Bifunctional Bioactive Polymer Surfaces with Micrometer and Submicrometer-sized Structure: The Effects of Structure Spacing and Elastic Modulus on Bioactivity
Abstract
1. Introduction
2. Results
2.1. Study Design
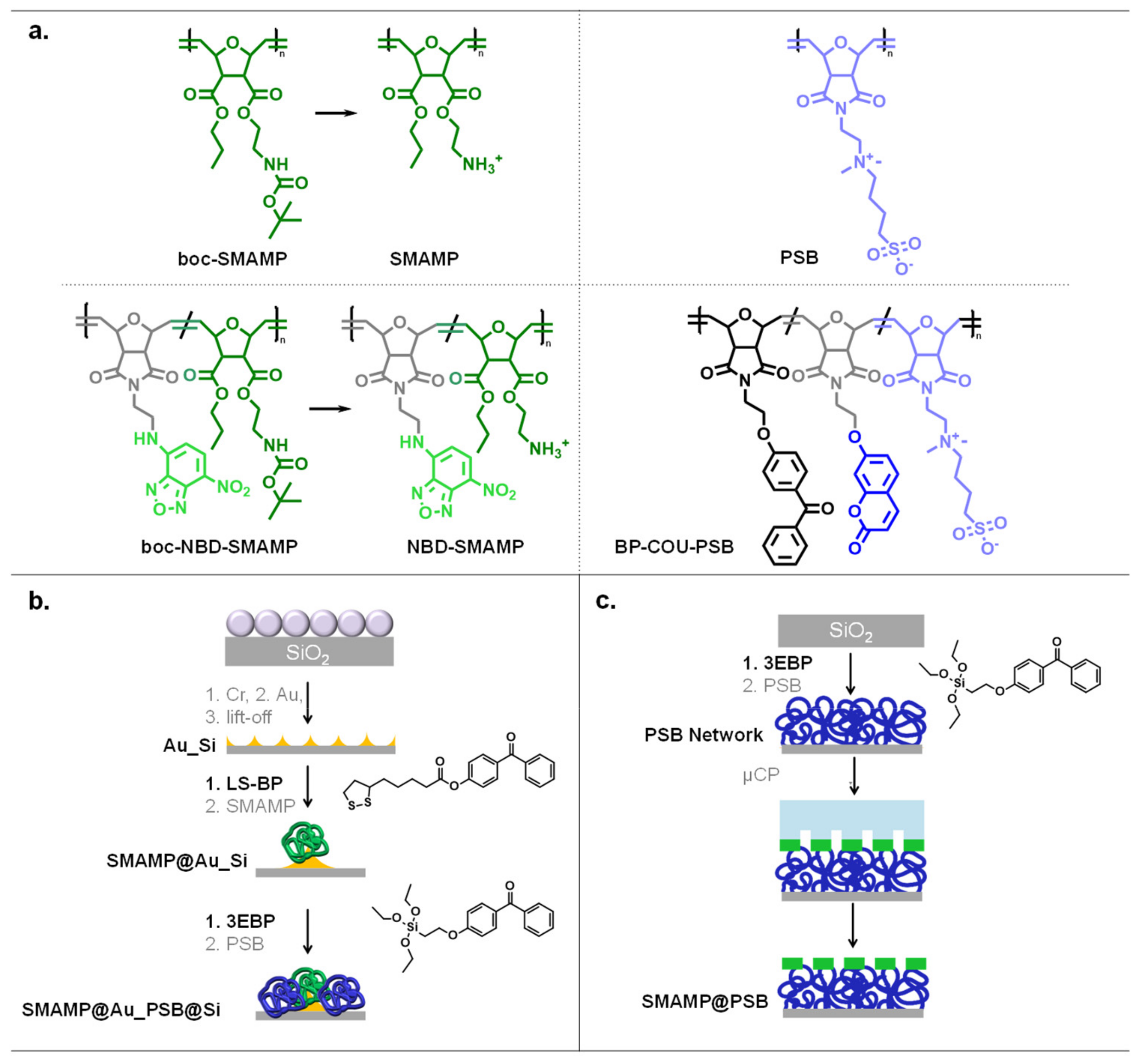
2.2. Surface Fabrication
2.2.1. Colloidal Lithography
2.2.2. Microcontact Printing
2.3. Physical Surface Characterization
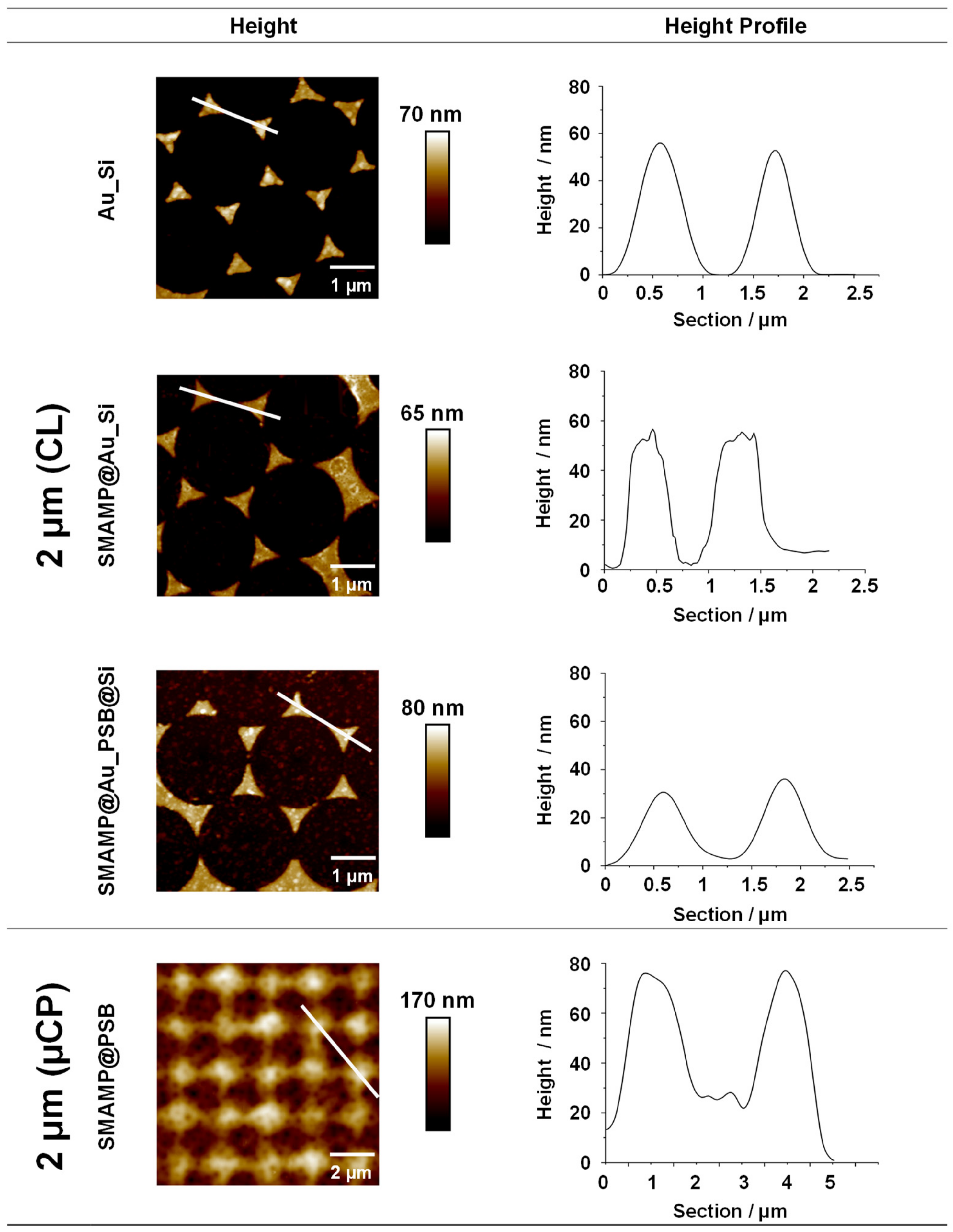
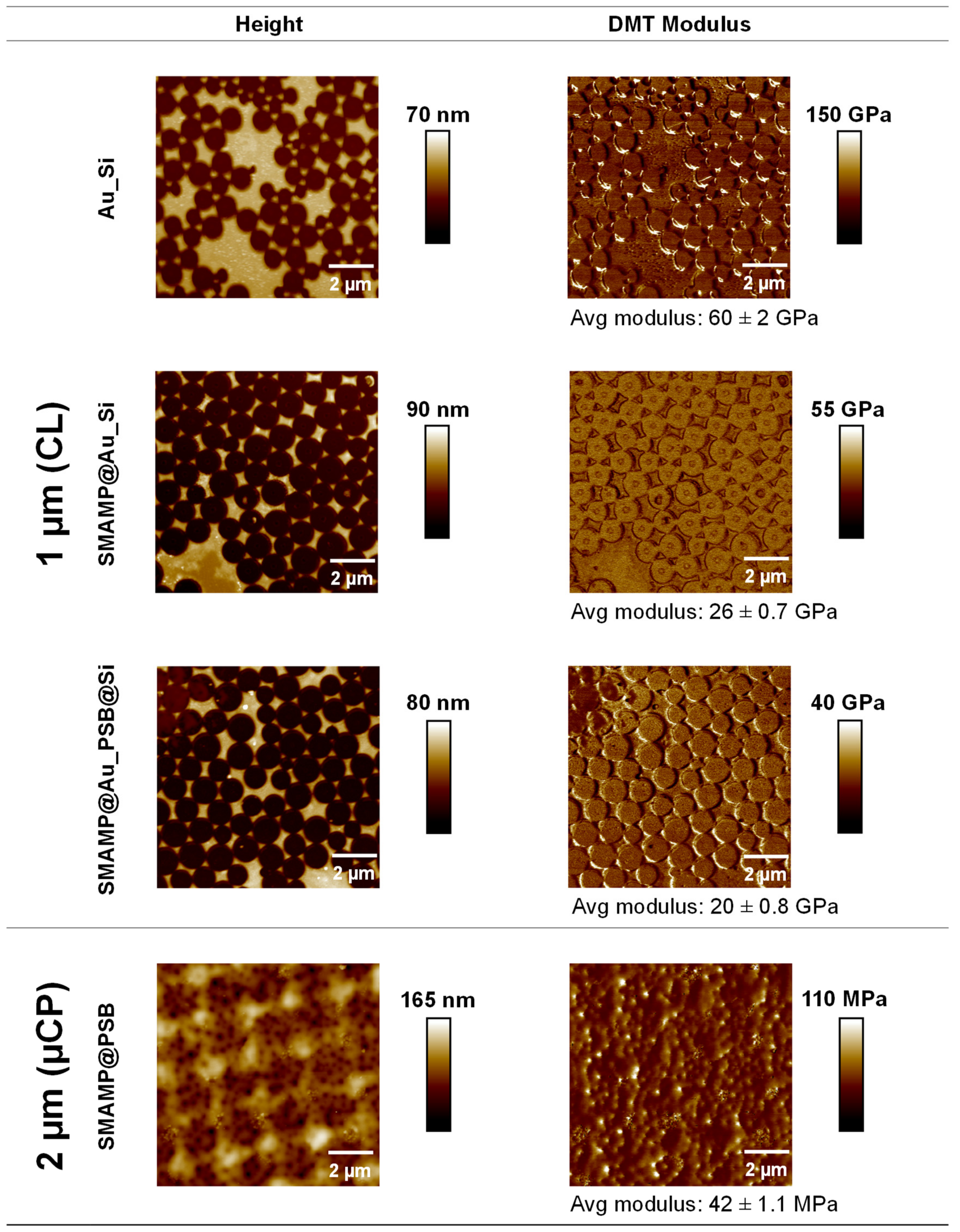
2.3.1. Atomic Force Microscopy and Contact Angle Measurements
2.3.2. Protein Adhesion Studies
2.4. Biological Surface Characterization
