Effect of Wine Matrix Composition on the Quantification of Volatile Sulfur Compounds by Headspace Solid-Phase Microextraction-Gas Chromatography-Pulsed Flame Photometric Detection
Abstract
:1. Introduction
2. Results and Discussion
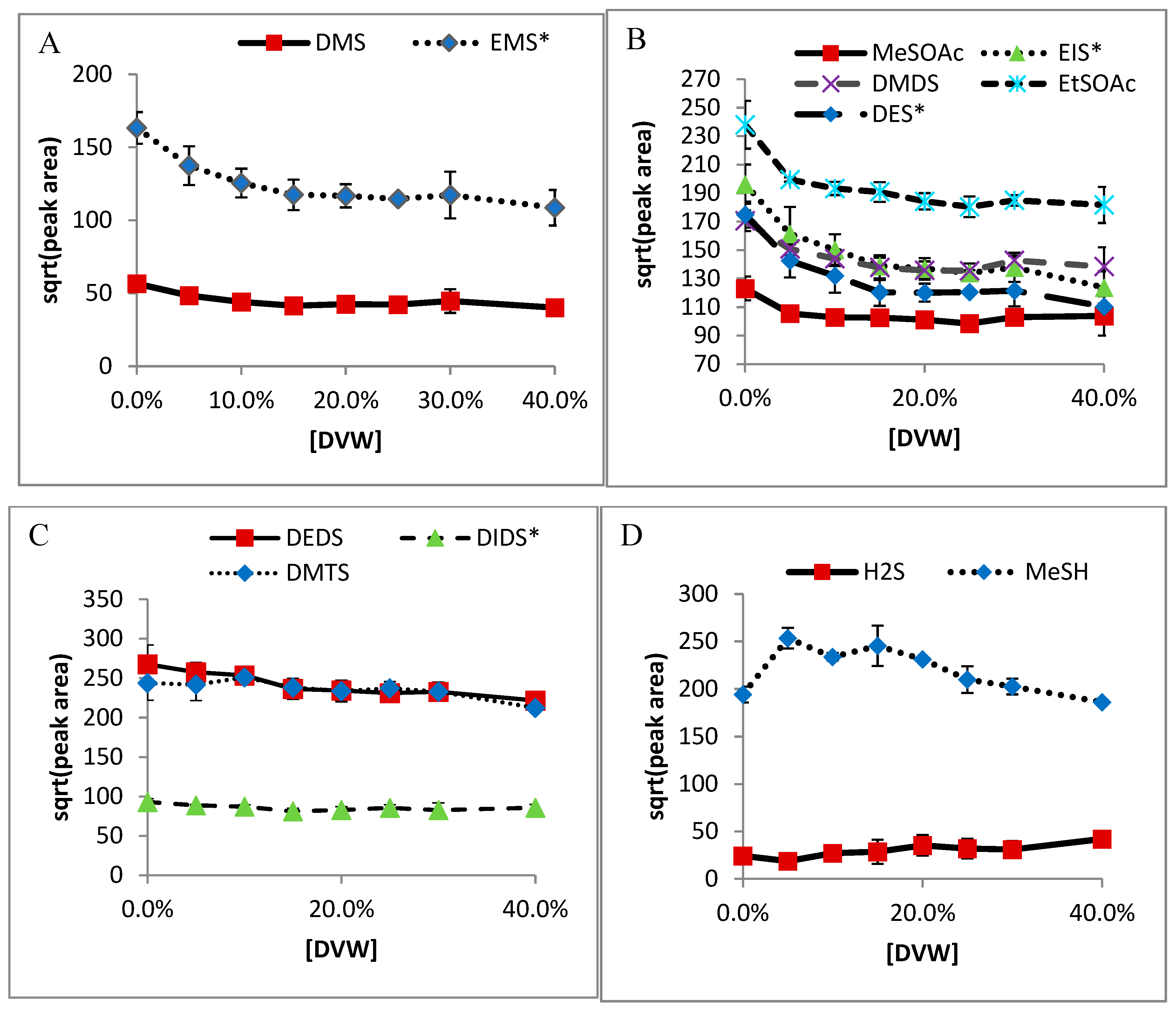
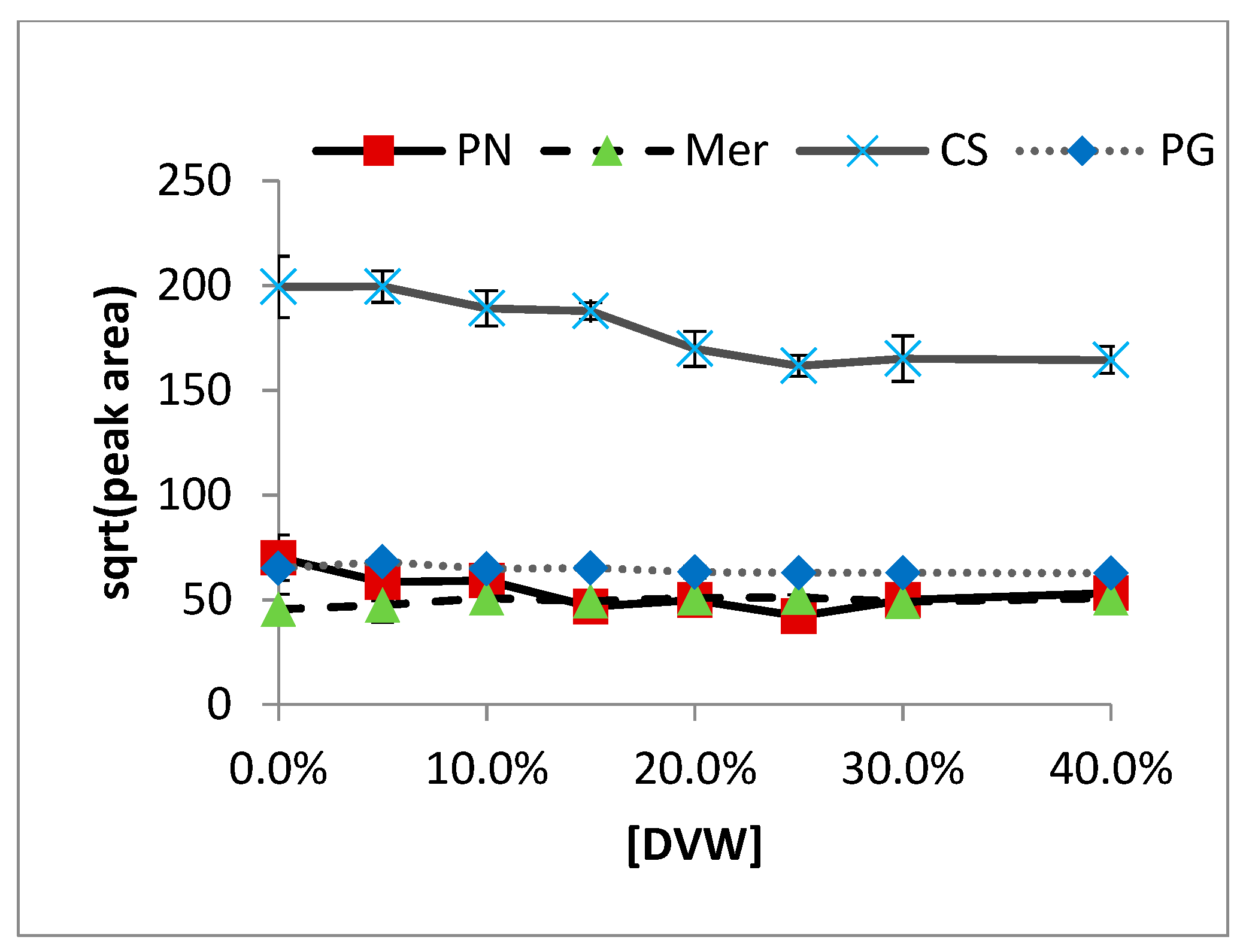
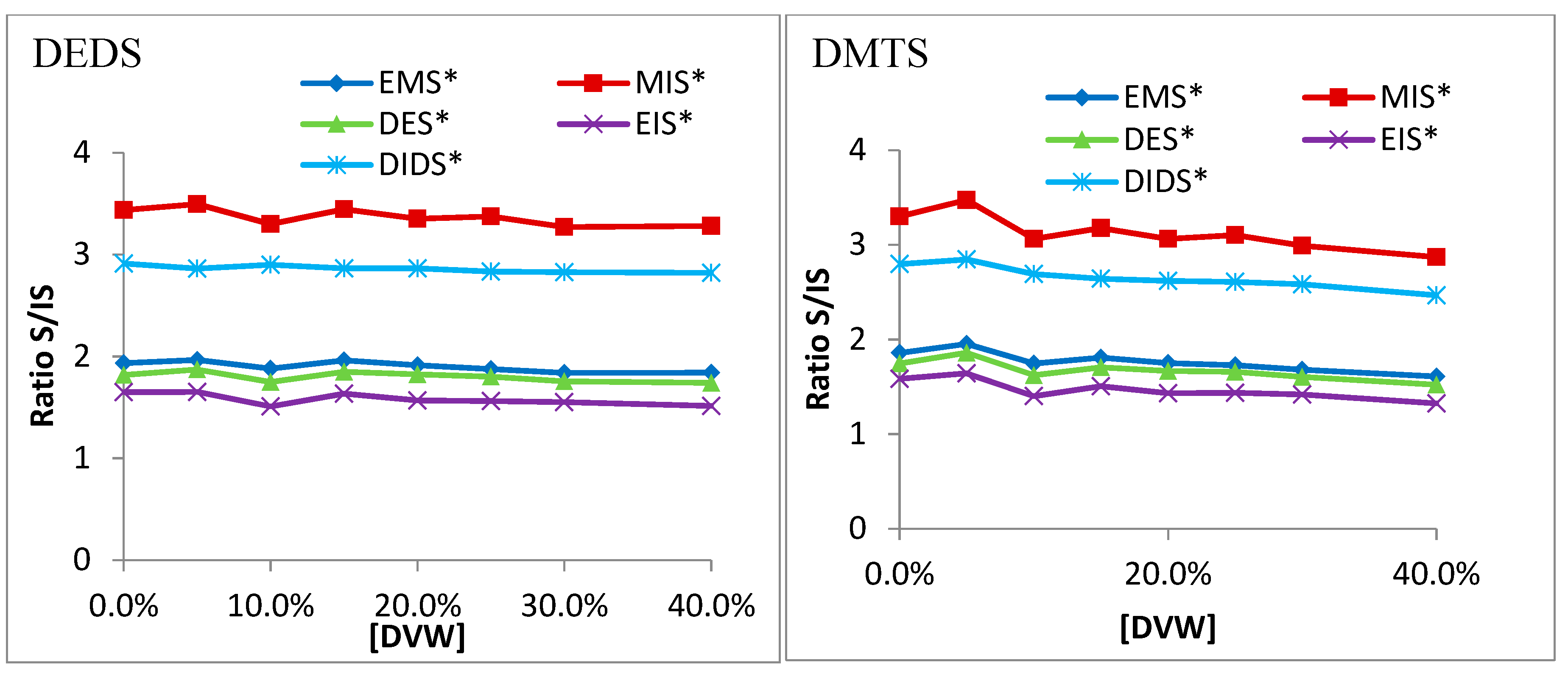
2.1. Non-Volatile Matrix Effects
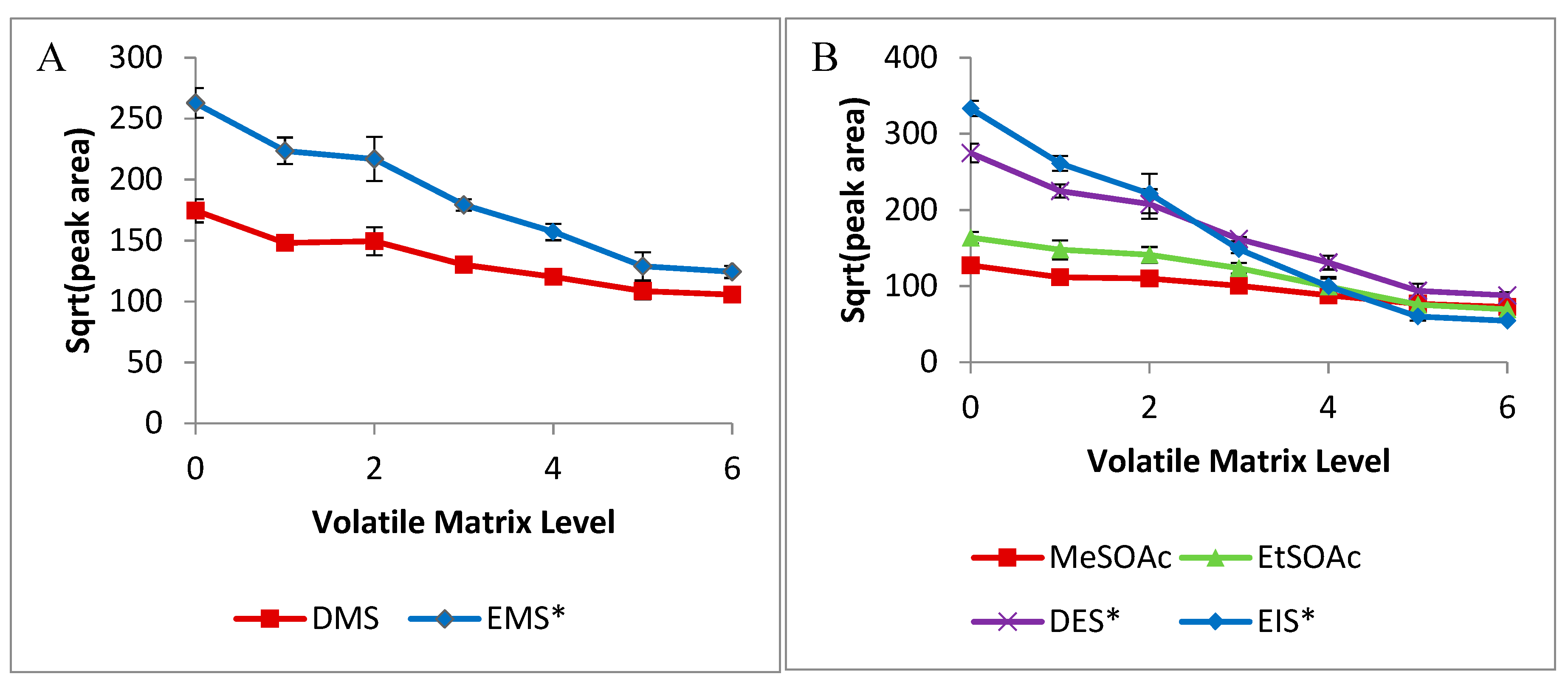
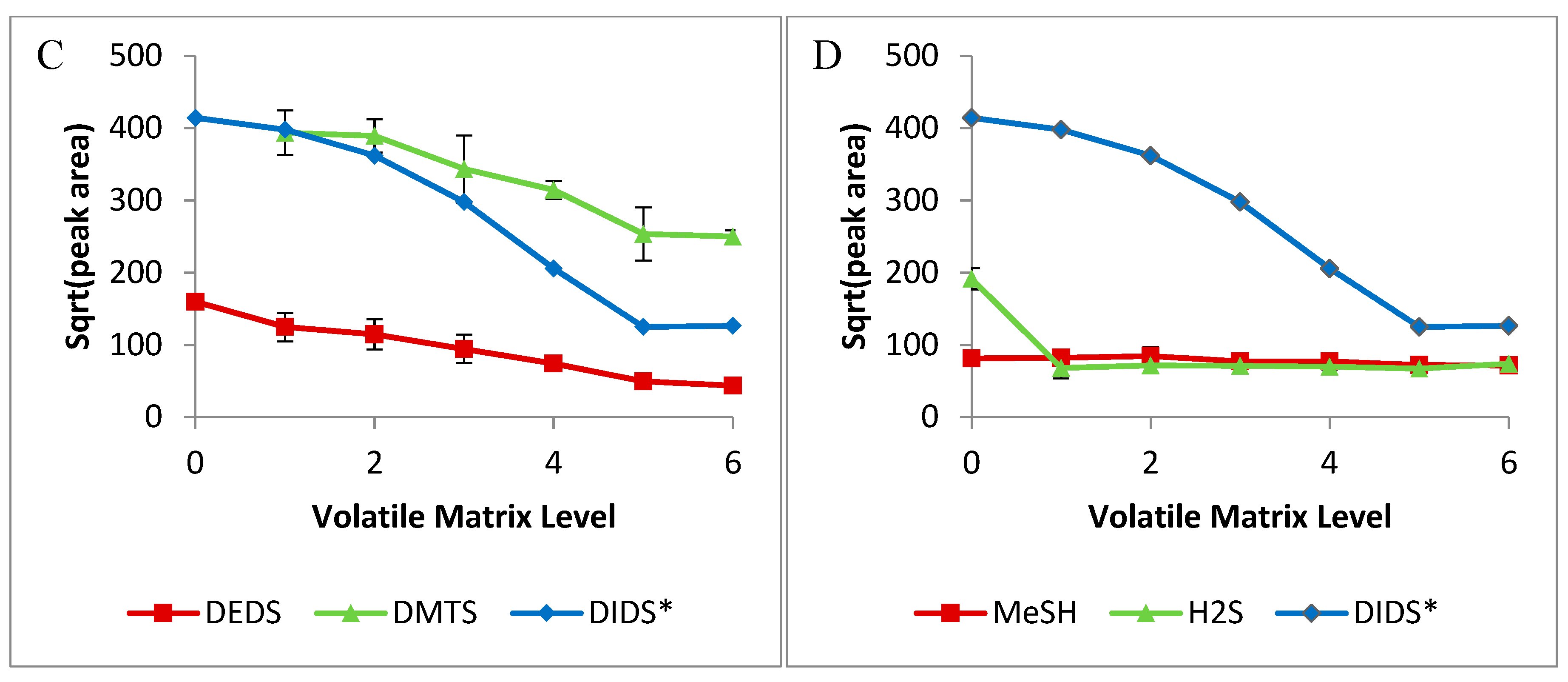
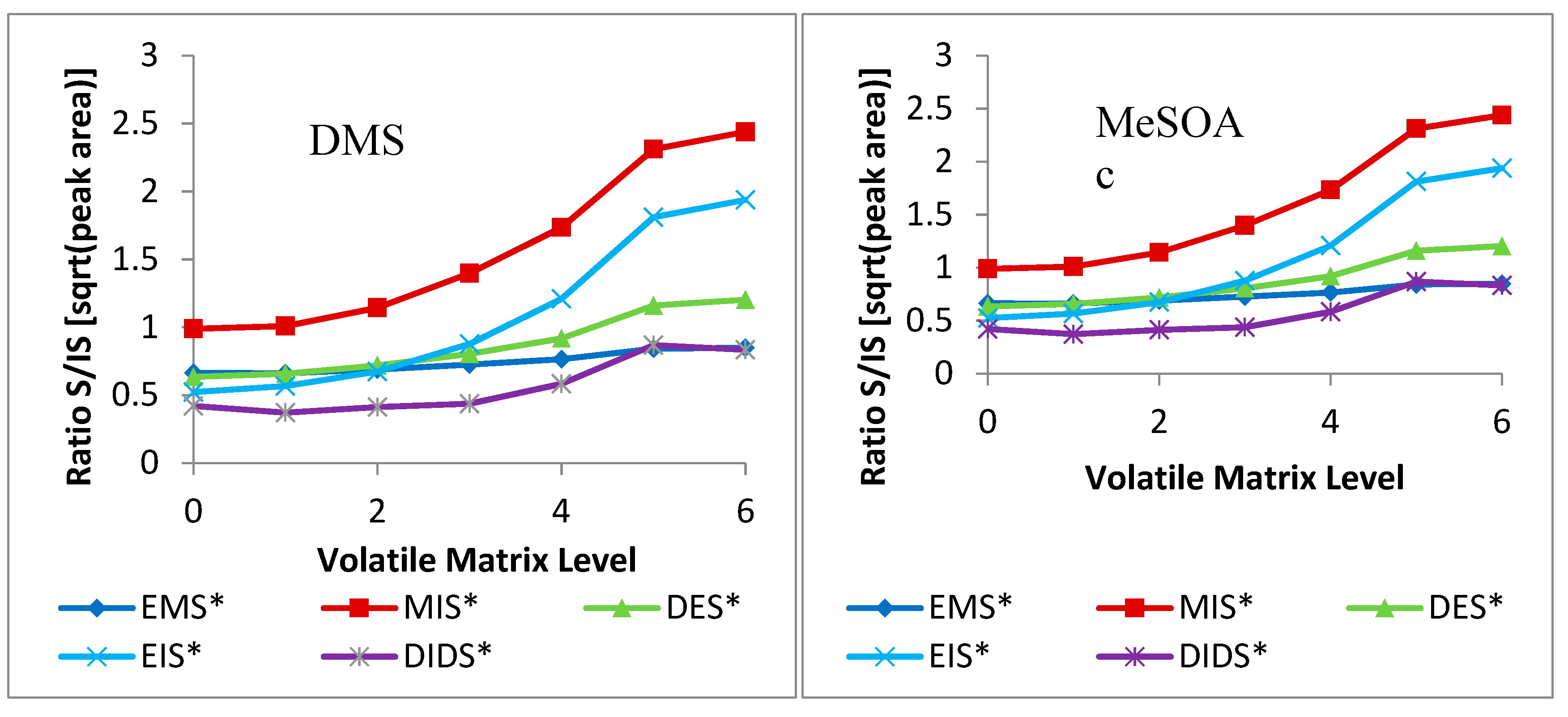
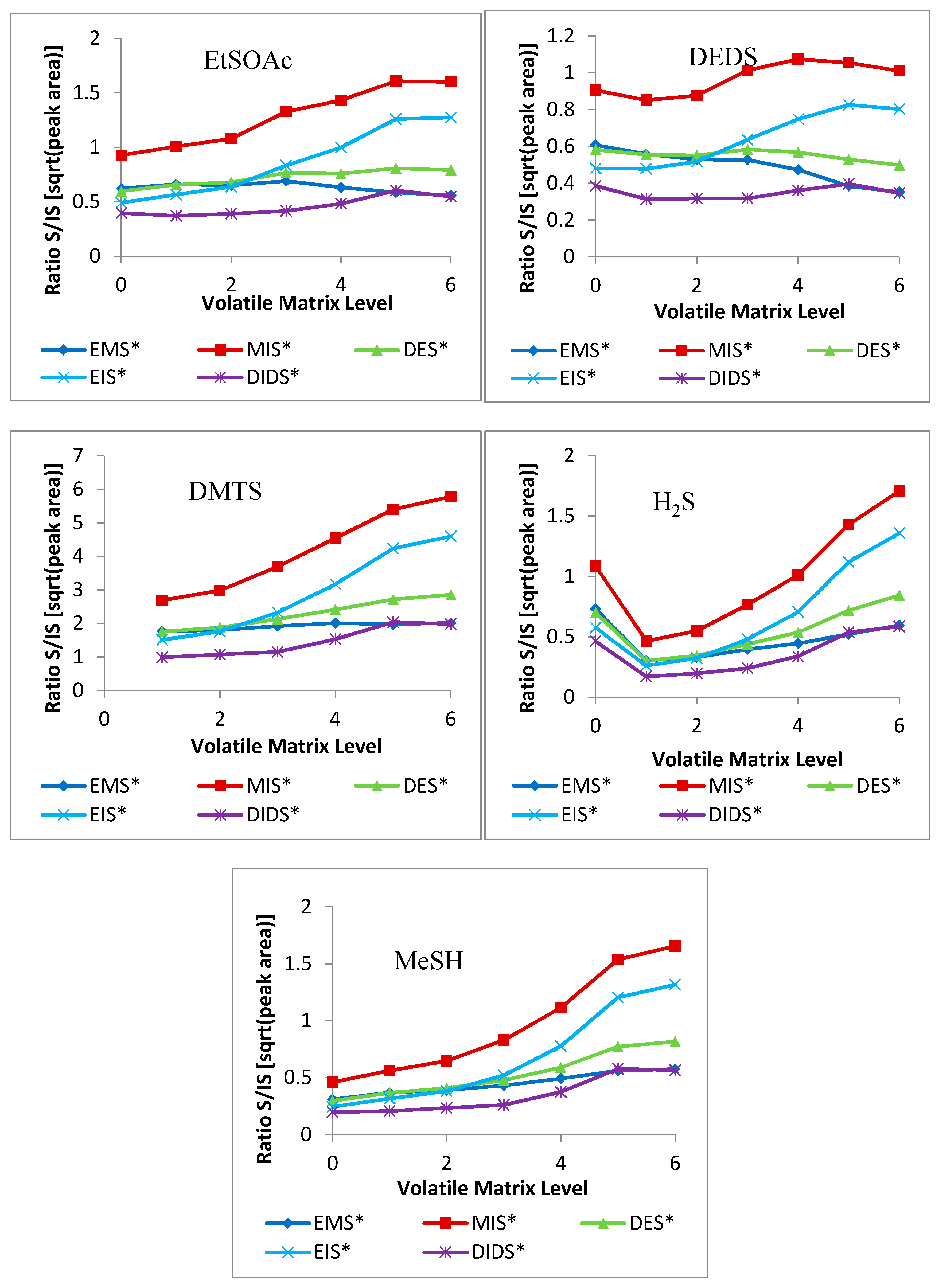
2.2. Volatile-Matrix Effects
3. Materials and Methods
3.1. Chemicals
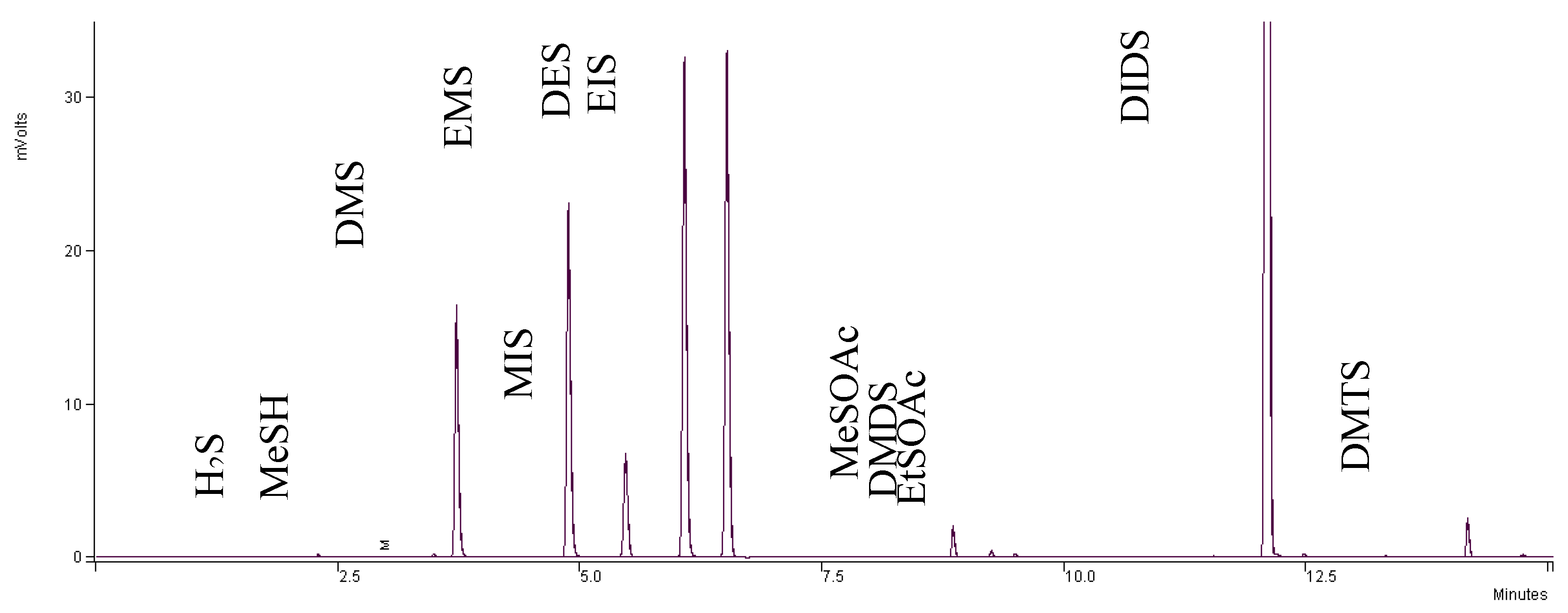
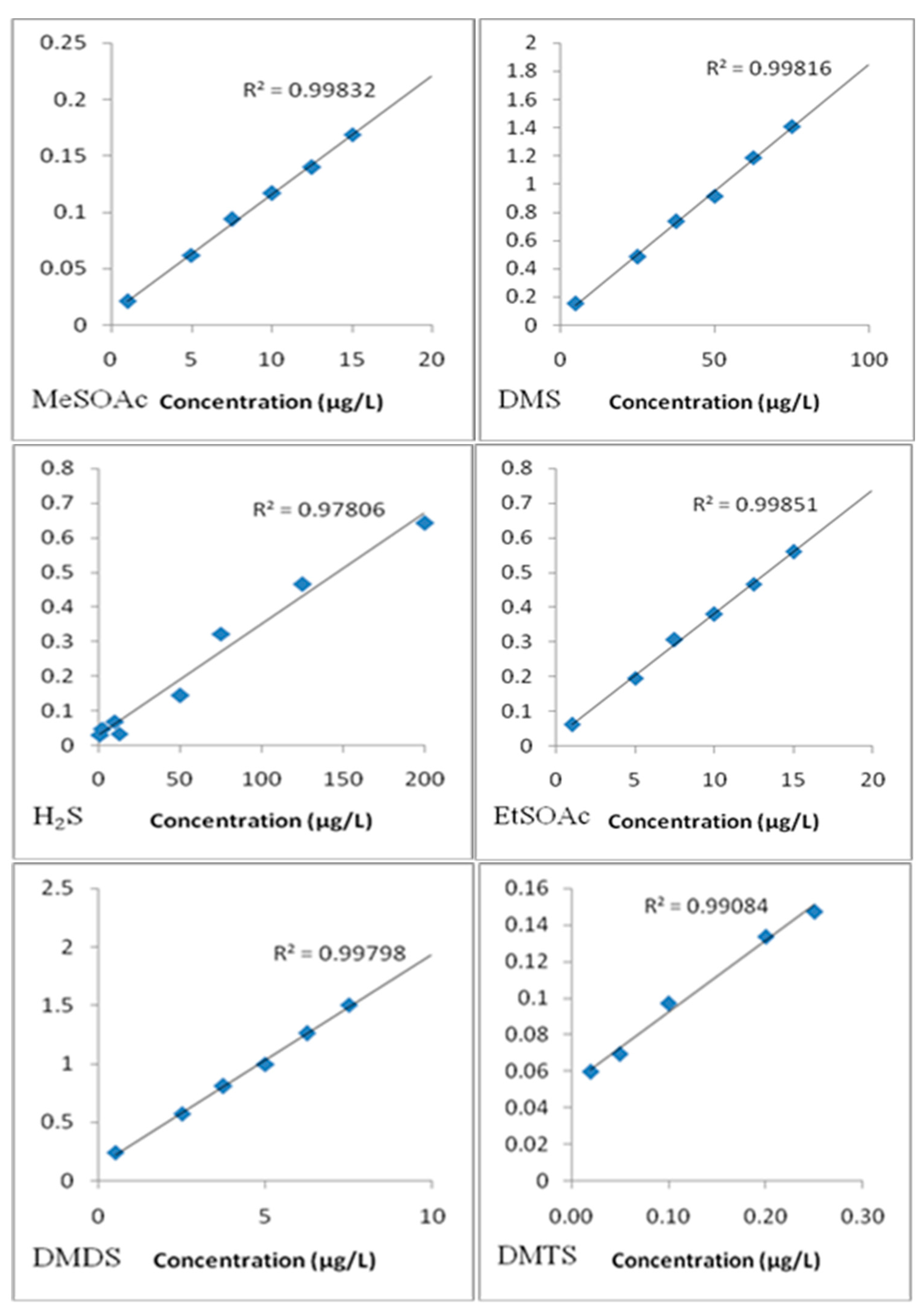
3.2. Calibration of Sulfur Compounds
3.3. Volatile-Matrix Effect
3.4. Non-Volatile Matrix Effect
3.5. Volatile Analysis
3.6. Wine Samples
3.7. SPME Conditions
3.8. GC-PFPD
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goniak, O.J.; Noble, A.C. Sensory Study of Selected Volatile Sulfur Compounds in White Wine. Am. J. Enol. Vitic. 1987, 38, 223–227. [Google Scholar]
