Assessment of By-Product from Botryosphaeria rhodina MAMB-05 as an Effective Biosorbent of Pb(II)
Abstract
1. Introduction
2. Results and Discussion
2.1. Fungal Biomass Preparation as Biosorbent
2.2. FT-IR Spectroscopy Study
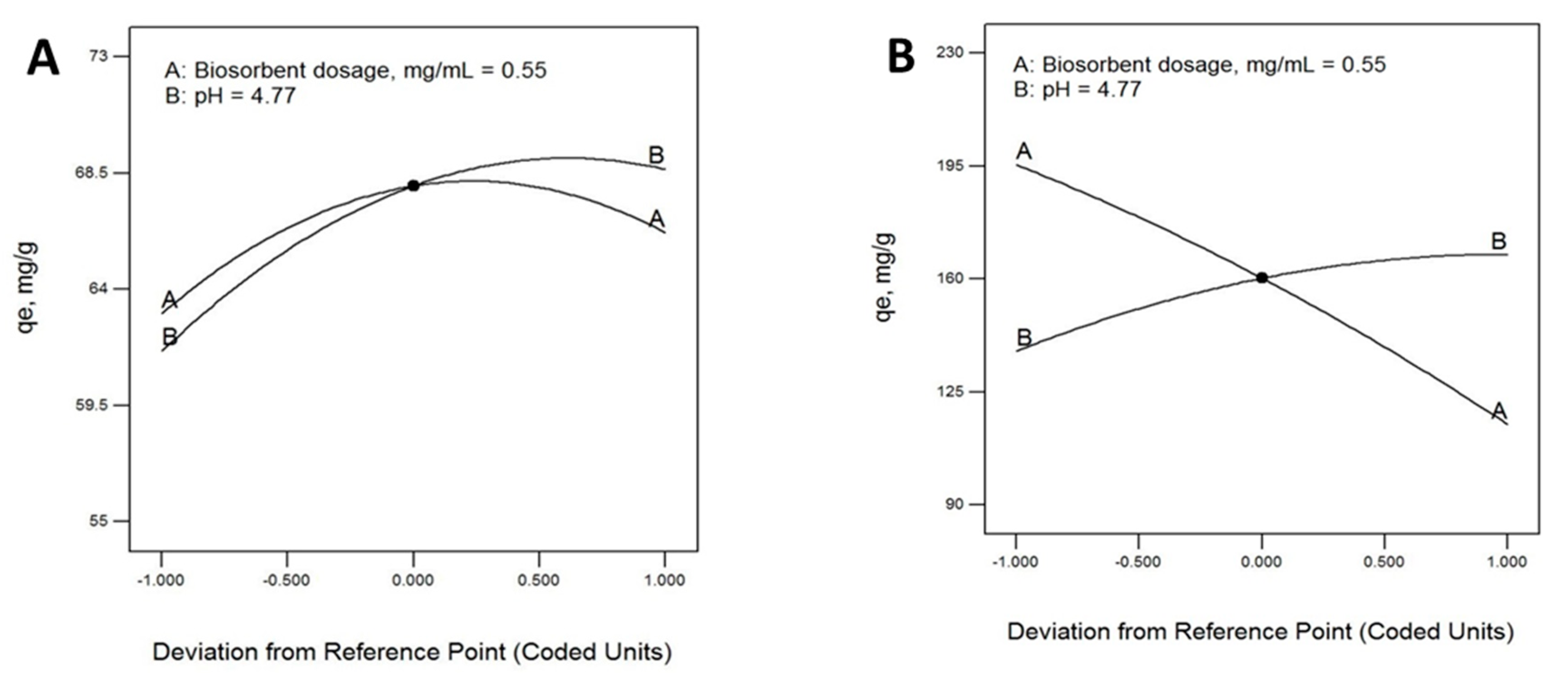
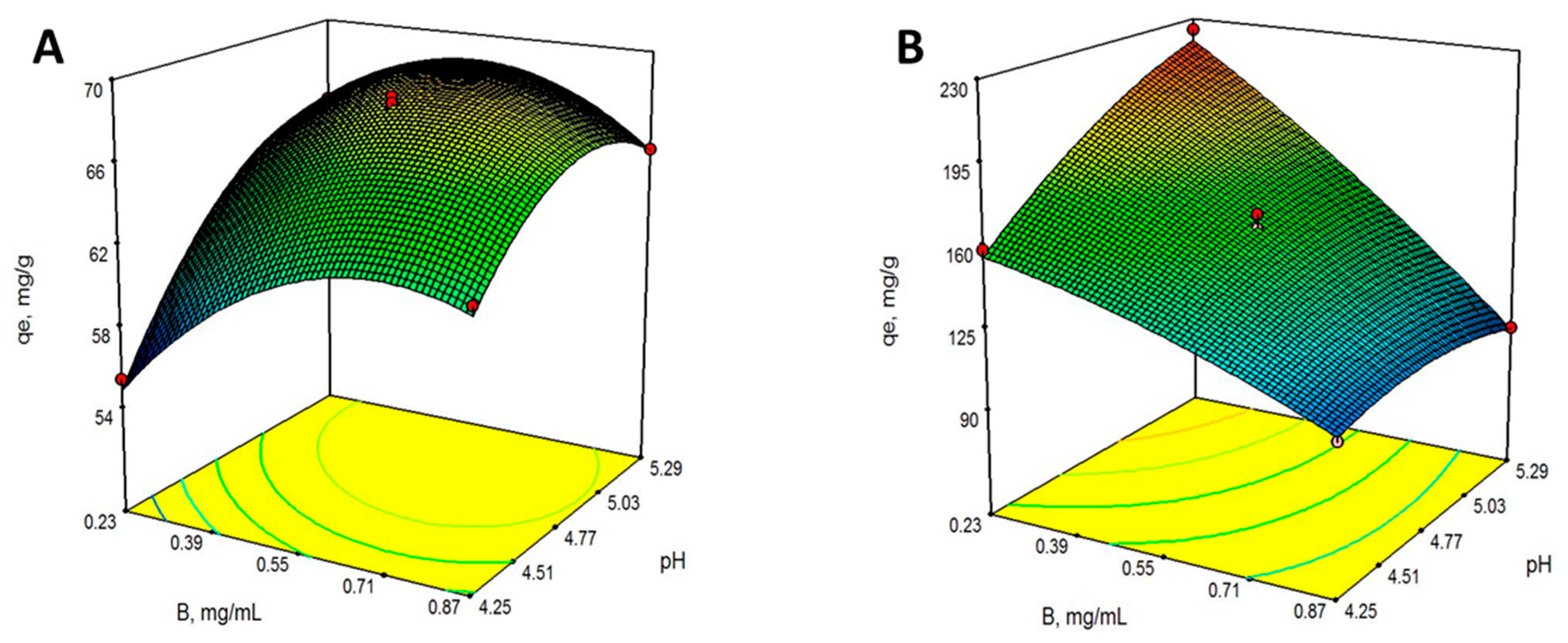
2.3. Optimum Operating Conditions for Pb(II) Bioadsorption: Response Surface Methodology
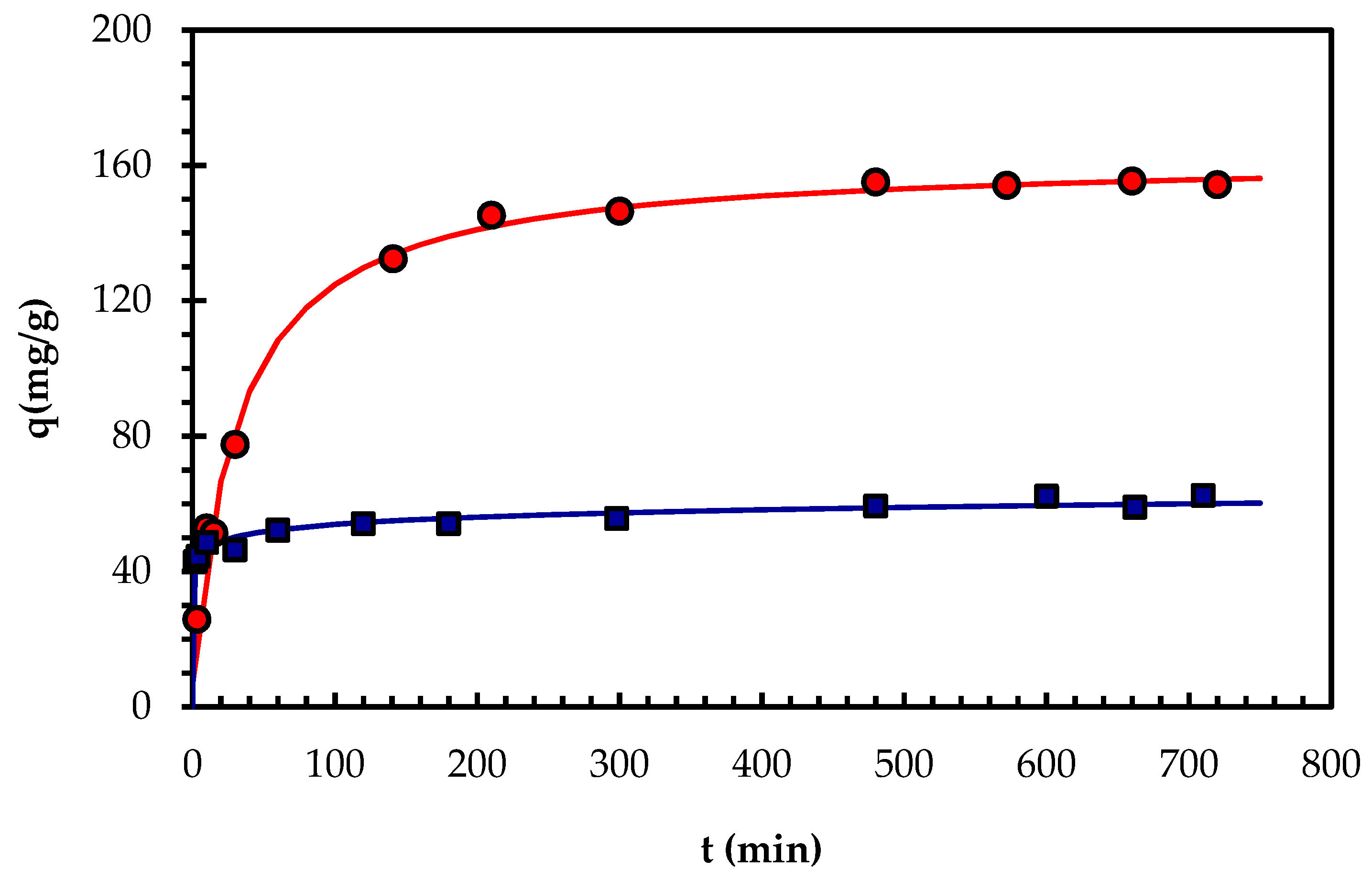