2.4.1. Antimicrobial Assay
2.4.2. Cell Compatibility
3. Discussion
4. Experimental
4.1. Surface Functionalization and Structuring by Colloidal Lithography
4.2. Surface Functionalization and Structuring by Microcontact Printing
4.3. Preparation of Reference Surfaces
4.4. Surface Characterization
4.4.1. Atomic Force Microscopy
4.4.2. Contact Angle Measurements
4.4.3. Surface Plasmon Resonance Spectroscopy
4.5. Biological Assays
4.5.1. Antimicrobial Activity Assay
4.5.2. Alamar Blue Assay and Optical Microscopy
4.5.3. Live-Dead Staining of Keratinocytes Grown on Functionalized Structured Surfaces
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Salwiczek, M.; Qu, Y.; Gardiner, J.; Strugnell, R.A.; Lithgow, T.; McLean, K.M.; Thissen, H. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 2014, 32, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef]
- Perera, S.; Bhushan, B.; Bandara, R.; Rajapakse, G.; Rajapakse, S.; Bandara, C. Morphological, antimicrobial, durability, and physical properties of untreated and treated textiles using silver-nanoparticles. Colloids Surf. A 2013, 436, 975–989. [Google Scholar] [CrossRef]
- AMR. Available online: https://amr-review.org/ (accessed on 15 November 2018).