- López, R.; Lapena, A.C.; Cacho, J.; Ferreira, V. Quantitative determination of wine highly volatile sulfur compounds by using automated headspace solid-phase microextraction and gas chromatography-pulsed flame photometric detection: Critical study and optimization of a new procedure. J. Chromatogr. A 2007, 1143, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wardencki, W. Problems with the determination of environmental sulphur compounds by gas chromatography. J. Chromatogr. A 1998, 793, 1–19. [Google Scholar] [CrossRef]
- Nedjma, M. Influence of complex media composition, Cognac’s brandy, or Cognac, on the gas chromatography analysis of volatile sulfur compounds—Preliminary results of the matrix effect. Am. J. Enol. Vitic. 1997, 48, 333–338. [Google Scholar]
- Hill, P.G.; Smith, R.M. Determination of sulphur compounds in beer using headspace solid-phase microextraction and gas chromatographic analysis with pulsed flame photometric detection. J. Chromatogr. A 2000, 872, 203–213. [Google Scholar] [CrossRef]
- Mestres, M.; Sala, C.; Martí, M.P.; Busto, O.; Guasch, J. Headspace solid-phase microextraction of sulphides and disulphides using Carboxen-polydimethylsiloxane fibers in the analysis of wine aroma. J. Chromatogr. A 1999, 835, 137–144. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P. Protection of volatiles in a wine with low sulfur dioxide by caffeic acid or glutathione. Am. J. Enol. Vitic. 2007, 58, 274–278. [Google Scholar]
- Negishi, O.; Negishi, Y.; Ozawa, T. Effects of food materials on removal of Allium-specific volatile sulfur compounds. J. Agric. Food Chem. 2002, 50, 3856–3861. [Google Scholar] [CrossRef]
- Blanchard, L.; Darriet, P.; Dubourdieu, D. Reactivity of 3-mercaptohexanol in red wine: Impact of oxygen, phenolic fractions, and sulfur dioxide. Am. J. Enol. Vitic. 2004, 55, 115–120. [Google Scholar]
- Abu-Lafi, S.; Dembicki, J.W.; Goldshlag, P.; Hanuš, L.O.; Dembitsky, V.M. The use of the ‘Cryogenic’ GC/MS and on-column injection for study of organosulfur compounds of the Allium sativum. J. Food Compos. Anal. 2004, 17, 235–245. [Google Scholar] [CrossRef]
- Ishihara, K.; Honma, N.; Uchiyama, T. Formation and reduction of volatile sulfur-containing compounds in synthetic media by salt-tolerant yeasts. Seibutsu-Kogaku Kaishi-J. Soc. Ferment. Bioeng. 1995, 73, 463–472. [Google Scholar]
