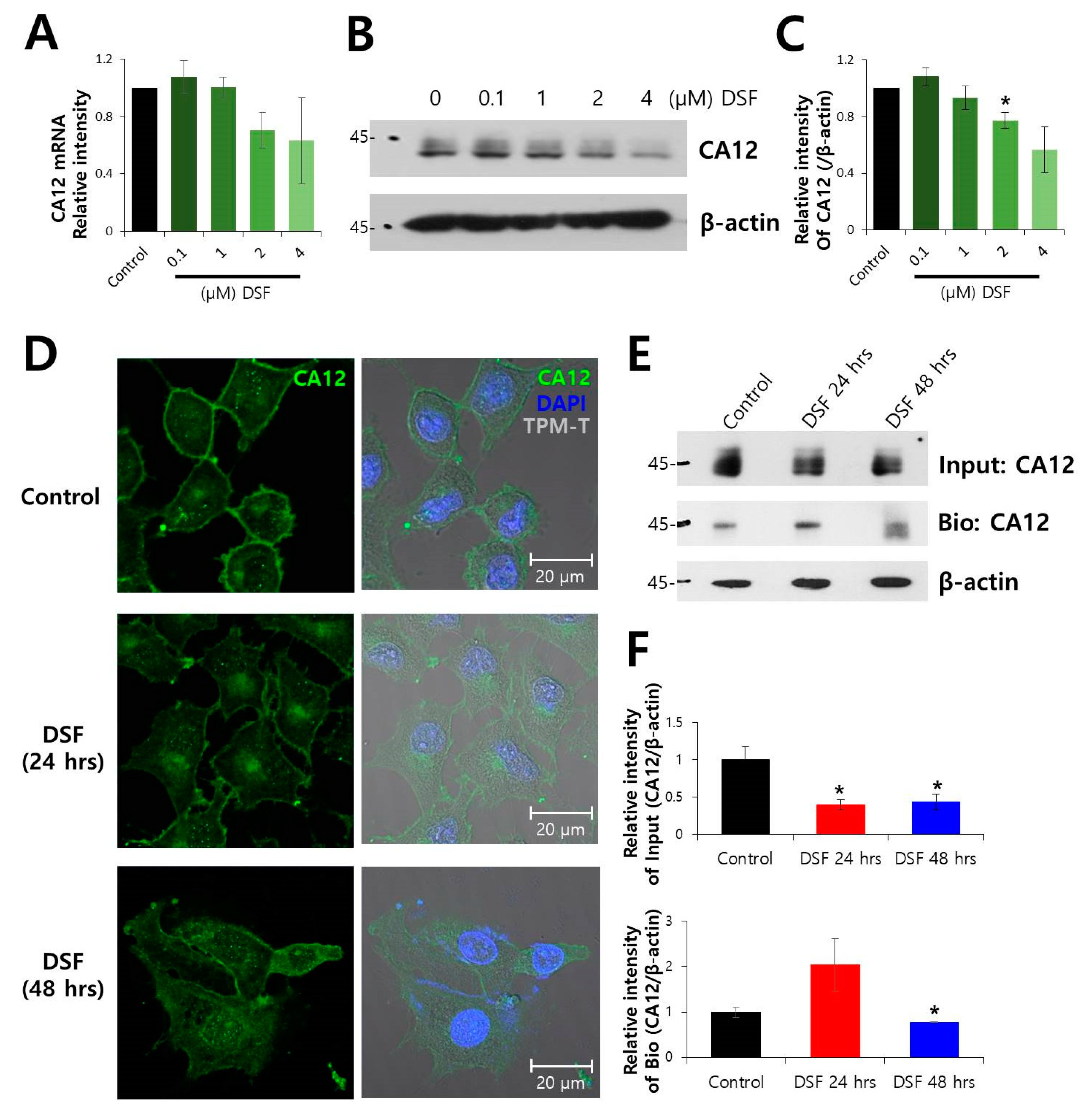
2.1. Surface Expression of CA12 Protein was Decreased by DSF Treatment
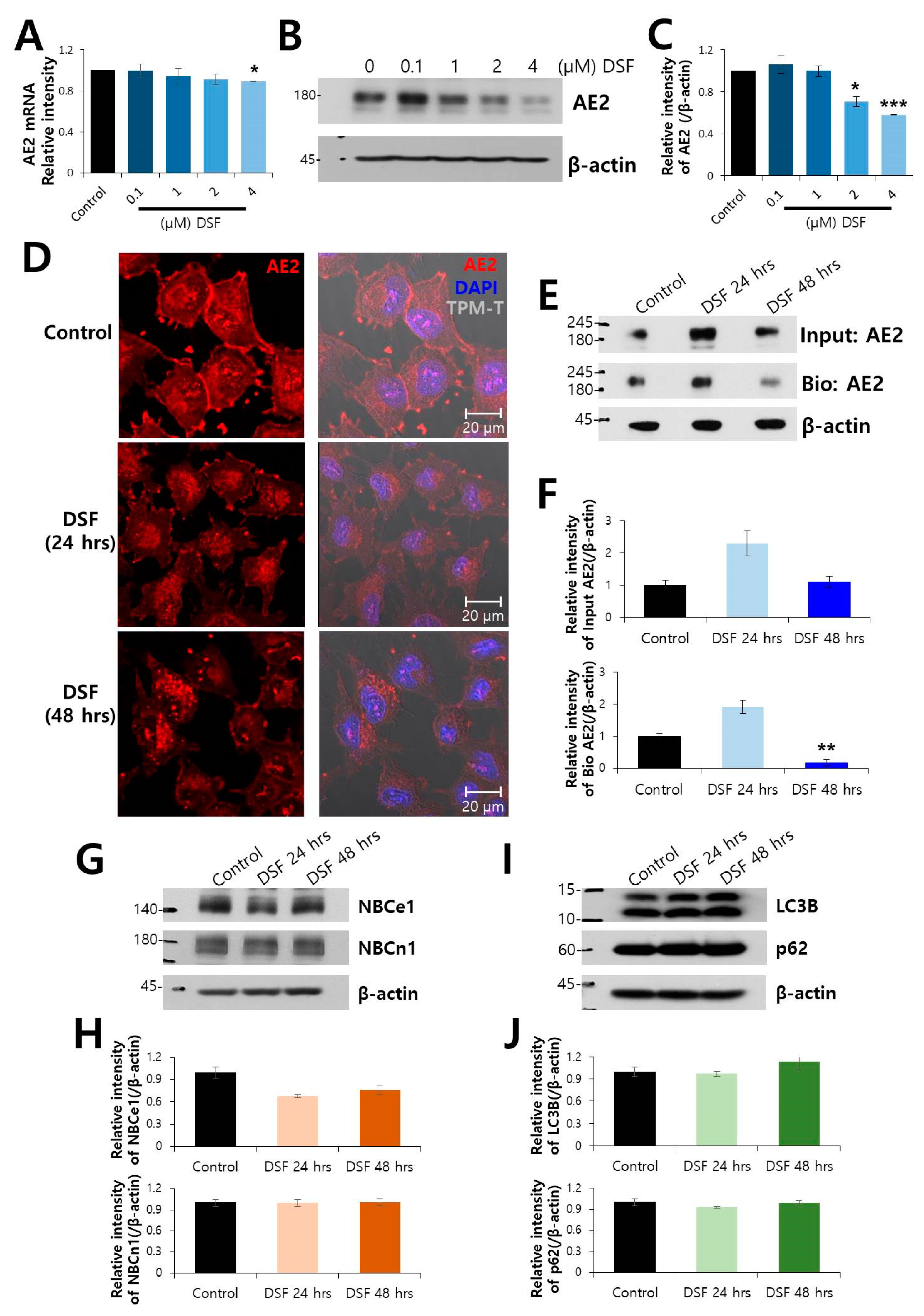
2.2. Surface Expression of AE2 Protein was Decreased by DSF Treatment
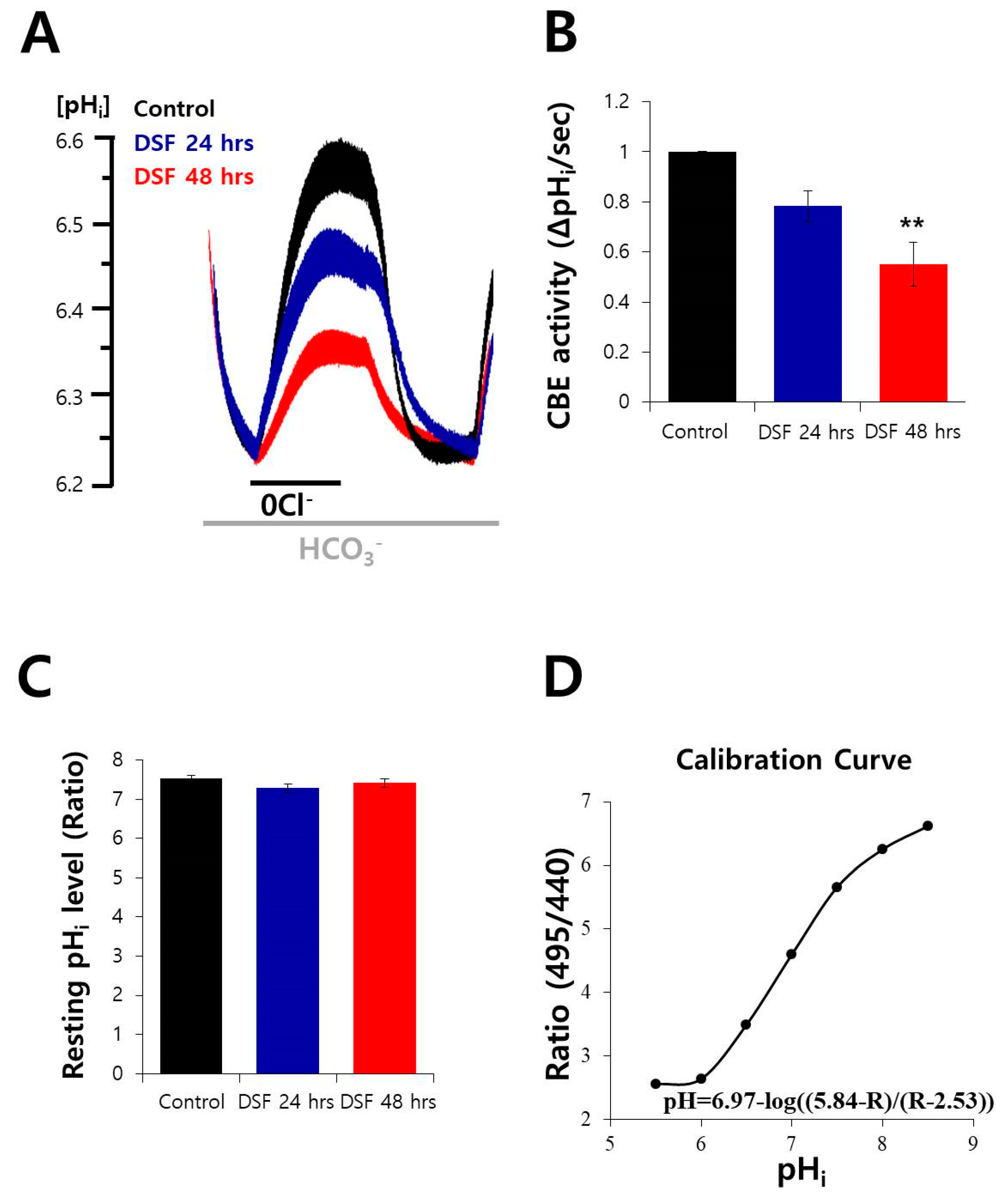
2.3. DSF Decreased CBE Activity in A549 Cells
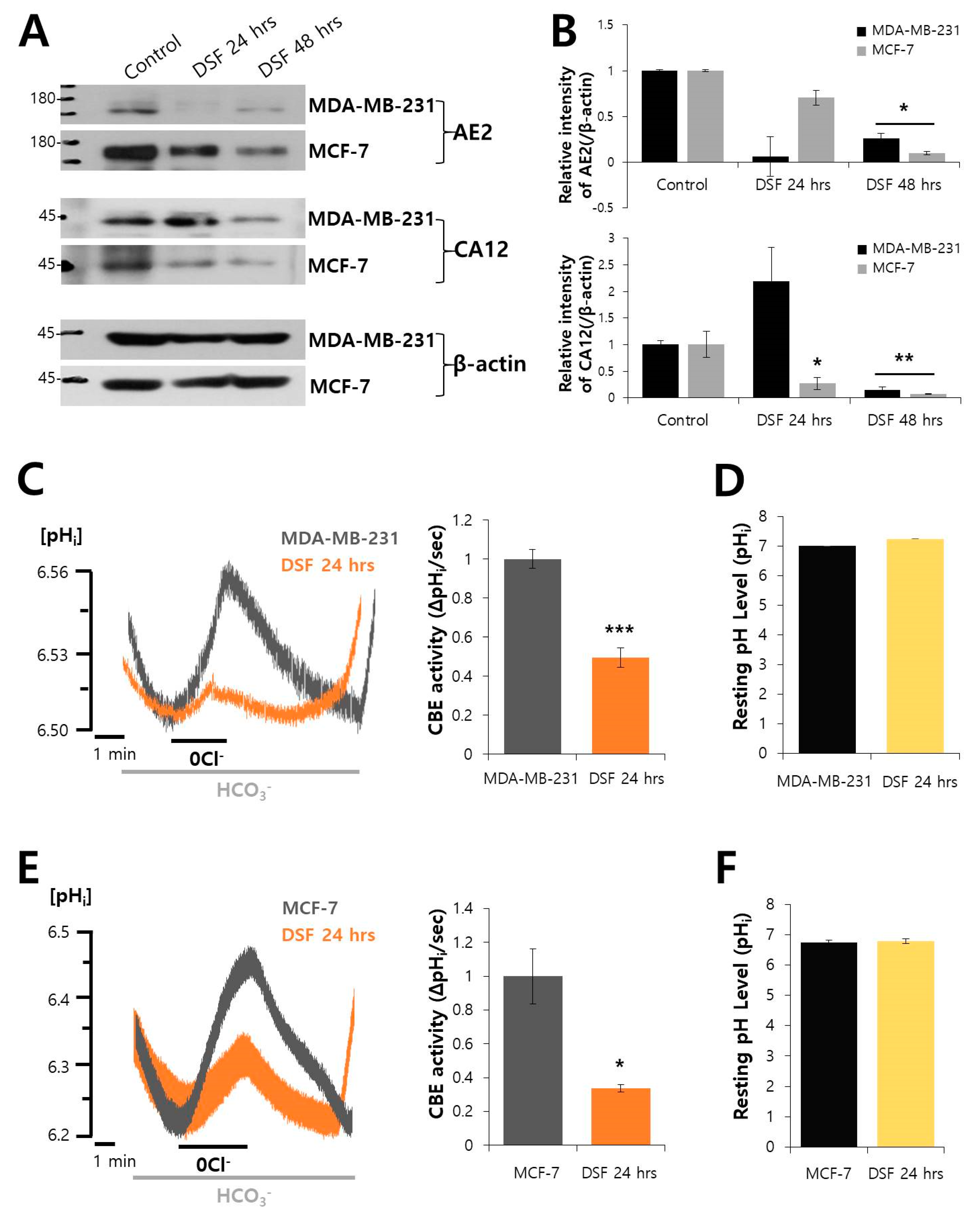
2.4. DSF Decreased the Expression of AE2 and CA12 and CBE Activity in Breast Cancer Cell Lines
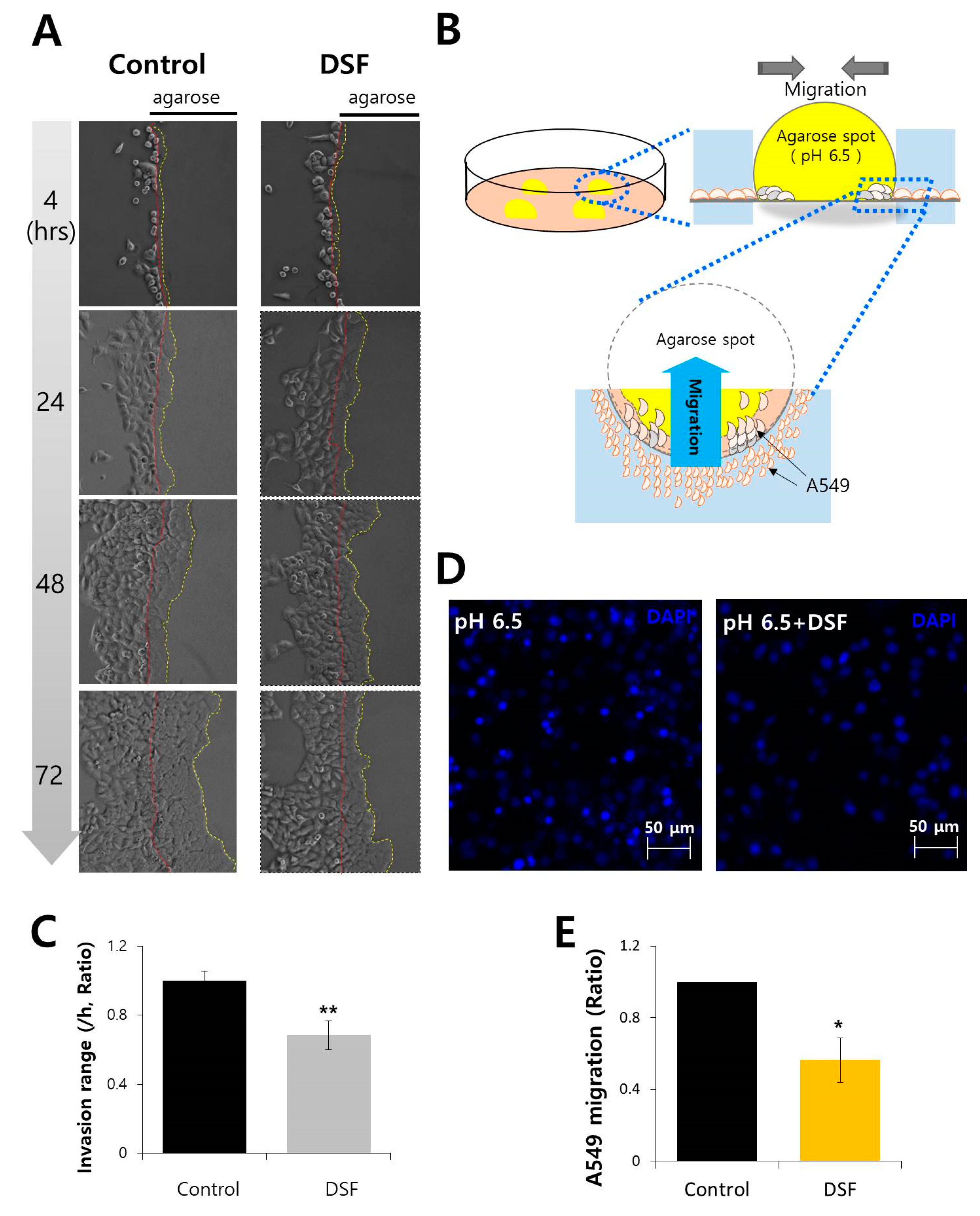
2.5. DSF Reduced Cancer Cell Invasion and Migration in Acidic Microenvironment
3. Materials and Methods
3.1. Immunofluorescence and Confocal Imaging
3.2. Agarose Spot Assay for Cell Invasion
3.3. Analysis of Cell Motility on Transwell System
3.4. DNA Transfection
3.5. Reverse Transcription-polymerase Chain Reaction (RT-PCR)
3.6. Surface Biotinylation and Western Blotting
3.7. Measurement of CBE Activity
3.8. pH Calibration
3.9. MTT Assay for Cell Viability
3.10. FACS Analysis
3.11. Statistical Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahadome, S.D.; Abraham, D.J.; Rayapureddi, S.; Saw, V.P.; Saban, D.R.; Calder, V.L.; Norman, J.T.; Ponticos, M.; Daniels, J.T.; Dart, J.K. Aldehyde dehydrogenase inhibition blocks mucosal fibrosis in human and mouse ocular scarring. JCI Insight 2016, 1, e87001. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, K.; Wang, Y.; Li, M.; Sun, X.; Liang, Z.; Wang, L.; Chen, L.; Yang, H.; Zhu, L. Disulfiram chelated with copper promotes apoptosis in human breast cancer cells by impairing the mitochondria functions. Scanning 2016, 38, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lee, T.S.; Park, J.B.; Park, K.K.; Choe, J.Y.; Sin, D.I.; Park, Y.Y.; Moon, Y.S.; Lee, K.G.; Yeo, J.H.; et al. Disulfiram suppresses invasive ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9 expression. J. Biochem. Mol. Biol. 2007, 40, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Shian, S.G.; Kao, Y.R.; Wu, F.Y.; Wu, C.W. Inhibition of invasion and angiogenesis by zinc-chelating agent disulfiram. Mol. Pharmacol. 2003, 64, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Hynninen, P.; Vaskivuo, L.; Saarnio, J.; Haapasalo, H.; Kivela, J.; Pastorekova, S.; Pastorek, J.; Waheed, A.; Sly, W.S.; Puistola, U.; et al. Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology 2006, 49, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Campia, I.; Jacobs, A.; Frei, A.P.; Ghigo, D.; Wollscheid, B.; Riganti, C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 2015, 6, 6776–6793. [Google Scholar] [CrossRef]