- Riga, E.K.; Vöhringer, M.; Widyaya, V.T.; Lienkamp, K. Polymer-Based surfaces designed to reduce biofilm formation: From antimicrobial polymers to strategies for long-term applications. Macromol. Rapid Commun. 2017, 38, 1700216-n/a. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Jaeger, W.; Laschewsky, A. Polymeric Betaines: Synthesis, characterization, and application. In Supramolecular Polymers Polymeric Betains Oligomers; Springer: Berlin, Germany, 2006; Volume 201, pp. 157–224. [Google Scholar]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating surfaces with Zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wu, Z.; Chen, H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zander, Z.K.; Becker, M.L. Antimicrobial and antifouling strategies for polymeric medical devices. Acs Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef]
- Hartleb, W.; Saar, J.S.; Zou, P.; Lienkamp, K. Just antimicrobial is not enough: Toward bifunctional polymer surfaces with dual antimicrobial and protein-repellent functionality. Macromol Chem Phys 2016, 217, 225–231. [Google Scholar] [CrossRef]
- Ye, G.; Lee, J.; Perreault, F.; Elimelech, M. Controlled architecture of dual-functional block copolymer brushes on thin-film composite membranes for integrated “defending” and “attacking” strategies against biofouling. Acs Appl. Mater. Interfaces 2015, 7, 23069–23079. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Hartleb, W.; Lienkamp, K. It takes walls and knights to defend a castle—synthesis of surface coatings from antimicrobial and antibiofouling polymers. J. Mater. Chem. 2012, 22, 19579–19589. [Google Scholar] [CrossRef]
- Ho, C.H.; Tobis, J.; Sprich, C.; Thomann, R.; Tiller, J.C. Nanoseparated polymeric networks with multiple antimicrobial properties. Adv. Mater. 2004, 16, 957–961. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Li, L.; Cheng, G.; Gong, X.; Zheng, J. Dual functionality of antimicrobial and antifouling of poly(N-hydroxyethylacrylamide)/salicylate hydrogels. Langmuir 2013, 29, 1517–1524. [Google Scholar] [CrossRef]