- Castro, R.; Natera, R.; Durán, E.; García-Barroso, C. Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products. Eur. Food Res. Technol. 2008, 228, 1–18. [Google Scholar] [CrossRef]
- Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Improved solid-phase extraction procedure for the isolation and in-sorbent pentafluorobenzyl alkylation of polyfunctional mercaptans: Optimized procedure and analytical applications. J. Chromatogr. A 2008, 1185, 9–18. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice, 1st ed.; Wiley-VCH Inc.: New York, NY, USA, 1997. [Google Scholar]
- Murray, R.A. Limitations to the use of solid-phase microextraction for quantitation of mixtures of volatile organic sulfur compounds. Anal. Chem. 2001, 73, 1646–1649. [Google Scholar] [CrossRef]
- Nedjma, M.; Maujean, A. Improved chromatographic analysis of volatile sulfur compounds by the static headspace technique on water-alcohol solutions and brandies with chemiluminescence detection. J. Chromatogr. A 1995, 704, 495–502. [Google Scholar] [CrossRef]
- Mestres, M.; Martı, M.P.; Busto, O.; Guasch, J. Simultaneous analysis of thiols, sulphides and disulphides in wine aroma by headspace solid-phase microextraction-gas chromatography. J. Chromatogr. A 1999, 849, 293–297. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Tsai, I.-M. Understanding Aroma Impacts of Four Important Volatile Sulfur Compounds in Oregon Pinot Noir Wines. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2006. [Google Scholar]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Spedding, D.J.; Raut, P. The influence of dimethyl sulfide and carbon-disulfide in the bouquet of wines. VITIS 1982, 21, 240–246. [Google Scholar]
- Fang, Y.; Qian, M.C. Sensitive quantification of sulfur compounds in wine by headspace solid-phase microextraction technique. J. Chromatogr. A 2005, 1080, 177–185. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Varietal | Year | H2S | MeSH | DMS | MeSOAc | DMDS | EtSOAc | DEDS | DMTS |
|---|---|---|---|---|---|---|---|---|---|
| Chardonnay | 2009 | 2.35 ± 0.29 | 5.64 ± 0.57 | 25.12 ± 0.60 | 6.03 ± 0.09 | 0.16 ± 0.01 | <0.1 | <0.01 | 0.18 ± 0.02 |
| Chardonnay | blend | 19.35 ± 4.51 | 7.07 ± 0.36 | 53.02 ± 0.12 | <1 | 0.06 ± 0.01 | <0.1 | <0.01 | 0.18 ± 0.01 |
