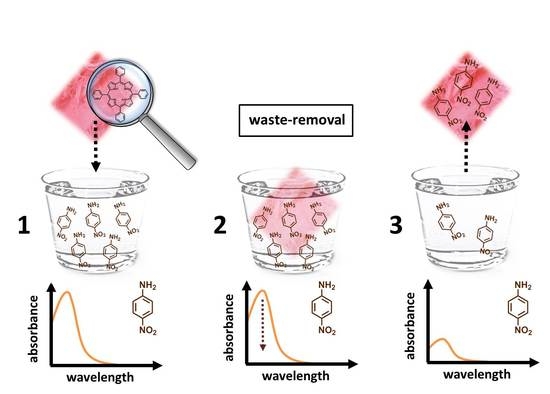
Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution
Abstract
:1. Introduction
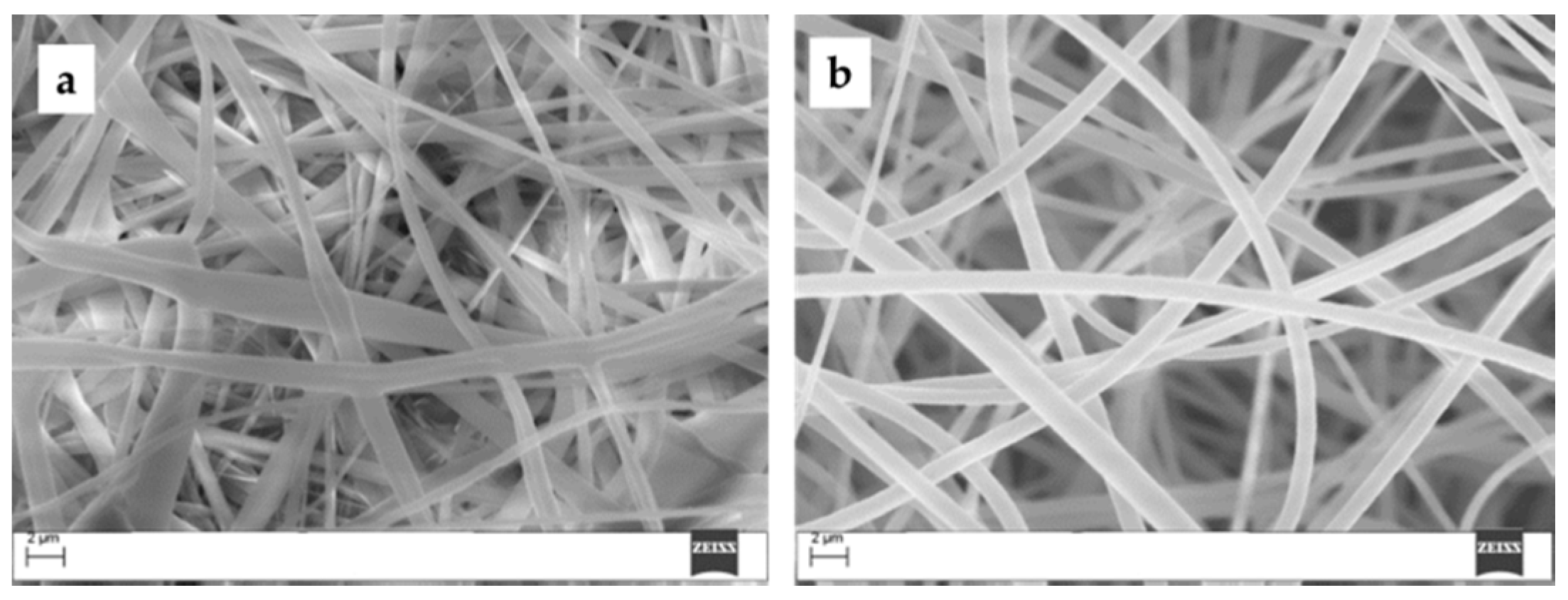
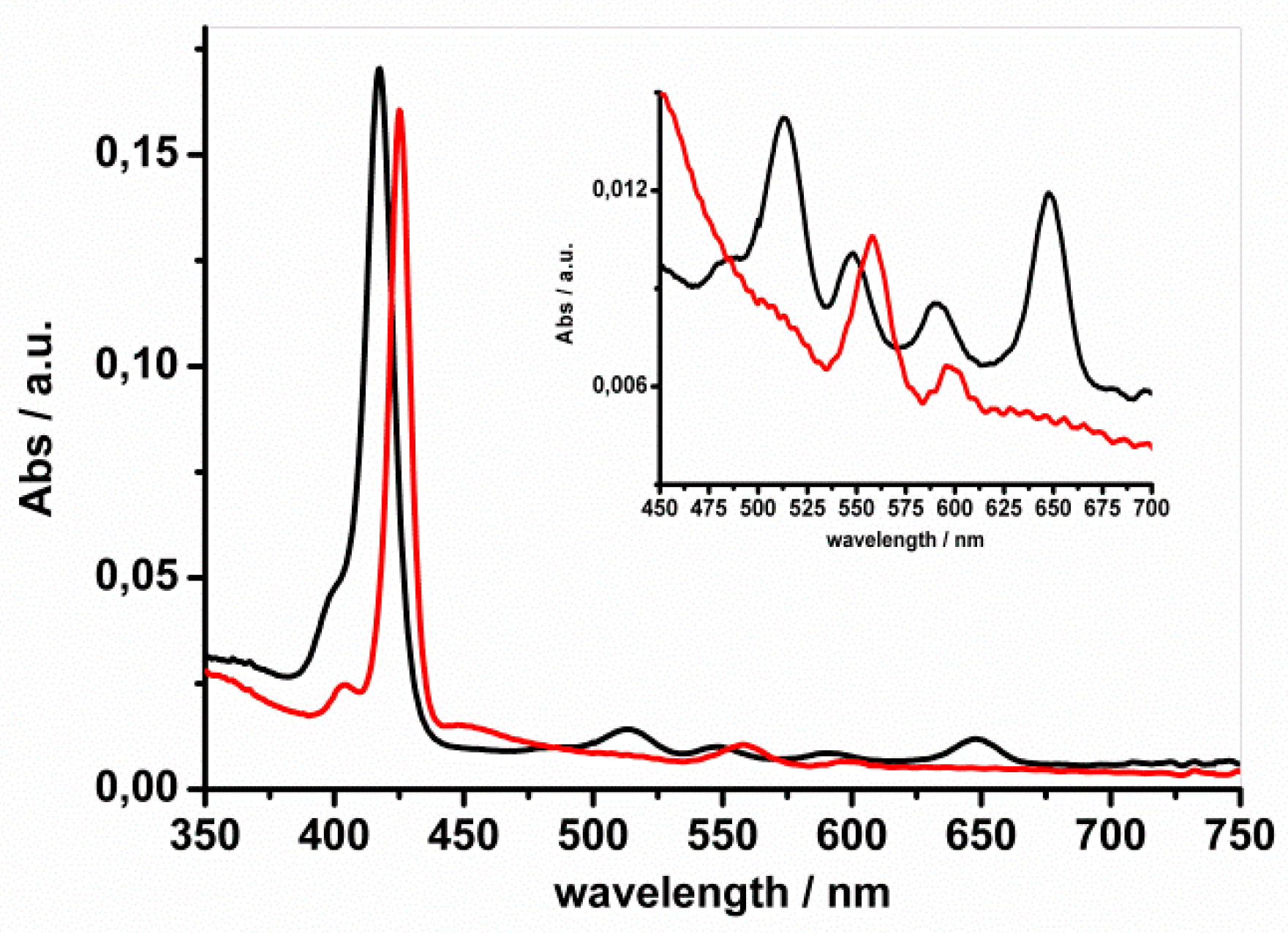
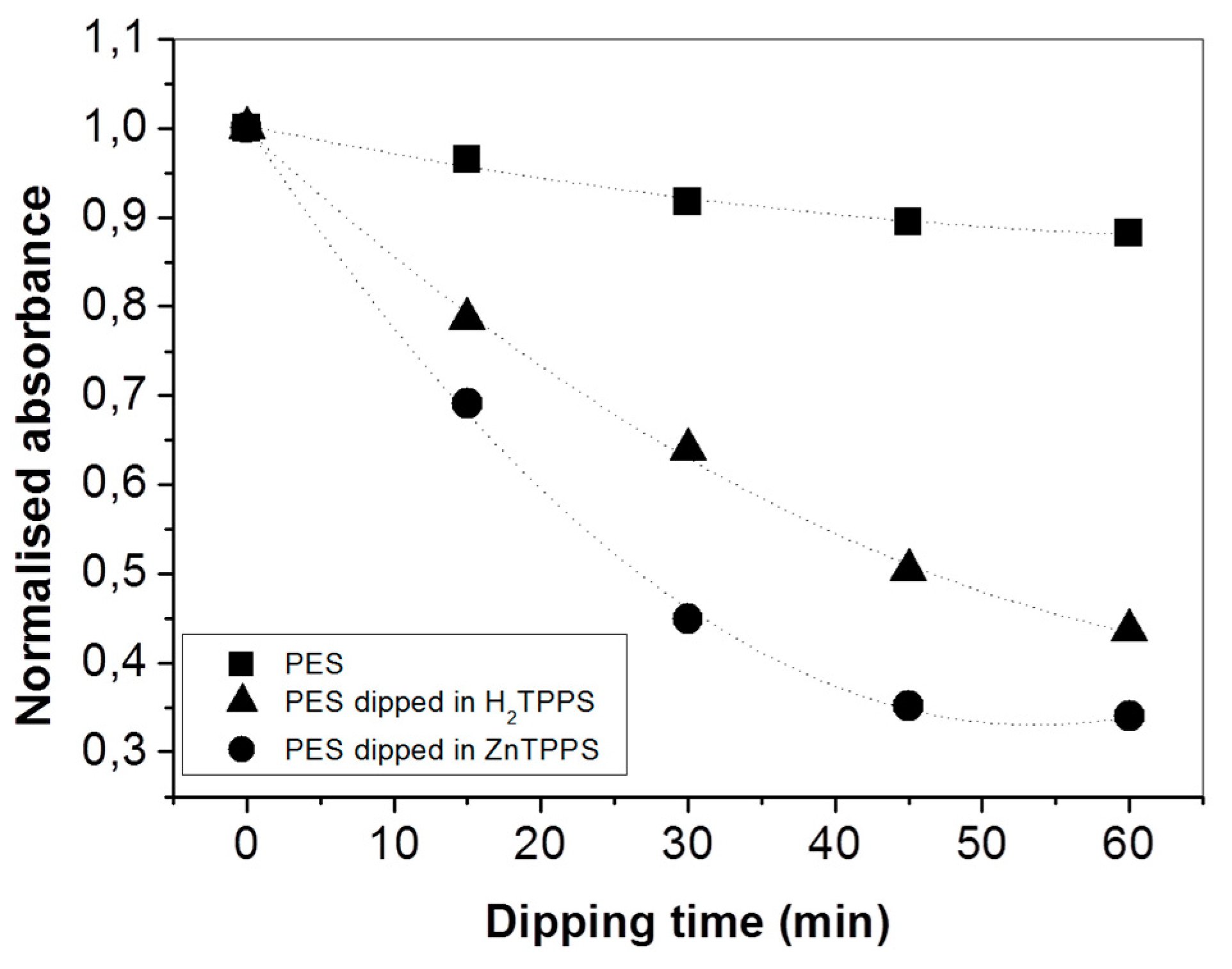
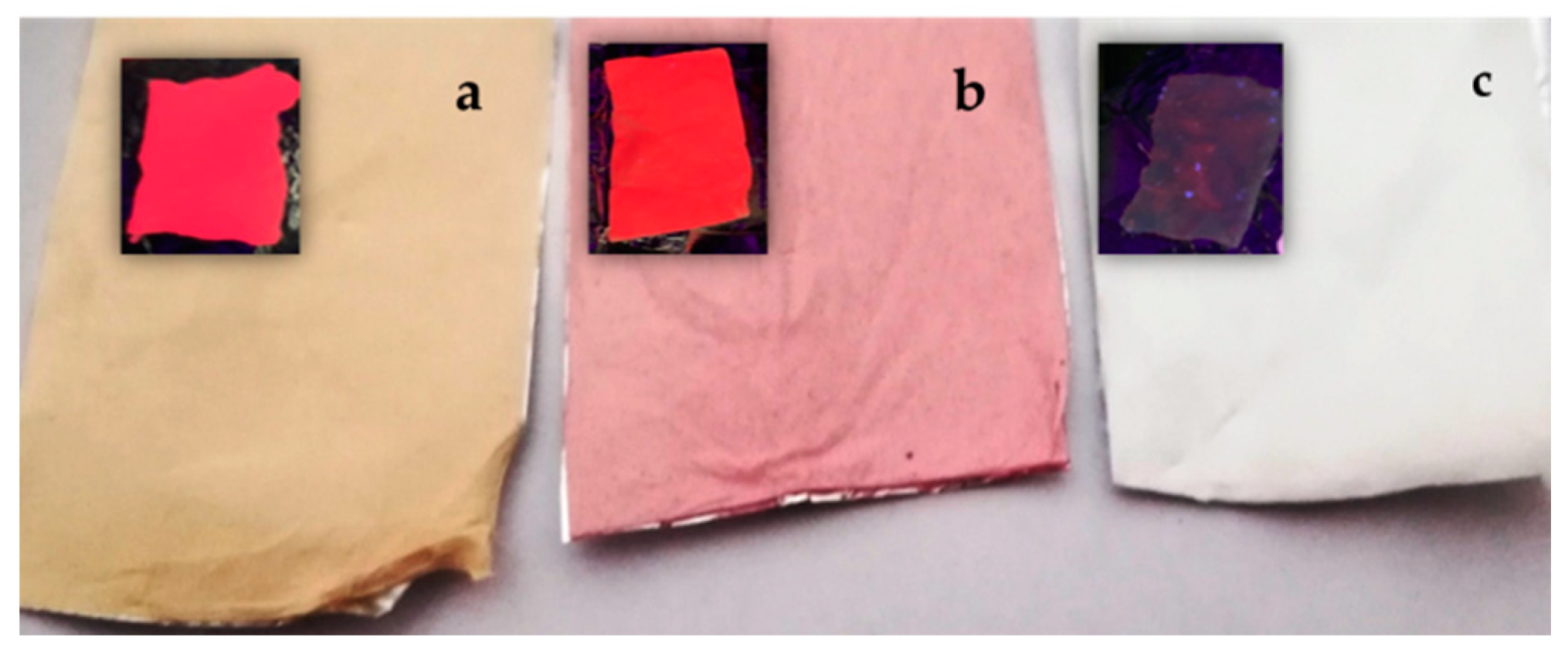
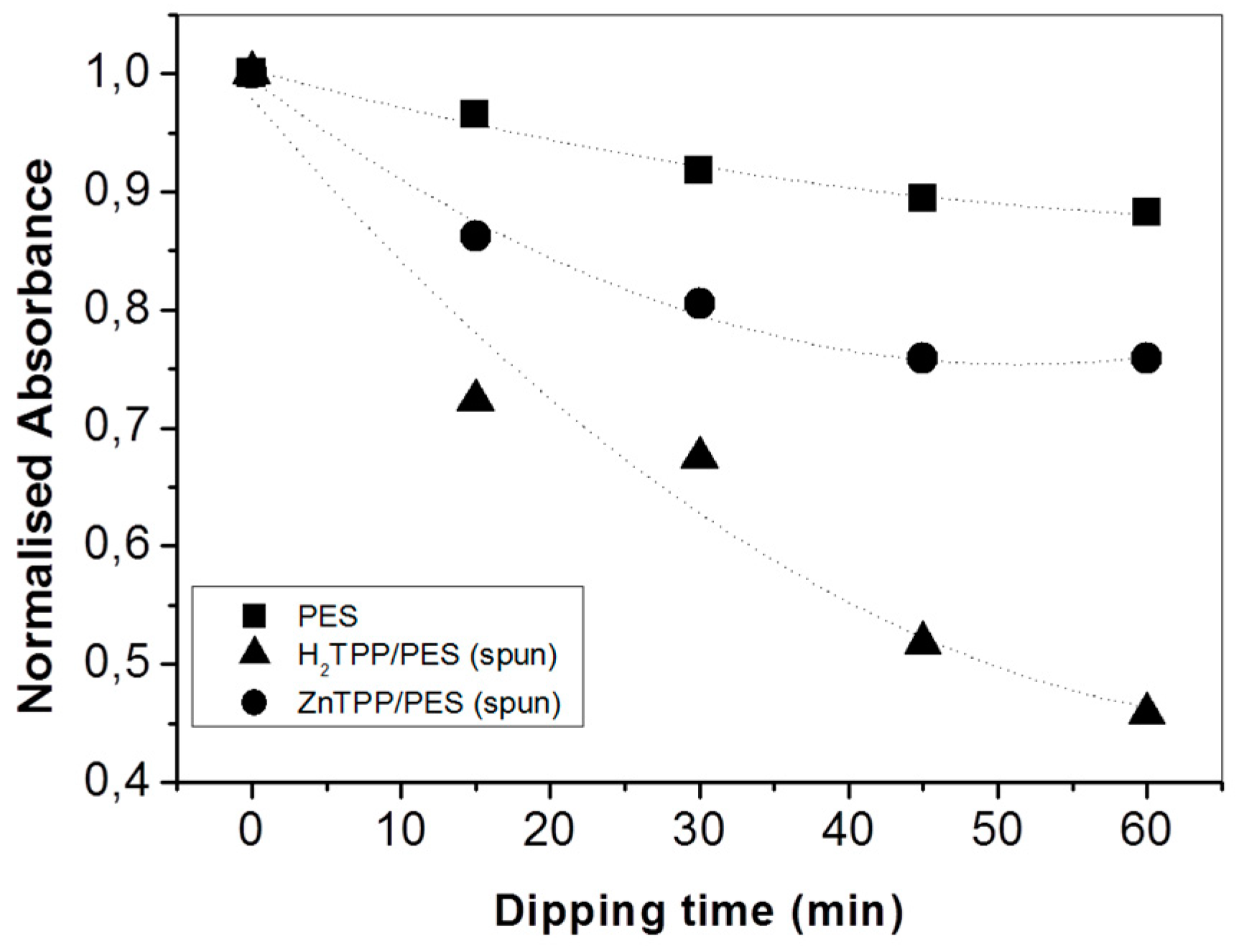
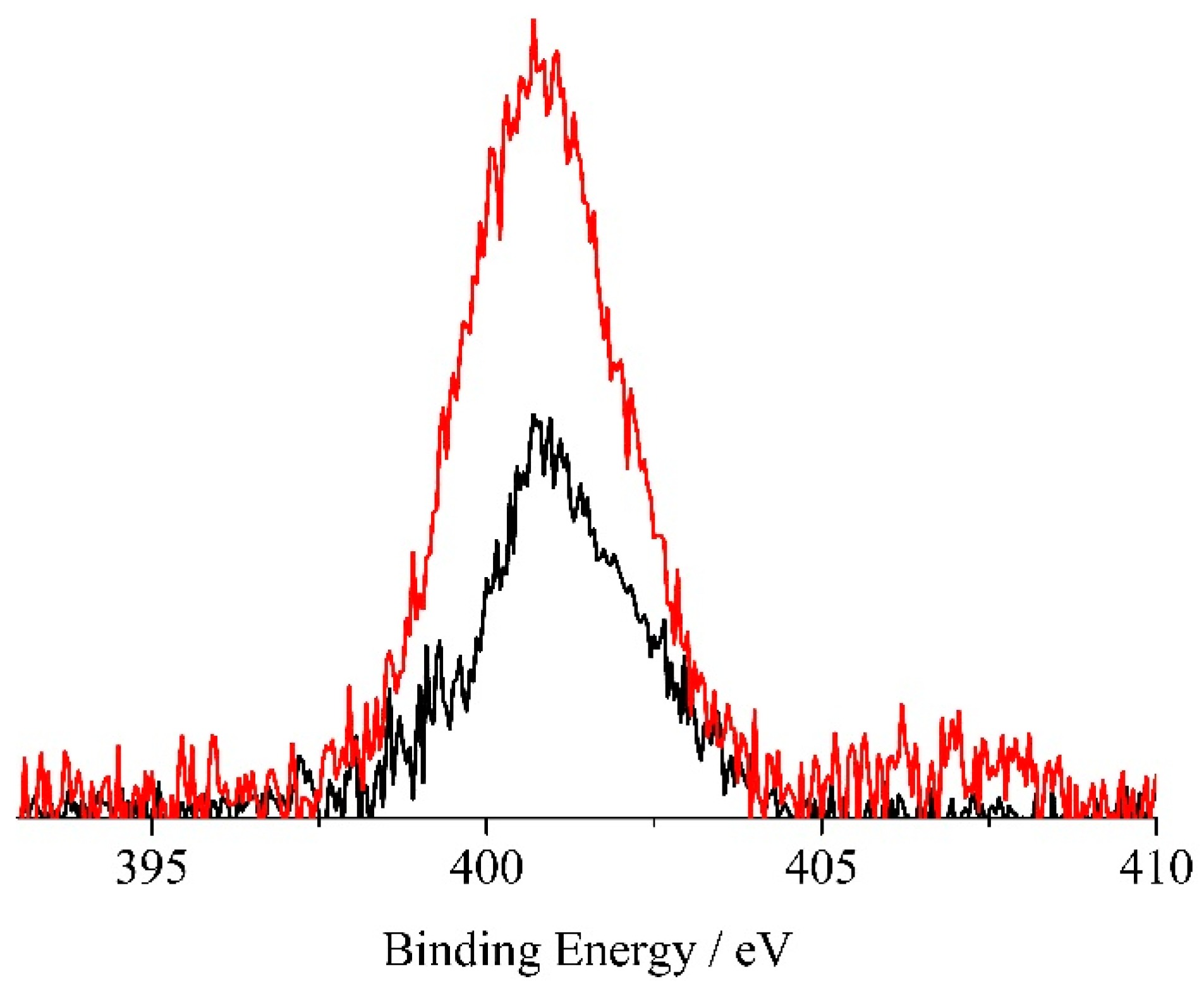
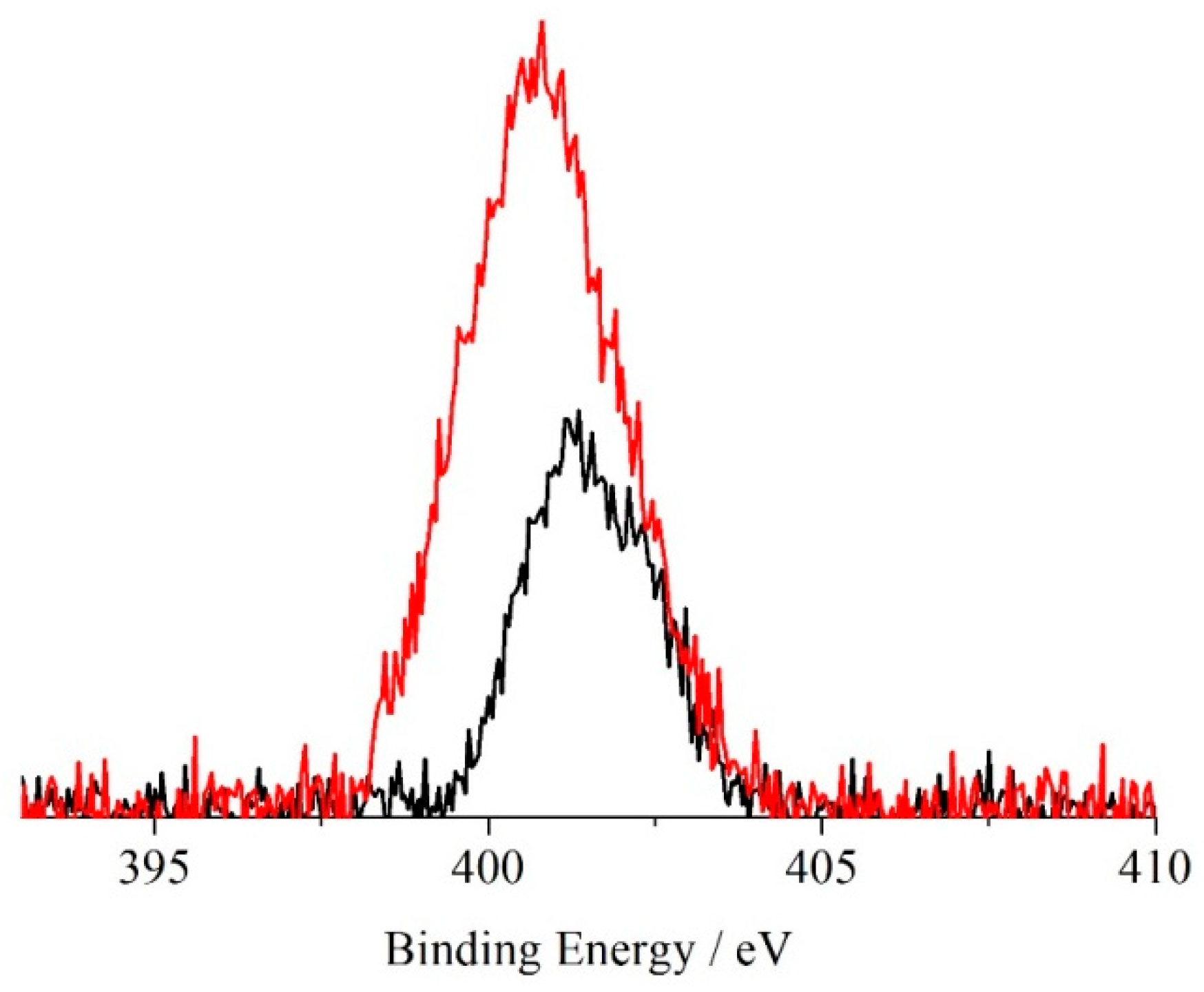
2. Results and Discussion
3. Materials and Methods
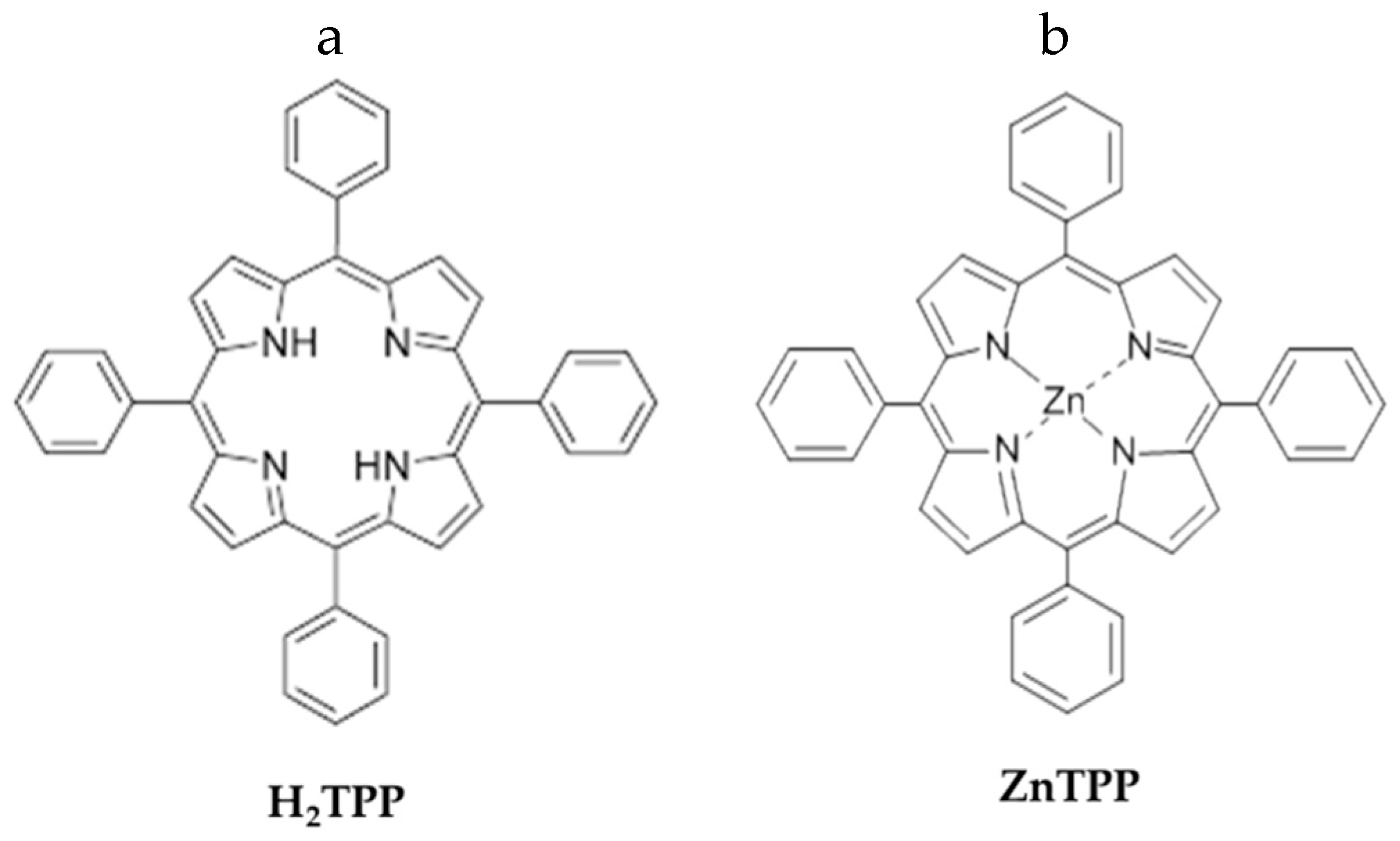
3.1. Porphyrin Impregnation of PES Mats by Dip-Coating
3.2. Porphyrin/PES Electrospinning
3.3. Para-Nitroaniline (p-NA) Absorption Measurements
3.4. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chung, K.T.; Chen, S.C.; Zhu, Y.Y.; Wong, T.Y.; Stevens, S.E. Toxic effects of some benzamines on the growth of Azotobacter vinelandii and other bacteria. Environ. Toxicol. Chem. 1997, 16, 1366–1369. [Google Scholar] [CrossRef]
- Saha, N.C.; Kaviraj, A.; Bhunia, F. Effects of Aniline—An Aromatic Amine to Some Freshwater Organisms. Ecotoxicology 2003, 12, 397–404. [Google Scholar]
- Nair, R.S.; Auletta, C.S.; Schroeder, R.E.; Johannsen, F.R. Chronic toxicity, oncogenic potential, and reproductive toxicity of p-nitroaniline in rats. Toxicol. Sci. 1990, 15, 607–621. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Huang, K. Adsorption of p-nitroaniline by phenolic hydroxyl groups modified hyper-cross-linked polymeric adsorbent and XAD-4: A comparative study. Chem. Eng. J. 2009, 155, 722–727. [Google Scholar] [CrossRef]