- Waheed, A.; Sly, W.S. Carbonic anhydrase XII functions in health and disease. Gene 2017, 623, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, B.; Zou, J.; Wang, W.; Chen, A.; Zhang, J.; Fu, Y.; Li, Z. High expression of carbonic anhydrase 12 (CA12) is associated with good prognosis in breast cancer. Neoplasma 2019, 66, 420–426. [Google Scholar] [CrossRef]
- Cohen, A.S.; Khalil, F.K.; Welsh, E.A.; Schabath, M.B.; Enkemann, S.A.; Davis, A.; Zhou, J.M.; Boulware, D.C.; Kim, J.; Haura, E.B.; et al. Cell-surface marker discovery for lung cancer. Oncotarget 2017, 8, 113373–113402. [Google Scholar] [CrossRef]
- Hong, J.H.; Muhammad, E.; Zheng, C.; Hershkovitz, E.; Alkrinawi, S.; Loewenthal, N.; Parvari, R.; Muallem, S. Essential role of carbonic anhydrase XII in secretory gland fluid and HCO3 (−) secretion revealed by disease causing human mutation. J. Physiol. 2015, 593, 5299–5312. [Google Scholar] [CrossRef]
- Hwang, S.; Kang, J.Y.; Kim, M.J.; Shin, D.M.; Hong, J.H. Carbonic anhydrase 12 mutation modulates membrane stability and volume regulation of aquaporin 5. J. Enzym. Inhib. Med. Chem. 2019, 34, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O. Tumor metabolism, cancer cell transporters, and microenvironmental resistance. J. Enzym. Inhib. Med. Chem. 2016, 31, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Hsieh, Y.H.; Wu, C.C.; Tsai, J.H.; Hsieh, Y.S.; Huang, C.Y.; Liu, J.Y. Overexpression of anion exchanger 2 in human hepatocellular carcinoma. Chin. J. Physiol. 2006, 49, 192–198. [Google Scholar] [PubMed]