- Cheng, G.; Xue, H.; Li, G.; Jiang, S. Integrated antimicrobial and nonfouling hydrogels to inhibit the growth of planktonic bacterial cells and keep the surface clean. Langmuir 2010, 26, 10425–10428. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Synchronizing nonfouling and antimicrobial properties in a zwitterionic hydrogel. Biomaterials 2012, 33, 8928–8933. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Xue, H.; Zhang, Z.; Chen, S.; Jiang, S. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities. Angew. Chem. 2008, 120, 8963–8966. [Google Scholar] [CrossRef]
- Cao, Z.; Mi, L.; Mendiola, J.; Ella-Menye, J.-R.; Zhang, L.; Xue, H.; Jiang, S. Reversibly switching the function of a surface between attacking and defending against bacteria. Angew. Chem. Int. Ed. 2012, 51, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Laloyaux, X.; Fautre, E.; Blin, T.; Purohit, V.; Leprince, J.; Jouenne, T.; Jonas, A.M.; Glinel, K. Temperature-responsive polymer brushes switching from bactericidal to cell-repellent. Adv. Mater. 2010, 22, 5024–5028. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, X.; Pang, C.; Luo, J.; Luo, Y.; Sun, R. Preparation and antimicrobial property of chitosan oligosaccharide derivative/rectorite nanocomposite. Carbohydr. Polym. 2013, 92, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.F.; Long, C.J.; Callow, M.E.; Finlay, J.A.; Callow, J.A.; Brennan, A.B. Engineered nanoforce gradients for inhibition of settlement (attachment) of swimming algal spores. Langmuir 2008, 24, 4931–4937. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Brennan, A.B. Bio-Inspired antifouling strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- May, R.M.; Magin, C.M.; Mann, E.E.; Drinker, M.C.; Fraser, J.C.; Siedlecki, C.A.; Brennan, A.B.; Reddy, S.T. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clin. Transl. Med. 2015, 4, 9. [Google Scholar] [CrossRef]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef]
- Song, F.; Brasch, M.E.; Wang, H.; Henderson, J.H.; Sauer, K.; Ren, D. How bacteria respond to material stiffness during attachment: A role of Escherichia coli flagellar motility. Acs Appl. Mater. Interfaces 2017, 9, 22176–22184. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ren, D. Stiffness of cross-linked poly (Dimethylsiloxane) affects bacterial adhesion and antibiotic susceptibility of attached cells. Langmuir 2014, 30, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Kolewe, K.W.; Peyton, S.R.; Schiffman, J.D. Fewer bacteria adhere to softer hydrogels. Acs Appl. Mater. Interfaces 2015, 7, 19562–19569. [Google Scholar] [CrossRef] [PubMed]