- Zheng, K.; Pan, B.; Zhang, Q.; Zhang, W.; Pan, B.; Han, Y.; Zhang, Q.; Wei, D.; Xu, Z.; Zhang, Q. Enhanced adsorption of p-nitroaniline from water by a carboxylated polymeric adsorbent. Sep. Purif. Technol. 2007, 57, 250–256. [Google Scholar] [CrossRef]
- Wu, G.; Wu, G.; Zhang, Q. Adsorptive removal of p-nitroaniline from aqueous solution by bamboo charcoal: Kinetics, isotherms, thermodynamics, and mechanisms. Desalin. Water Treat. 2016, 57, 26448–26460. [Google Scholar] [CrossRef]
- Jianguo, C.; Aimin, L.; Hongyan, S.; Zhenghao, F.; Chao, L.; Quanxinget, Z. Equilibrium and kinetic studies on the adsorption of aniline compounds from aqueous phase onto bifunctional polymeric adsorbent with sulfonic groups. Chemosphere 2005, 6, 502–509. [Google Scholar] [CrossRef]
- He, C.; Huang, K.; Huang, J. Surface modification on a hyper-cross-linked polymeric adsorbent by multiple phenolic hydroxyl groups to be used as a specific adsorbent for adsorptive removal of p-nitroaniline from aqueous solution. J. Colloid Interf. Sci. 2010, 342, 462–466. [Google Scholar] [CrossRef]
- Xiao, G.; Wen, R.; Wei, D.; Wu, D. Effects of the steric hindrance of micropores in the hyper-cross-linked polymeric adsorbent on the adsorption of p-nitroaniline in aqueous solution. J. Hazard. Mater. 2014, 280, 97–103. [Google Scholar] [CrossRef]
- Hu, D.; Xiao, Y.; Liu, H.; Wang, H.; Li, J.; Zhou, B.; Liu, P.; Shen, M.; Shi, X. Loading of Au/Ag bimetallic nanoparticles within electrospun PVA/PEI nanofibers for catalytic applications. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 552, 9–15. [Google Scholar] [CrossRef]
- Fang, X.; Ma, H.; Xiao, S.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Facile immobilization of gold nanoparticles into electrospun polyethyleneimine/polyvinyl alcohol nanofibers for catalytic applications. J. Mater. Chem. 2011, 21, 4493–4501. [Google Scholar] [CrossRef]