2.4. Biosorption Kinetics of Pb(II)
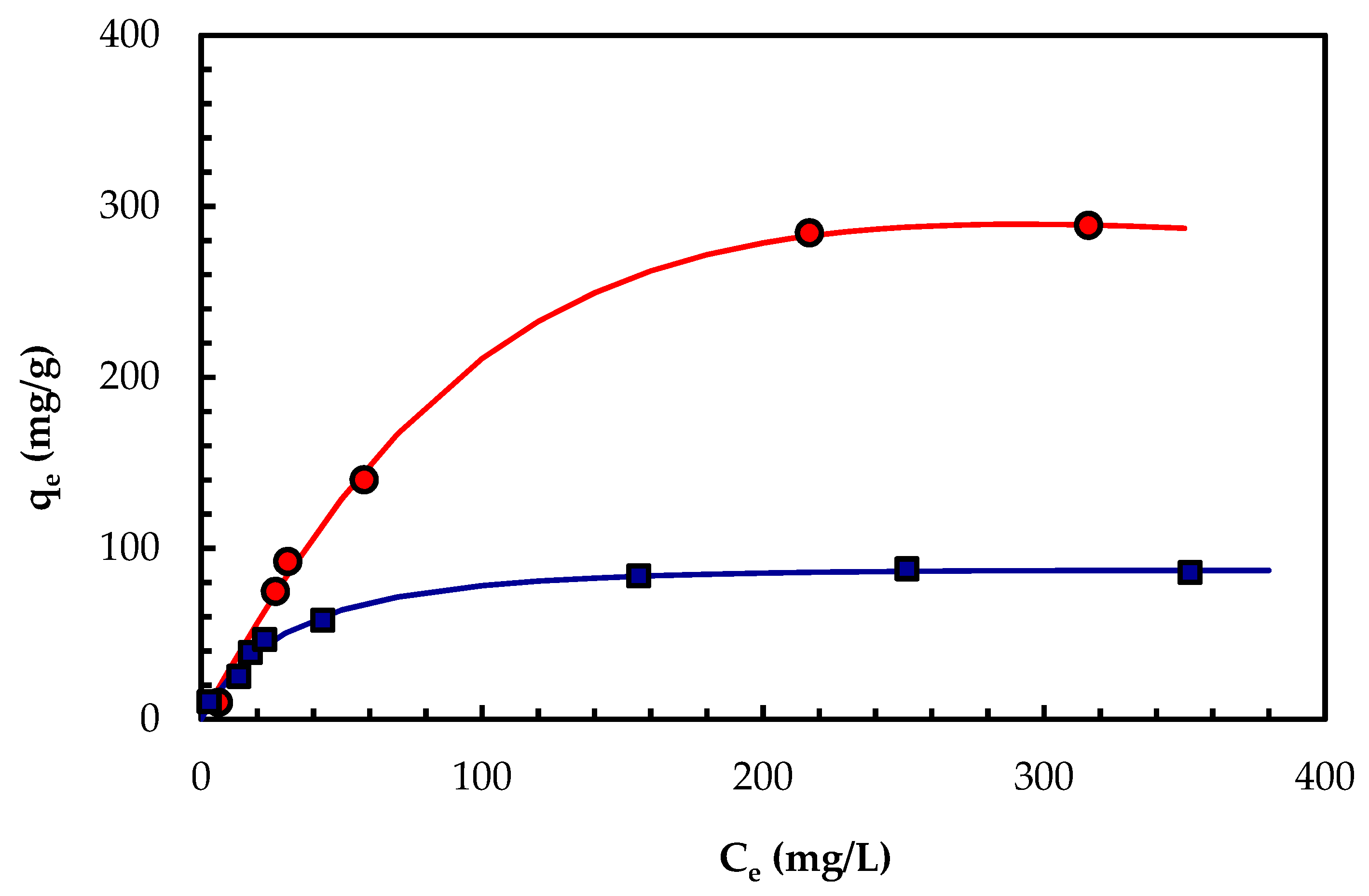
2.5. Biosorption Isotherm for PBPb(II)
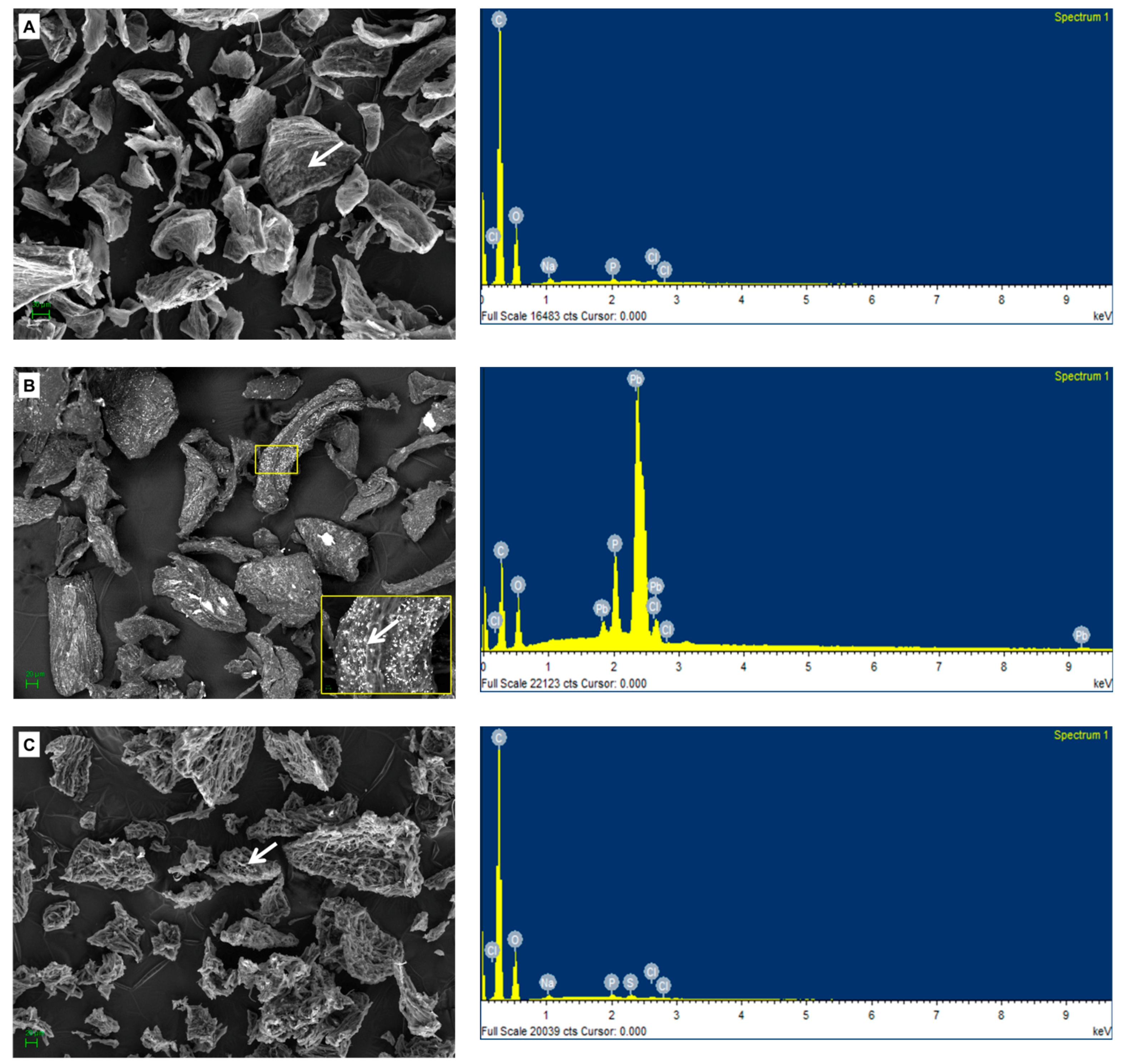
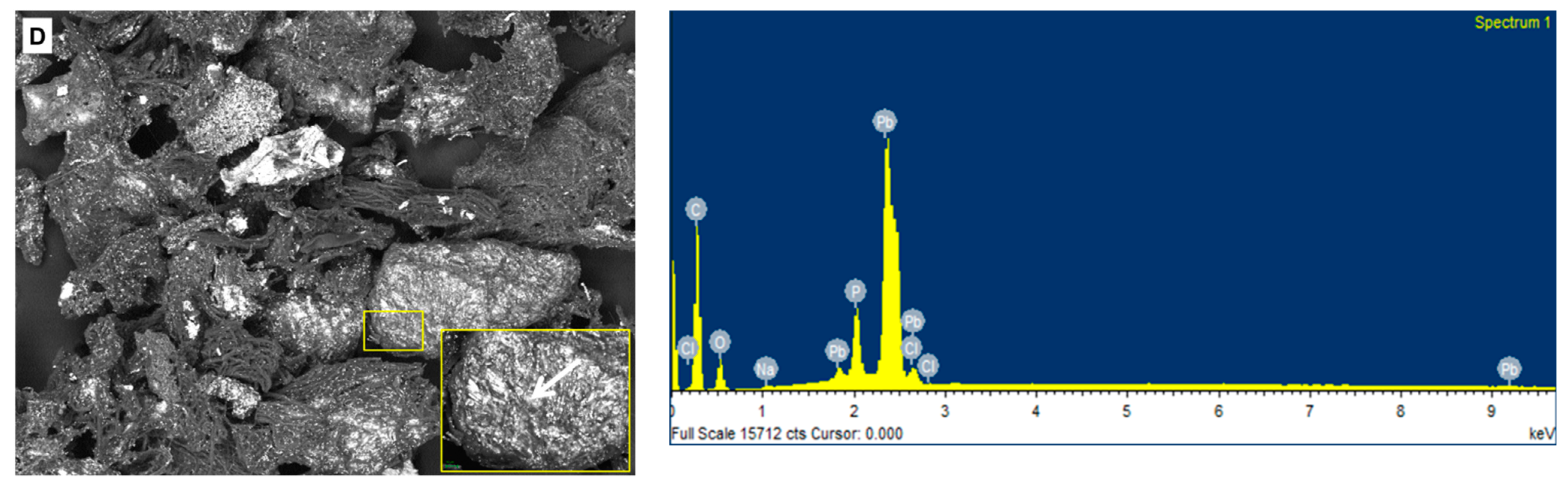
2.6. Chemical and SEM Analysis of the Biomass Preparations from Botryosphaeria rhodina MAMB-05
3. Materials and Methods
3.1. Production of Fungal Biomass
3.2. Fungal Biomass Characterization: FT-IR Analysis
3.3. Optimizing Conditions for Biosorption of Pb(II)
3.4. Biosorption Kinetics
3.5. Equilibrium Study
3.6. Field Emission-Scanning Electron Microscopy-Energy Dispersive X-Ray Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Binkowski, L.J. The influence of environmental conditions on lead transfer from spent gunshot to sediments and water: Other routes for Pb poisoning. Chemosphere 2017, 187, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, M.A.S.; Filippelli, G.M.; Brown, S.; Paz-Ferreiro, J.; Reichman, S.M.; Netherway, P.; Truskewycz, A.; Ball, A.S.; Mielke, H.W. Case studies and evidence-based approaches to addressing urban soil lead contamination. Appl. Geochem. 2017, 83, 14–30. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente, Gobierno de España. Registro Estatal de Emisiones y Fuentes Contaminantes: Emisiones por Sustancias Contaminantes. 2017. Available online: http://www.prtr-es.es (accessed on 10 September 2019).