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata mechanical stiffness can regulate adhesion of viable bacteria. Biomacromolecules 2008, 9, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Ploux, L.; Ponche, A.; Anselme, K. Bacteria/Material interfaces: Role of the material and cell wall properties. J. Adhes. Sci. Technol. 2010, 24, 2165–2201. [Google Scholar] [CrossRef]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The influence of surface modification on bacterial adhesion to titanium-based substrates. Acs Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater. 2012, 8, 72–81. [Google Scholar] [CrossRef]
- Hasan, J.; Webb, H.; Truong, V.K.; Pogodin, S.; Baulin, V.; Watson, G.; Watson, J.; Crawford, R.; Ivanova, E. Selective Bactericidal Activity of Nanopatterned Superhydrophobic Cicada Psaltoda Claripennis Wing Surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Hasan, J.; Raj, S.; Yadav, L.; Chatterjee, K. Engineering a nanostructured “super surface” with superhydrophobic and superkilling properties. Rsc Adv. 2015, 5, 44953–44959. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Thérien-Aubin, H.; Ben-Sasson, M.; Ober, C.K.; Nielsen, M.; Elimelech, M. Control of biofouling on reverse osmosis polyamide membranes modified with biocidal nanoparticles and antifouling polymer brushes. J. Mater. Chem. B 2014, 2, 1724–1732. [Google Scholar] [CrossRef]
- Vöhringer, M.; Hartleb, W.; Lienkamp, K. Surface structuring meets orthogonal chemical modifications: Toward a technology platform for site-selectively functionalized polymer surfaces and BioMEMS. Acs Biomater. Sci. Eng. 2017, 3, 909–921. [Google Scholar] [CrossRef]
- Widyaya, V.T.; Müller, C.; Al-Ahmad, A.; Lienkamp, K. Three-Dimensional, bifunctional microstructured polymer hydrogels made from polyzwitterions and antimicrobial polymers. Langmuir 2019, 35, 1211–1226. [Google Scholar] [CrossRef]