- Jin, W.J.; Lee, H.K.; Jeong, R.H.; Park, W.H.; Youk, J.H. Preparation of polymer nanofibers containing silver nanoparticles by using poly(N-vinylpyrrolidone). Macromol. Rapid Commun. 2005, 26, 1903–1907. [Google Scholar] [CrossRef]
- Yun, K.M.; Hogan, C.J., Jr.; Matsubayashi, Y.; Kawabe, M.; Iskandar, F.; Okuyama, K. Nanoparticle filtration by electrospun polymer fibers. Chem. Eng. Sci. 2007, 62, 4751–4759. [Google Scholar] [CrossRef]
- Qin, X.; Wang, S. Electrospun nanofibers from crosslinked poly (vinyl alcohol) and its filtration efficiency. J. Appl. Polym. Sci. 2008, 109, 951–956. [Google Scholar] [CrossRef]
- Strain, I.N.; Wu, Q.; Pourrahimi, A.M.; Hedenqvist, M.S.; Olsson, R.T.; Andersson, R.L. Electrospinning of recycled PET to generate tough mesomorphic fibre membranes for smoke filtration. J. Mater. Chem. A 2015, 3, 1632–1640. [Google Scholar] [CrossRef]
- Jiang, S.; Hou, H.; Agarwal, S.; Greiner, A. Polyimide nanofibers by “Green” electrospinning via aqueous solution for filtration applications. ACS Sustain. Chem. Eng. 2016, 4, 4797–4804. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.-H.; Morshed, M.; Ghasemi-Mobarakeh, L.; Ramakrishna, S.; Prabhakaran, M.P. Electrospun poly(ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Pinto, S.C.; Rodrigues, A.R.; Saraiva, J.A.; Lopes-da-Silva, J.A. Catalytic activity of trypsin entrapped in electrospun poly(ϵ-caprolactone) nanofibers. Enzyme Microb. Technol. 2015, 79, 8–18. [Google Scholar] [CrossRef]