- Tirima, S.; Bartrem, C.; Lindern, I.V.; Braun, M.V.; Lind, D.; Anka, S.M.; Abdullahi, A. Food contamination as a pathway for lead exposure in children during the 2010–2013 lead poisoning epidemic in Zamfara. Nigeria. J. Environ. Sci. 2017, 67, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Padararu, C.; Ibanescu, D.; Tofan, L. Biosorption of lead ions from aqueous effluents by rapeseed biomass. New Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept: Definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Barros, O.; Costa, L.; Costa, F.; Lago, A.; Rocha, V.; Vipotnik, Z.; Silva, B.; Tavares, T. Recovery of rare earth elements from wastewater towards a circular economy. Molecules 2019, 24, 1005. [Google Scholar] [CrossRef] [PubMed]
- Dekker, R.F.H.; Barbosa, A.M. Effect of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp. Enzym. Microb. Technol. 2001, 28, 81–88. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Steluti, R.M.; Dekker, R.F.H.; Cardoso, M.S.; Corradi da Silva, M.L. Structural characterization of Botryosphaeran: A (1→3;1→6)-β-D-glucan produced by the ascomyceteous fungus, Botryosphaeria sp. Carbohydr. Res. 2003, 338, 1691–1698. [Google Scholar] [CrossRef]
- Dekker, R.F.H.; Barbosa, A.M.; Sargent, K. The effect of lignin-related compounds on growth and production of laccases by the ligninolytic ascomycete, Botryosphaeria sp. Enzym. Microb. Technol. 2002, 30, 374–380. [Google Scholar] [CrossRef]
- Giese, E.C.; Dekker, R.F.H.; Barbosa-Dekker, A.M. Biosorption of lanthanum and samarium by viable and autoclaved mycelium of Botryosphaeria rhodina MAMB-05. Biotechnol. Prog. 2019, 35, e2783. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. A Convenient Growth Medium for Neurospora crassa. Microb. Genet. Bull. 1956, 13, 42–43. [Google Scholar]
- Crognale, S.; Bruno, M.; Fidaleo, M.; Moresi, M.; Petruccioli, M. Production of β-glucan and related glucan-hydrolases by Botryosphaeria rhodina. J. Appl. Microbiol. 2007, 102, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Corradi da Silva, M.L.; Fukuda, E.K.; Vasconcelos, A.F.D.; Dekker, R.F.H.; Matias, A.C.; Monteiro, N.K.; Cardoso, M.S.; Barbosa, A.M.; Silveira, J.L.M.; Sassaki, G.L.; et al. Structural characterization of the cell wall D-glucans isolated from the mycelium of Botryosphaeria rhodina MAMB-05. Carbohydr. Res. 2008, 343, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Dumas, P. From structure to cellular mechanism with infrared microspectroscopy. Curr. Opin. Struct. Biol. 2010, 20, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jin, R.-N.; Omer, A.M.; Ouyang, X.-K. Adsorption of Pb(II) from fish sauce using carboxylated cellulose nanocrystal: Isotherm, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2017, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Q.; Li, B.; Liu, B.; Wu, G.; Ibrahim, M.; Xie, G.; Li, H.; Sun, G. Differentiation in MALDI-TOF MS and FTIR spectra between two closely related species Acidovorax oryzae and Acidovorax citrulli. BMC Microbiol. 2012, 12, 182. [Google Scholar] [CrossRef]
- Wang, N.; Ouyang, X.-K.; Yang, L.-Y.; Omer, A.M. Fabrication of a magnetic cellulose nanocrystal/metal−organic framework composite for removal of Pb(II) from water. ACS Sustain. Chem. Eng. 2017, 5, 10447–10458. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Gao, B.; Shi, X.; Sun, Y.; Wu, J. Efficient biosorption of Pb(II) from aqueous solutions by a PAH-degrading strain Herbaspirillum chlorophenolicum FA1. J. Ind. Eng. Chem. 2018, 57, 64–71. [Google Scholar] [CrossRef]
- Vijayaraghvan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Kirova, G.; Velkova, Z.; Stoytcheva, M.; Hristova, Y.; Iliev, I.; Gochev, V. Biosorption of Pb(II) ions from aqueous solutions by waste biomass of Streptomyces fradiae pretreated with NaOH. Biotechnol. Biotechnol. Equip. 2015, 29, 689–695. [Google Scholar] [CrossRef]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Kinetic studies of competitive heavy metal adsorption by sphagnum peat. Environ. Technol. 1996, 17, 71–77. [Google Scholar] [CrossRef]