- Elsayed, S.M.; Paschke, S.; Rau, S.J.; Lienkamp, K. Surface structuring combined with chemical surface functionalization: An effective tool to manipulate cell adhesion. Molecules 2019, 24, 909. [Google Scholar] [CrossRef]
- Widyaya, V.T.; Riga, E.K.; Müller, C.; Lienkamp, K. Submicrometer-Sized, 3D surface-attached polymer networks by microcontact printing: Using UV-cross-linking efficiency to tune structure height. Macromolecules 2018, 51, 1409–1417. [Google Scholar] [CrossRef]
- Kurowska, M.; Eickenscheidt, A.; Guevara-Solarte, D.-L.; Widyaya, V.T.; Marx, F.; Al-Ahmad, A.; Lienkamp, K. A simultaneously antimicrobial, protein-repellent, and cell-compatible polyzwitterion network. Biomacromolecules 2017, 18, 1373–1386. [Google Scholar] [CrossRef]
- Zou, P.; Laird, D.; Riga, E.K.; Deng, Z.; Perez-Hernandez, H.-R.; Guevara-Solarte, D.L.; Steinberg, T.; Al-Ahmad, A.; Lienkamp, K. Antimicrobial and cell-compatible surface-attached polymer networks—How the correlation of chemical structure to physical and biological data leads to a modified mechanism of action. J. Mater. Chem. B 2015, 3, 6224–6238. [Google Scholar] [CrossRef]
- Colak, S.; Tew, G.N. Amphiphilic polybetaines: The effect of side-chain hydrophobicity on protein adsorption. Biomacromolecules 2012, 13, 1233–1239. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Zou, P.; Guevara-Solarte, D.L.; Hellwig, E.; Steinberg, T.; Lienkamp, K. Development of a standardized and safe airborne antibacterial assay, and its evaluation on antibacterial biomimetic model surfaces. PloS ONE 2014, 9, e111357. [Google Scholar] [CrossRef] [PubMed]
- Wörz, A.; Berchtold, B.; Moosmann, K.; Prucker, O.; Rühe, J. Protein-resistant polymer surfaces. J. Mater. Chem. 2012, 22, 19547–19561. [Google Scholar] [CrossRef]
- Pandiyarajan, C.K.; Prucker, O.; Zieger, B.; Rühe, J. Influence of the molecular structure of surface-attached poly (N-alkyl Acrylamide) coatings on the interaction of surfaces with proteins, cells and blood platelets. Macromol. Biosci. 2013, 13, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering microscale topographies to control the cell–substrate interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Hari Narayana, S.N.G.; Kasiviswanathan, U.; Agarwal, T.; Senthilguru, K.; Mukhopadhyay, D.; Pal, K.; Giri, S.; Maiti, T.K.; Banerjee, I. Substrate stiffness does affect the fate of human keratinocytes. RSC Adv. 2016, 6, 3539–3551. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