- Kabay, G.; Kaleli, G.; Sultanova, Z.; Ölmez, T.T.; Şeker, U.Ö.Ş.; Mutlu, M. Biocatalytic protein membranes fabricated by electrospinning. React. Funct. Polym. 2016, 103, 26–32. [Google Scholar] [CrossRef]
- Huang, X.J.; Yu, A.G.; Xu, Z.K. Covalent immobilization of lipase from Candida rugosa onto poly(acrylonitrile-co-2-hydroxyethyl methacrylate) electrospun fibrous membranes for potential bioreactor application. Bioresour. Technol. 2008, 99, 5459–5465. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, G.; Gangemi, C.M.A.; D’Urso, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Combined Approach to Remove and Fast Detect Heavy Metals in Water Based on PES-TiO2 Electrospun Mats and Porphyrin Chemosensors. ACS Omega 2018, 3, 7182–7190. [Google Scholar] [CrossRef] [PubMed]
- Neubert, S.; Pliszka, D.; Thavasi, V.; Wintermantel, E.; Ramakrishna, S. Conductive electrospun PANi-PEO/TiO2 fibrous membrane for photo catalysis. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 640–646. [Google Scholar] [CrossRef]
- Sarbatly, R.; Krishnaiah, D.; Kamin, Z. A review of polymer nanofibres by electrospinning and their application in oil-water separation for cleaning up marine oil spills. Mar. Pollut. Bull. 2016, 106, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhou, Y.N.; Jiang, Z.D.; Luo, Z.H. Electrospun Fibrous Mat with pH-Switchable Superwettability That Can Separate Layered Oil/Water Mixtures. Langmuir 2016, 32, 13358–13366. [Google Scholar] [CrossRef]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Zhong, K.; Shen, J.; Jullok, N.; Sotto, A.; Van der Bruggen, B. Enhancement of polyethersulfone (PES) membrane doped by monodisperse Stöber silica for water treatment. Chem. Eng. Process. Process Intensif. 