- Hwang, J.M.; Kao, S.H.; Hsieh, Y.H.; Li, K.L.; Wang, P.H.; Hsu, L.S.; Liu, J.Y. Reduction of anion exchanger 2 expression induces apoptosis of human hepatocellular carcinoma cells. Mol. Cell. Biochem. 2009, 327, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Song, L.J.; Liu, R.J.; Zeng, Z.; Alper, S.L.; Cui, H.J.; Lu, Y.; Zheng, L.; Yan, Z.W.; Fu, G.H. Gastrin inhibits a novel, pathological colon cancer signaling pathway involving EGR1, AE2, and P-ERK. J. Mol. Med. 2012, 90, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, P.P.; Wu, J.; Shen, W.W.; Wu, Y.L.; Fu, A.F.; Zheng, L.; Jin, X.L.; Fu, G.H. Expression of anion exchanger 2 in human gastric cancer. Exp. Oncol. 2008, 30, 81–87. [Google Scholar] [PubMed]
- Zhang, L.J.; Lu, R.; Song, Y.N.; Zhu, J.Y.; Xia, W.; Zhang, M.; Shao, Z.Y.; Huang, Y.; Zhou, Y.; Zhang, H.; et al. Knockdown of anion exchanger 2 suppressed the growth of ovarian cancer cells via mTOR/p70S6K1 signaling. Sci. Rep. 2017, 7, 6362. [Google Scholar] [CrossRef]
- Stock, C.; Schwab, A. Ion channels and transporters in metastasis. Biochim. Biophys. Acta 2015, 1848, 2638–2646. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Hong, J.H. Governing effect of regulatory proteins for Cl(-)/HCO3(-) exchanger 2 activity. Channels 2016, 10, 214–224. [Google Scholar] [CrossRef][Green Version]