| Chardonnay | blend | 22.25 ± 5.52 | 6.85 ± 0.50 | 30.77 ± 0.57 | 6.67 ± 1.55 | 0.64 ± 0.08 | <0.1 | 0.01 ± 0.001 | 0.32 ± 0.04 |
| Moscato | 2010 | 1.15 ± 0.23 | <0.1 | 4.08 ± 0.04 | 24.02 ± 14.11 | 0.02 ± 0.01 | 2.88 ± 1.96 | 0.02 ± 0.004 | 0.03 ± 0.002 |
| Pinot Gris | 2010 | 8.82 ± 0.11 | 0.66 ± 0.08 | 25.44 ± 0.02 | <1 | <0.01 | <0.1 | <0.01 | 0.05 ± 0.004 |
| Riesling | 2009 | 1.11 ± 0.14 | 2.56 ± 0.05 | 11.48 ± 0.62 | <1 | 0.01 ± 0.001 | <0.1 | <0.01 | 0.14 ± 0.03 |
| Riesling | 2009 | 5.47 ± 1.13 | <0.1 | 14.53 ± 0.55 | <1 | <0.01 | <0.1 | <0.01 | 0.03 ± 0.002 |
| Sauv. Blanc | 2009 | <1 | <0.1 | 13.57 ± 1.33 | 15.19 ± 13.45 | 1.06 ± 0.02 | 5.00 ± 0.43 | 0.08 ± 0.003 | 0.04 ± 0.000 |
| White (blend) | blend | 25.39 ± 3.82 | 3.31 ± 0.41 | 17.84 ± 0.99 | <1 | <0.01 | <0.1 | <0.01 | 0.04 ± 0.004 |
| Cab. Sauv. | 2009 | <1 | 1.25 ± 0.09 | 59.46 ± 0.01 | 21.38 ± 0.15 | 0.16 ± 0.03 | 0.71 ± 0.02 | <0.01 | 0.18 ± 0.007 |
| Cab. Sauv. | 2008 | <1 | 3.21 ± 0.28 | 84.51 ± 5.82 | 7.75 ± 0.17 | 0.27 ± 0.02 | <0.1 | <0.01 | 0.02 ± 0.002 |
| Cab. Sauv. | 2007 | 1.13 ± 0.20 | 1.95 ± 0.14 | 55.12 ± 0.01 | 2.07 ± 0.04 | 0.03 ± 0.001 | <0.1 | <0.01 | 0.02 ± 0.002 |