2016, 107, 194–205. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Ognibene, G.; Cristaldi, D.A.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scirè, S.; Fragalà, M.E. Photoactivity of hierarchically nanostructured ZnO-PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Zhu, W.P.; Sun, S.P.; Gao, J.; Fu, F.J.; Chung, T.S. Dual-layer polybenzimidazole/polyethersulfone (PBI/PES) nanofiltration (NF) hollow fiber membranes for heavy metals removal from wastewater. J. Membr. Sci. 2014, 456, 117–127. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J. Membr. Sci. 2012, 415–416, 250–259. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, F.A.; Baron, M.G. Polymeric matrices for immobilising zinc tetraphenylporphyrin in absorbance based gas sensors. Sens. Actuators B Chem. 2003, 90, 276–285. [Google Scholar] [CrossRef]
- Castillero, P.; Roales, J.; Lopes-Costa, T.; Sánchez-Valencia, J.R.; Barranco, A.; González-Elipe, A.R.; Pedrosa, J.M. Optical gas sensing of ammonia and amines based on protonated porphyrin/TiO2 composite thin films. Sensors 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, C.M.A.; Ognibene, G.; Randazzo, R.; D’Urso, A.; Purrello, R.; Fragalà, M.E. Easy sensing of lead and zinc in water using smart glass based on cationic porphyrin layers. New J. Chem. 2018, 42, 8717–8723. [Google Scholar] [CrossRef]
- Itagaki, Y.; Nakashima, S.; Sadaoka, Y. Optical humidity sensor using porphyrin immobilized Nafion composite films. Sens. Actuators B Chem. 2009, 142, 44–48. [Google Scholar] [CrossRef]