- Lauritzen, G.; Stock, C.M.; Lemaire, J.; Lund, S.F.; Jensen, M.F.; Damsgaard, B.; Petersen, K.S.; Wiwel, M.; Ronnov-Jessen, L.; Schwab, A.; et al. The Na+/H+ exchanger NHE1, but not the Na+, HCO3(-) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett. 2012, 317, 172–183. [Google Scholar] [CrossRef]
- Parks, S.K.; Pouyssegur, J. The Na(+)/HCO3(−) Co-Transporter SLC4A4 Plays a Role in Growth and Migration of Colon and Breast Cancer Cells. J. Cell. Physiol. 2015, 230, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Sato, Y.; Nakanuma, Y. An impaired biliary bicarbonate umbrella may be involved in dysregulated autophagy in primary biliary cholangitis. Lab. Investig. J. Tech. Methods Pathol. 2018, 98, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, H.; Rappoport, J. An agarose spot assay for chemotactic invasion. Biotechniques 2010, 48, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.A.; Shin, D.M.; Hong, J.H. Chloride Influx of Anion Exchanger 2 Was Modulated by Calcium-Dependent Spinophilin in Submandibular Glands. Front. Physiol. 2018, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Nehrke, K. Intracellular pH measurements in vivo using green fluorescent protein variants. Methods Mol. Biol. 2006, 351, 223–239. [Google Scholar] [PubMed]