- Ezzouhri, L.; Ruiz, E.; Castro, E.; Moya, M.; Espínola, F.; Cherrad, L.; Er-Raioui, H.; Lairini, K. Mechanisms of lead uptake by fungal biomass isolated from heavy metals habitats. Afinidad 2010, 67, 39–44. [Google Scholar]
- Sag, Y.; Yalc¸uk, A.; Kutsal, T. Mono and multi-component biosorption of heavy metal ions on Rhizopus arrhizus in a CFST. Process. Biochem. 2000, 35, 787–799. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.Z.L.; Basibuyuk, M.; Forster, C.F. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour. Technol. 2004, 92, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Wang, X.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of lead from aqueous solution by activated carbon prepared from Enteromorpha prolifera by zinc chloride activation. J. Hazard. Mater. 2010, 183, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.; Paul, S.; Bera, D.; Chattopadhyay, P. Bioaccumulation of Pb (II) from aqueous solutions by Bacillus cereus M116. J. Hazard. Subst. Res. 2006, 5, 1. [Google Scholar]
- Dursun, A.Y. A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger. Biochem. Eng. J. 2006, 28, 187–195. [Google Scholar] [CrossRef]
- Wang, X.J.; Xia, S.Q.; Chen, L.; Zhao, J.F.; Chovelon, J.M.; Nicole, J.R. Biosorption of cadmium(II) and lead(II) ions from aqueous solutions onto dried activated sludge. J. Environ. Sci. 2006, 18, 840–844. [Google Scholar] [CrossRef]
- Pavasant, P.; Apiratikul, R.; Sungkhum, V.; Suthiparinyanont, P.; Wattanachira, S.; Marhaba, T.F. Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 2006, 97, 2321–2329. [Google Scholar] [CrossRef]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Filote, C.; Volf, I.; Santos, S.C.R.; Botelho, C.M.S. Bioadsorptive removal of Pb(II) from aqueous solution by the biorefinery waste of Fucus spiralis. Sci Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Borra, M.; Tamburrino, S.; Saggiomo, M.; Viola, A.; Biffali, E.; Balestra, C.; Sprovieri, M.; Casotti, R. A Bacillus sp. isolated from sediments of the Sarno River mouth, Gulf of Naples (Italy) produces a biofilm biosorbing Pb(II). Sci. Total Environ. 2016, 562, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Apiratikul, R.; Madacha, V.; Pavasant, P. Kinetic and mass transfer analyses of metal biosorption by Caulerpa lentillifera. Desalination 2011, 278, 303–311. [Google Scholar] [CrossRef]
- Begum, S.A.S.; Tharakeswar, Y.; Kalyan, Y.; Naidu, G.R. Biosorption of Cd(II), Cr(VI) & Pb(II) from aqueous solution using Mirabilis jalapa as adsorbent. J. Encapsulation Adsorpt. Sci. 2015, 5, 93–104. [Google Scholar]
- Nguyen, T.T.L.; Lee, H.R.; Hong, S.H.; Jang, J.R.; Choe, W.-S.; Yoo, I.K. Selective lead adsorption by recombinant Escherichia coli displaying a lead-binding peptide. Appl. Biochem. Biotechnol. 2013, 169, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, R.; Ansari, T.M.; Khalid, A.M. Fourier Transform Infrared Spectroscopic characterization and optimization of Pb(II) biosorption by fish (Labeo rohita) scales. J. Hazard. Mater. 2008, 156, 64–73. [Google Scholar] [CrossRef]
- Akar, T.; Tunali, S.; Kiran, I. Botrytis cinerea as a new fungal biosorbent for removal of Pb(II) from aqueous solutions. Biochem. Eng. J. 2005, 25, 227–235. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Ajala, M.A.; Agarry, S.E. Evaluation of biosorptive capacity of banana (Musa paradisiaca) stalk for lead(II) removal from aqueous solution. J. Environ. Prot. 2014, 5, 1451–1465. [Google Scholar] [CrossRef]