- Topal, S.Z.; Onal, E.; Gürek, A.G.; Hirel, C.; Ertekin, K.; Oter, O. Significant sensitivity and stability enhancement of tetraphenylporphyrin- based optical oxygen sensing material in presence of perfluorochemicals. J. Porphyr. Phthalocyanines 2013, 17, 431–439. [Google Scholar] [CrossRef]
- Adilov, S.; Thalladi, V.R. Layered porphyrin coordination polymers based on zinc⋯nitro recognition: Reversible intercalation of nitrobenzene. Cryst. Growth Des. 2007, 7, 481–484. [Google Scholar] [CrossRef]
- Vogel, G.C.; Searby, L.A. Lewis acid-base interactions of zinc alpha, beta, gamma, delta -tetraphenylporphine with several neutral donors. Inorg. Chem. 1973, 12, 936–939. [Google Scholar] [CrossRef]
- Mizutani, T.; Wada, K.; Kitagawa, S. Molecular recognition of amines and amino esters by zinc porphyrin receptors: Binding mechanisms and solvent effects. J. Org. Chem. 2000, 65, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Xu, J.; Boyer, C. Exploiting Metalloporphyrins for Selective Living Radical Polymerization Tunable over Visible Wavelengths. J. Am. Chem. Soc. 2015, 137, 9174–9185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappa, M.; Valentine, J.S. The Influence of Axial Ligands on Metalloporphyrin Visible Absorption Spectra. Complexes of Tetraphenylporphinatozinc. J. Am. Chem. Soc. 1978, 100, 5075–5080. [Google Scholar] [CrossRef]
- Gulino, A. Structural and Electronic Characterization of Self-assembled Molecular Nanoarchitectures by X-ray Photoelectron Spectroscopy. Anal. Bioanal. Chem. 2013, 405, 1479–1495. [Google Scholar] [CrossRef]