- Rochon, P.; Jourdain, M.; Mangalaboyi, J.; Fourrier, F.; Soulie-Begu, S.; Buys, B.; Dehlin, G.; Lesage, J.C.; Chambrin, M.C.; Mordon, S. Evaluation of BCECF fluorescence ratio imaging to properly measure gastric intramucosal pH variations in vivo. J. Biomed. Opt. 2007, 12, 064014. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, N.K.; Lloyd, M.C.; Proemsey, J.B.; Bui, M.M.; Kim, J.; Gillies, R.J.; Morse, D.L. Evaluation of CAIX and CAXII Expression in Breast Cancer at Varied O2 Levels: CAIX is the Superior Surrogate Imaging Biomarker of Tumor Hypoxia. Mol. Imaging Biol. 2016, 18, 219–231. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin. Investig. Drugs 2018, 27, 963–970. [Google Scholar] [CrossRef]
- Svastova, E.; Witarski, W.; Csaderova, L.; Kosik, I.; Skvarkova, L.; Hulikova, A.; Zatovicova, M.; Barathova, M.; Kopacek, J.; Pastorek, J.; et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J. Biol. Chem. 2012, 287, 3392–3402. [Google Scholar] [CrossRef]
- Celay, J.; Lozano, T.; Concepcion, A.R.; Beltran, E.; Rudilla, F.; Garcia-Barchino, M.J.; Robles, E.F.; Rabal, O.; de Miguel, I.; Panizo, C.; et al. Targeting the anion exchanger 2 with specific peptides as a new therapeutic approach in B lymphoid neoplasms. Haematologica 2018, 103, 1065–1072. [Google Scholar] [CrossRef]