| Cab. Sauv. | blend | <1 | 0.37 ± 0.03 | 42.04 ± 0.59 | 19.46 ± 0.04 | <0.01 | 1.62 ± 0.13 | <0.01 | 0.06 ± 0.004 |
| Malbec | 2009 | <1 | 3.70 ± 0.44 | 45.22 ± 0.92 | 18.10 ± 0.16 | 0.48 ± 0.04 | 0.72 ± 0.01 | <0.01 | 0.29 ± 0.01 |
| Merlot | blend | <1 | 1.15 ± 0.03 | 42.24 ± 1.56 | 34.00 ± 0.23 | <0.01 | 2.98 ± 0.02 | <0.01 | 0.08 ± 0.01 |
| Merlot | 2009 | <1 | <0.1 | 34.99 ± 0.26 | 17.90 ± 0.31 | <0.01 | 0.93 ± 0.08 | <0.01 | 0.08 ± 0.002 |
| Merlot | 2008 | <1 | 1.01 ± 0.05 | 51.26 ± 4.15 | 11.13 ± 4.12 | 0.54 ± 0.14 | 0.22 ± 0.31 | <0.01 | 0.09 ± 0.003 |
| Pinot noir | 2009 | <1 | 2.87 ± 0.51 | 19.44 ± 0.56 | 18.74 ± 0.46 | 0.08 ± 0.019 | 0.39 ± 0.01 | <0.01 | 0.15 ± 0.04 |
| Pinot Noir | blend | 9.60 ± 0.76 | 1.19 ± 0.26 | 20.28 ± 0.25 | 22.56 ± 0.14 | <0.01 | 1.52 ± 0.07 | <0.01 | 0.10 ± 0.05 |
| Zinfandel | 2009 | <1 | 2.13 ± 0.21 | 64.04 ± 0.11 | 29.28 ± 0.05 | 0.04 ± 0.000 | 2.43 ± 0.10 | <0.01 | 0.23 ± 0.01 |
| Red (blend) | blend | <1 | 1.52 ± 0.18 | 33.49 ± 1.89 | 42.95 ± 0.30 | <0.01 | 5.68 ± 0.05 | <0.01 | 0.13 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, P.M.; Qian, M.C. Effect