- Gulino, A.; Bazzano, S.; Mineo, P.; Scamporrino, E.; Vitalini, D.; Fragalà, I. Characterization, optical recognition behavior, sensitivity and selectivity of silica surfaces functionalized with a porphyrin monolayer. Chem. Mater. 2005, 17, 521–526. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, R.; Liu, Z. Formation of a Porphyrin Monolayer Film by Axial Ligation of Protoporphyrin IX Zinc to an Amino-Terminated Silanized Glass Surface. Langmuir 2000, 16, 1158–1162. [Google Scholar] [CrossRef]
- Sarno, D.M.; Matienzo, L.J.; Jones, W.E. X-ray Photoelectron Spectroscopy as a Probe of Intermolecular Interactions in Porphyrin Polymer Thin Films. Inorg. Chem. 2001, 40, 6308–6315. [Google Scholar] [CrossRef]
- Offord, D.A.; Sachs, S.B.; Ennis, M.S.; Eberspacher, T.A.; Griffin, J.H.; Chidsey, C.E.D.; Collman, J.P. Synthesis and Properties of Metalloporphyrin Monolayers and Stacked Multilayers Bound to an Electrode via Site Specific Axial Ligation to a Self-Assembled Monolayer. J. Am. Chem. Soc. 1998, 120, 4478–4487. [Google Scholar] [CrossRef]
- Yamashige, H.; Matsuo, S.; Kurisaki, T.; Perera, R.C.C.; Wakita, H. Local structure of nitrogen atoms in a porphine ring of meso-phenyl substituted porphyrin with an electron-withdrawing group using X-ray photoelectron spectroscopy and X-ray absorption spectroscopy. Anal. Sci. 2005, 21, 635–639. [Google Scholar] [CrossRef]
- Chen, M.; Feng, X.; Zhang, L.; Ju, H.; Xu, Q.; Zhu, J.; Gottfried, J.M.; Ibrahim, K.; Qian, H.; Wang, J. Direct Synthesis of Nickel(II) Tetraphenylporphyrin and Its Interaction with a Au(111) Surface: A Comprehensive Study. J. Phys. Chem. C 2010, 114, 9908–9916. [Google Scholar] [CrossRef]
- Bekyarova, E.; Itkis, M.E.; Ramesh, P.; Berger, C.; Sprinkle, M.; de Heer, W.A.; Haddon, R.C. Chemical Modification of Epitaxial Graphene: Spontaneous Grafting of Aryl Groups. J. Am. Chem. Soc. 2009, 131, 1336–1337. [Google Scholar] [CrossRef]