- Shiozaki, A.; Hikami, S.; Ichikawa, D.; Kosuga, T.; Shimizu, H.; Kudou, M.; Yamazato, Y.; Kobayashi, T.; Shoda, K.; Arita, T.; et al. Anion exchanger 2 suppresses cellular movement and has prognostic significance in esophageal squamous cell carcinoma. Oncotarget 2018, 9, 25993–26006. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds, chemicals, and cell lines are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Shin, D.M.; Hong, J.H. Drug Repurposing as an Antitumor Agent: Disulfiram-Mediated Carbonic Anhydrase 12 and Anion Exchanger 2 Modulation to Inhibit Cancer Cell Migration. Molecules 2019, 24, 3409. https://doi.org/10.3390/molecules24183409
Hwang S, Shin DM, Hong JH. Drug Repurposing as an Antitumor Agent: Disulfiram-Mediated Carbonic Anhydrase 12 and Anion Exchanger 2 Modulation to Inhibit Cancer Cell Migration. Molecules. 2019; 24(18):3409. https://doi.org/10.3390/molecules24183409
Chicago/Turabian StyleHwang, Soyoung, Dong Min Shin, and Jeong Hee Hong. 2019. "Drug Repurposing as an Antitumor Agent: Disulfiram-Mediated Carbonic Anhydrase 12 and Anion Exchanger 2 Modulation to Inhibit Cancer Cell Migration" Molecules 24, no. 18: 3409. https://doi.org/10.3390/molecules24183409
APA StyleHwang, S., Shin, D. M., & Hong, J. H. (2019). Drug Repurposing as an Antitumor Agent: Disulfiram-Mediated Carbonic Anhydrase 12 and Anion Exchanger 2 Modulation to Inhibit Cancer Cell Migration. Molecules, 24(18), 3409. https://doi.org/10.3390/molecules24183409