of Wine Matrix Composition on the Quantification of Volatile Sulfur Compounds by Headspace Solid-Phase Microextraction-Gas Chromatography-Pulsed Flame Photometric Detection. Molecules 2019, 24, 3320. https://doi.org/10.3390/molecules24183320
Davis PM, Qian MC. Effect of Wine Matrix Composition on the Quantification of Volatile Sulfur Compounds by Headspace Solid-Phase Microextraction-Gas Chromatography-Pulsed Flame Photometric Detection. Molecules. 2019; 24(18):3320. https://doi.org/10.3390/molecules24183320
Chicago/Turabian StyleDavis, Peter M., and Michael C. Qian. 2019. "Effect of Wine Matrix Composition on the Quantification of Volatile Sulfur Compounds by Headspace Solid-Phase Microextraction-Gas Chromatography-Pulsed Flame Photometric Detection" Molecules 24, no. 18: 3320. https://doi.org/10.3390/molecules24183320
APA StyleDavis, P. M., & Qian, M. C. (2019). Effect of Wine Matrix Composition on the Quantification of Volatile Sulfur Compounds by Headspace Solid-Phase Microextraction-Gas Chromatography-Pulsed Flame Photometric Detection. Molecules, 24(18), 3320. https://doi.org/10.3390/molecules24183320