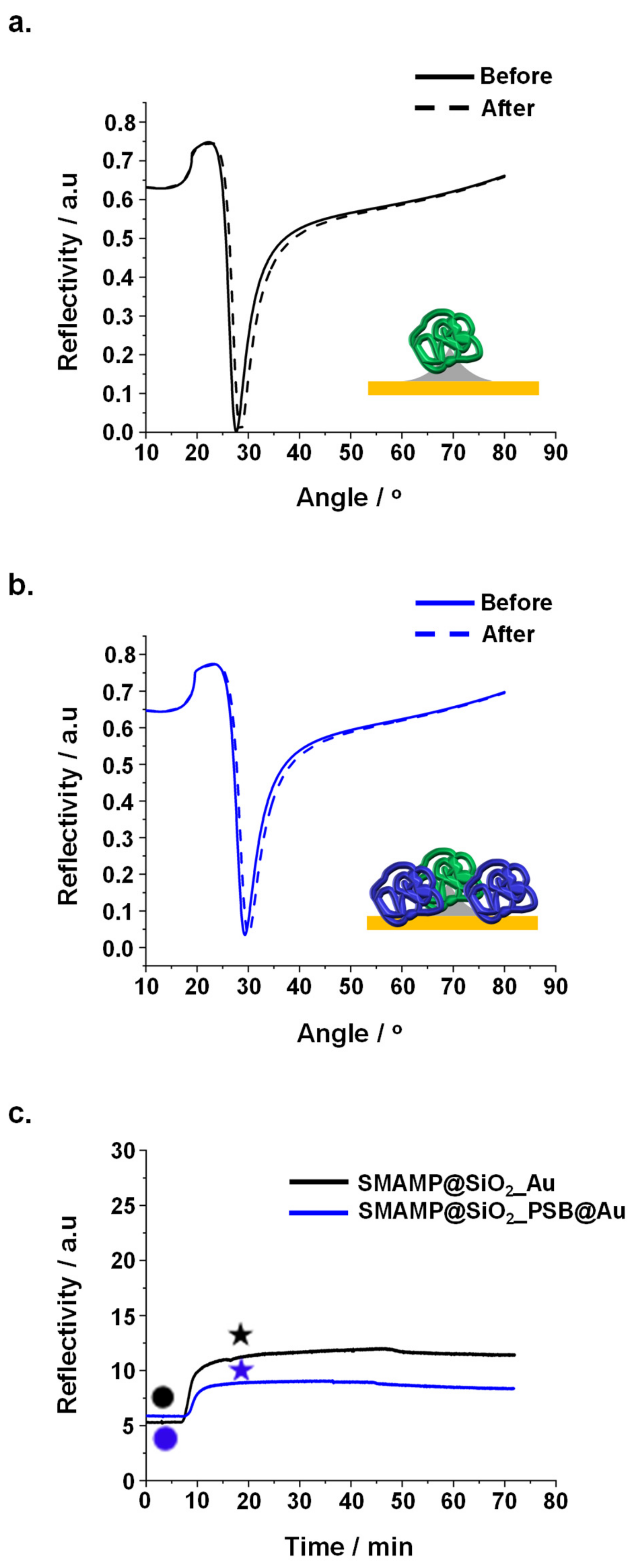
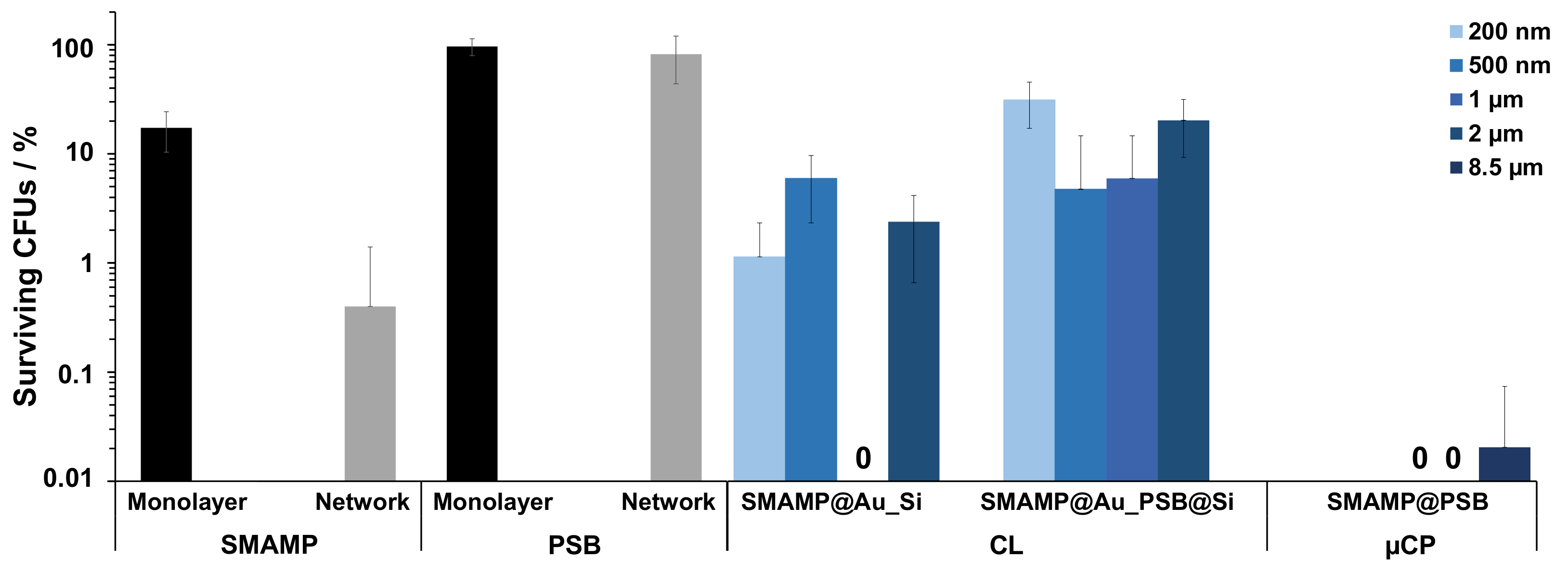
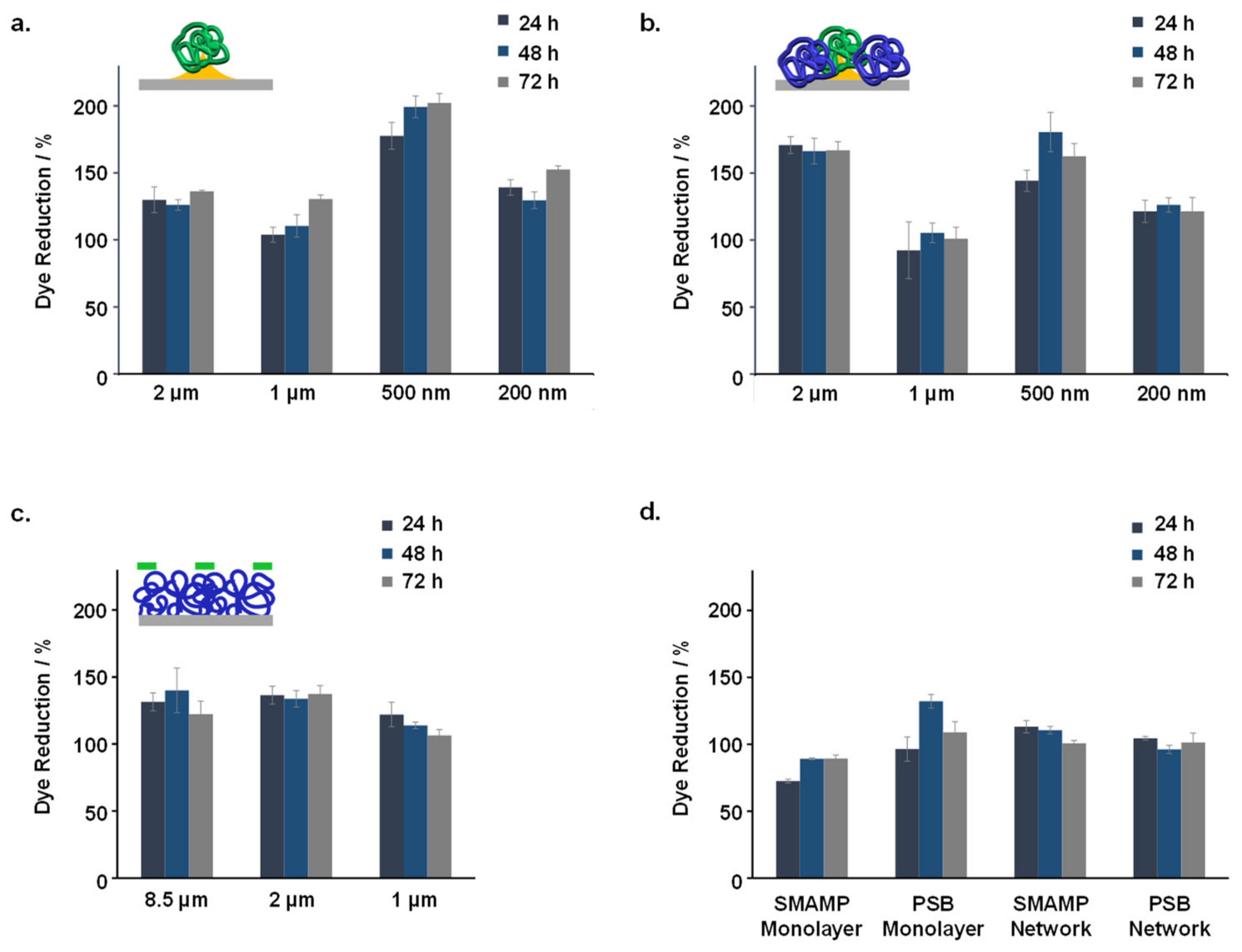
| Sample | Adhered Fibrinogen (ng mm−2) | Elastic Modulus (MPa) |
|---|---|---|
| PSB monolayer | 11 | n.a |
| SMAMP monolayer PSB network SMAMP network | 13 0 6.6 | n.a 236 ± 18 58 ± 0.4 |
| CL materials: SMAMP@SiO2_Au | ||
| 200 nm | 10.8 | n.a |
| 500 nm | 2.8 | n.a |
| 1 µm | 16.3 | (26 ± 0.7) × 103 |
| 2 µm | 7.0 | n.a |
| CL materials: SMAMP@SiO2_PSB@Au | ||
| 200 nm | 0.0 | n.a |
| 500 nm | 0.0 | n.a |
| 1 µm | 0.2 | (20 ± 0.8) × 103 |
| 2 µm | 0.5 | n.a |
| µCP materials: SMAMP@PSB | ||
| 1 µm | 0.0 | n.a |
| 2 µm | 0.0 | 42 ± 1.1 |
| 8.5 µm | 0.0 | n.a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, S.M.; Widyaya, V.T.; Shafi, Y.; Eickenscheidt, A.; Lienkamp, K. Bifunctional Bioactive Polymer Surfaces with Micrometer and Submicrometer-sized Structure: The Effects of Structure Spacing and Elastic Modulus on Bioactivity. Molecules 2019, 24, 3371. https://doi.org/10.3390/molecules24183371
Elsayed SM, Widyaya VT, Shafi Y, Eickenscheidt A, Lienkamp K. Bifunctional Bioactive Polymer Surfaces with Micrometer and Submicrometer-sized Structure: The Effects of Structure Spacing and Elastic Modulus on Bioactivity. Molecules. 2019; 24(18):3371. https://doi.org/10.3390/molecules24183371
Chicago/Turabian StyleElsayed, Sarah M., Vania Tanda Widyaya, Yasir Shafi, Alice Eickenscheidt, and Karen Lienkamp. 2019. "Bifunctional Bioactive Polymer Surfaces with Micrometer and Submicrometer-sized Structure: The Effects of Structure Spacing and Elastic Modulus on Bioactivity" Molecules 24, no. 18: 3371. https://doi.org/10.3390/molecules24183371
APA StyleElsayed, S. M., Widyaya, V. T., Shafi, Y., Eickenscheidt, A., & Lienkamp, K. (2019). Bifunctional Bioactive Polymer Surfaces with Micrometer and Submicrometer-sized Structure: The Effects of Structure Spacing and Elastic Modulus on Bioactivity. Molecules, 24(18), 3371. https://doi.org/10.3390/molecules24183371