- Salehi, P.; Asghari, B.; Mohammadi, F. Biosorption of Ni(II), Cu(II) and Pb(II) by Punica geranatum from aqueous solutions. J. Water Resour. Prot. 2010, 2, 701–705. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Moya, M.; Ruiz, E. Biosorption of Pb(II) ions by Klebsiella sp. 3S1 isolated from a wastewater treatment plant: Kinetics and mechanisms studies. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Steluti, R.M.; Giese, E.C.; Pigato, M.M.; Sumiya, A.F.G.; Covizzi, L.G.; Job, A.E.; Cardoso, M.S.; Corradi da Silva, M.L.; Dekker, R.F.H.; Barbosa, A.M. Comparison of Botryosphaeran production by the ascomyceteous fungus Botryosphaeria sp., grown on different carbohydrate carbon sources, and their partial structural features. J. Basic Microbiol. 2004, 44, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.W.; Porter, J.F.; McKay, G. Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res. 2001, 35, 605–612. [Google Scholar] [CrossRef]
- Langmuir, I. Adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Adsorption in solutions. Phys. Chem. 1906, 57, 384–410. [Google Scholar]
- Sips, R. Combined form of Langmuir and Freundlich equations. J. Chem. Phys. 1948, 16, 490–549. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. C 1959, 63, 1024–1039. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E. Biosorption of Ag(I) from aqueous solutions by Klebsiella sp. 3S1. J. Hazard. Mater. 2017, 329, 166–177. [Google Scholar] [CrossRef]
Sample Availability: Samples of the fungal biomass are available from the authors. |
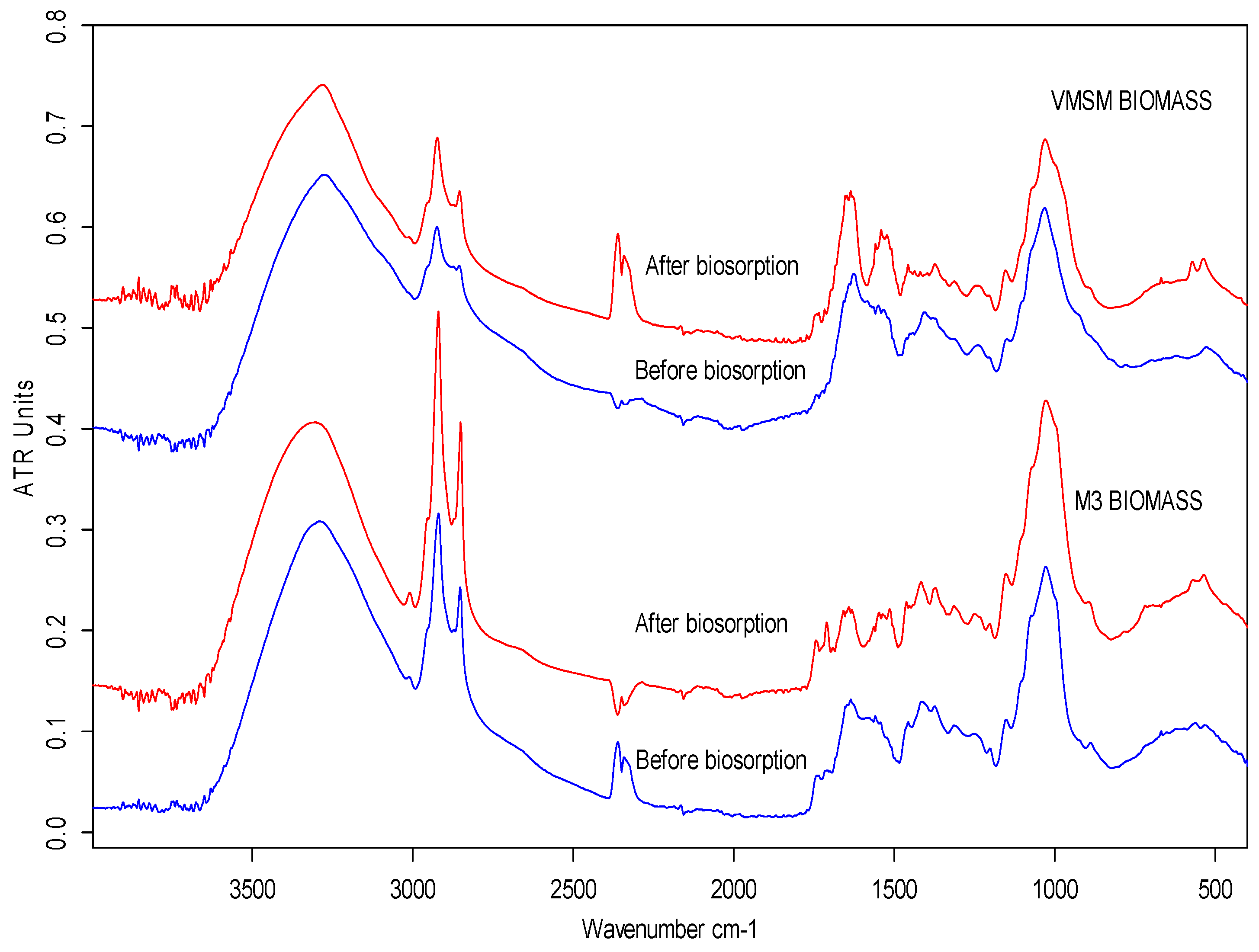
| M3 Biomass (peaks cm−1) | VMSM Biomass (peaks cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| Before | After | Shift | Before | After | Shift | Functional Groups | Possible Assignment |
| 3290 | 3303 | 13 | O-H, N-H | Stretching vibrations of amino and hydroxyl groups | |||
| - | 3012 | 3012 | -CH3 | –CH3 asymmetric stretching | |||
| 1792 | 1786 | 6 | 1797 | 1792 | 5 | C = O | C = O stretching |
| 1771 | 1778 | 7 | C = O | C = O stretching | |||
| - | 1734 | 1734 | C = O | C = O stretching | |||
| 1723 | 1717 | 6 | C = O | C = O stretching | |||
| 1700 | 1691 | 9 | -CO. C-N, N-H | -CO, -CN stretching and -NH bending vibration (amide I) | |||
| 1652 | 1658 | 6 | 1657 | 1652 | 5 | -CO. C-N, N-H | -CO, -CN stretching and -NH bending vibration (amide I) |
| 1646 | 1649 | 3 | - | 1647 | 1647 | -CO. C-N, N-H | -CO, -CN stretching and -NH bending vibration (amide I) |
| 1636 | 1642 | 6 | 1640 | 1636 | 4 | -CO. C-N, N-H | -CO, -CN stretching and -NH bending vibration (amide I) |
| - | 1631 | 1631 | -CO | -CO stretching amide I | |||
| 1559 | 1564 | 5 | 1565 | 1558 | 7 | N-H, C-N | C–N stretching in amide II group and N–H bending vibration |
| 1542 | 1547 | 5 | 1549 | 1541 | 8 | N-H, C-N | C–N stretching in amide II group and N–H bending vibration. |
| 1523 | 1536 | 13 | - | 1522 | 1522 | N-H, C-N | C–N stretching in amide II group and N–H bending vibration. |
| - | 1509 | 1509 | N-H, C-N | C–N stretching in amide II group and N–H bending vibration. | |||
| 1490 | 1514 | 28 | -CH | -CH bending | |||
| 1484 | 1480 | 4 | -CH2, -CO | -CH bending, symmetric C = O | |||
| - | 1462 | 1462 | 1461 | 1457 | 4 | -CH2, -OH, C = O | -CH bend. Deformations, OH bend. in carboxylic groups, sym. C = O |
| 1457 | 1453 | 4 | -CH2, -CO | -CH bending, symmetric C = O | |||
| - | 1441 | 1441 | 1442 | 1437 | 5 | -CH2, -CO | -CH bending, symmetric C = O |
| - | 1419 | 1419 | -CH, C-N, C = O | C-H stretching, C-H rocking, C-N stretching | |||
| 1405 | 1397 | 14 | N-H, C-N, COO− | N-H bend. in the amine group; C-N stretch.; C = O str (sym) of COO− | |||
| 1397 | 1374 | 5 | -CH | C-H bending vibrations | |||
| - | 1334 | 1334 | -CH | C-H bending vibrations | |||
| 1239 | 1243 | 4 | -PO2−, -CO | P = O stretching of phosphodiester, or monoester phosphate groups; C-O-C, C-O-P, P-O-P ring vibrations of polysaccharides | |||
| - | 1205 | 1205 | C-O-C, C-O-P, P-O-P | C-O-C, C-O-P, P-O-P ring vibrations of polysaccharides | |||
| 888 | 893 | 5 | PO2−, -CH | C-O-C, C-O-P, P-O-P ring vibrations of polysaccharides; C-H bending (aromatic compounds) | |||
| - | 782 | 782 | Deformational modes of the CCO groups; Nitro compounds and disulfide groups | ||||
| - | 718 | 718 | -CH2 | C-H rocking | |||
| 668 | 673 | 5 | 673 | 668 | 5 | Deformational modes of the CCO groups; Nitro compounds and disulfide groups | |
| 621 | 617 | 4 | Deformational modes of the CCO groups; Nitro compounds and disulfide groups | ||||