- Briggs, D.; Grant, J.T. Surface Analysis by Auger and X-Ray Photoelectron Spectroscopy; IMPublications: Chichester, UK, 2003. [Google Scholar]
- Li, K.; Zheng, Z.; Feng, J.; Zhang, J.; Luo, X.; Zhao, G.; Huang, X. Adsorption of p-nitroaniline from aqueous solutions onto activated carbon fiber prepared from cotton stalk. J. Hazard. Mater. 2009, 166, 1180–1185. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds H2TPP/PES and ZnTPP/PES are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangemi, C.M.A.; Iudici, M.; Spitaleri, L.; Randazzo, R.; Gaeta, M.; D’Urso, A.; Gulino, A.; Purrello, R.; Fragalà, M.E. Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution. Molecules 2019, 24, 3344. https://doi.org/10.3390/molecules24183344
Gangemi CMA, Iudici M, Spitaleri L, Randazzo R, Gaeta M, D’Urso A, Gulino A, Purrello R, Fragalà ME. Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution. Molecules. 2019; 24(18):3344. https://doi.org/10.3390/molecules24183344
Chicago/Turabian StyleGangemi, Chiara Maria Antonietta, Mario Iudici, Luca Spitaleri, Rosalba Randazzo, Massimiliano Gaeta, Alessandro D’Urso, Antonino Gulino, Roberto Purrello, and Maria Elena Fragalà. 2019. "Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution" Molecules 24, no. 18: 3344. https://doi.org/10.3390/molecules24183344
APA StyleGangemi, C. M. A., Iudici, M., Spitaleri, L., Randazzo, R., Gaeta, M., D’Urso, A., Gulino, A., Purrello, R., & Fragalà, M. E. (2019). Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution. Molecules, 24(18), 3344. https://doi.org/10.3390/molecules24183344