| 595 | 603 | 8 | Deformational modes of the CCO groups; Nitro compounds and disulfide groups | ||||
| - | 571 | 571 | -CH | C-H bending vibrations (aromatics) | |||
| 562 | 569 | 7 | Deformational modes of the CCO groups; Nitro compounds and disulfide groups | ||||
| 532 | 536 | 4 | 527 | 537 | 10 | Deformational modes of the CCO groups; Nitro compounds and disulfide groups | |
| 476 | 467 | 9 | 427 | 420 | 7 | Deformational modes of the CCO groups; Nitro compounds and disulfide groups | |
| Factors | Responses | ||
|---|---|---|---|
| B 1 (g/L) | pH | qe 2 VMSM Biomass (mg/g) | qe M3 Biomass (mg/g) |
| 0.55 | 4.77 | 159.05 | 67.96 |
| 0.55 | 4.77 | 159.03 | 67.53 |
| 0.55 | 4.03 | 56.69 | |
| 1.00 | 4.77 | 94.93 | 63.25 |
| 0.55 | 4.77 | 158.44 | 67.96 |
| 0.55 | 4.77 | 159.22 | 68.45 |
| 0.55 | 5.51 | 162.95 | |
| 0.87 | 4.25 | 107.59 | 62.05 |
| 0.23 | 4.25 | 158.61 | 55.39 |
| 0.10 | 4.77 | 202.10 | 58.70 |
| 0.23 | 5.29 | 224.91 | 65.65 |
| 0.55 | 4.77 | 165.65 | 68.15 |
| 0.87 | 5.29 | 105.76 | 65.06 |
| Source of Biomass | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| M3 | |||||
| Model | 243.16 | 5 | 48.63 | 201.31 | <0.0001 |
| B: Biosorbent dose | 19.55 | 1 | 19.55 | 80.91 | 0.0001 |
| pH | 59.47 | 1 | 59.47 | 246.19 | <0.0001 |
| B pH | 13.14 | 1 | 13.14 | 54.40 | 0.0003 |
| B2 | 73.85 | 1 | 73.85 | 305.72 | <0.0001 |
| pH2 | 35.04 | 1 | 35.04 | 145.03 | <0.0001 |
| Residual | 1.45 | 6 | 0.24 | ||
| Lack of fit | 1.00 | 2 | 0.50 | 4.46 | 0.0958 |
| Pure error | 0.45 | 4 | 0.11 | ||
| Cor total | 244.61 | 11 | |||
| C.V.% | 0.77 | ||||
| R-Squared | 0.9941 | ||||
| Adj. R-Squared | 0.9891 | ||||
| Pred. R-Squared | 0.9599 | ||||
| Adeq. Precision | 37.850 | ||||
| VMSM | |||||
| Model | 155,26.71 | 5 | 3105.34 | 167.13 | <0.0001 |
| B: Biosorbent dose | 129,38.88 | 1 | 129,38.88 | 696.37 | <0.0001 |
| pH | 1075.23 | 1 | 1075.23 | 57.87 | 0.0003 |
| B pH | 1160.42 | 1 | 1160.42 | 62.45 | 0.0002 |
| B2 | 166.59 | 1 | 166.59 | 8.97 | 0.0242 |
| pH2 | 244.48 | 1 | 244.48 | 13.16 | 0.0110 |
| Residual | 111.48 | 6 | 18.58 | ||
| Lack of fit | 75.06 | 2 | 37.53 | 4.12 | 0.1067 |
| Pure error | 36.42 | 4 | 9.11 | ||
| Cor total | 156,38.19 | 11 | |||
| C.V.% | 2.78 | ||||
| R-Squared | 0.9929 | ||||
| Adj. R-Squared | 0.9869 | ||||
| Pred. R-Squared | 0.9211 | ||||
| Adeq. Precision | 41.515 |
| Biosorbent (Biomass Type) | qm (mg/g) | Reference |
|---|---|---|
| Penicillium sp. (fungus) | 60.77 | [24] |
| Rhizopus arrhizus (fungus) | 48.79 | [25] |
| Ceratophyllum demursum (aquatic plant) | 44.80 | [26] |
| Activated carbon-Enteromorpha prolifera (green alga) | 146.85 | [27] |
| Bacillus cereus M116 (bacterium) | 70.42 | [28] |
| Aspergillus niger (fungus) | 34.92 | [29] |
| Dried activated sludge | 131.60 | [30] |
| Caulerpa lentillifera (marine green macroalga) | 28.99 | [31] |
| Cladophora fascicularis (green alga) | 227.70 | [32] |
| Herbaspirillum. chlorophenolicum FA1 (bacterium) | 151.52 | [20] |
| Fucus spiralis (residual biomaterial) | 132 | [33] |
| Bacillus pumilus (bacterium) | 93.24 | [34] |
| Caulerpa lentillifera (marine alga) | 36.67 | [35] |
| Mirabilis jalapa (vegetal leaves) | 38.46 | [36] |
| Escherichia coli (recombinant bacterium) | 108.99 | [37] |
| Labeo rohita (fish scales) | 196.8 | [38] |
| Botrytis cinerea (fungus) | 107.10 | [39] |
| Musa paradisiaca (vegetal stalks) | 13.53 | [40] |
| Punica geranatum (vegetal leaves) | 18.4 | [41] |
| Klebsiella sp. 3S1(bacterium) | 140.19 | [42] |
| Botryosphaeria rhodina MAMB-05 - VMSM | 403.4 | this study |
| B. rhodina MAMB-05 - M3 | 96.05 | this study |
| Parameter | Biomass Preparation | |||
|---|---|---|---|---|
| VMSM | M3 | |||
| Pseudo-first order, or Lagergren [44] | ||||
| q = 0 at t = 0 and q = q para t = t | qe | 149.4 | 55.059 | |
| k1 | 0.02864 | 0.6216 | ||
| r2 | 0.977 | 0.899 | ||
| Σ(q-qcal)2 | 847 | 317 | ||
| q = qi at t = 0 and q = q at t = t | qe | 150.4 | 55.07 | |
| qi | 12.69 | 0.5672 | ||
| k1 | 0.02211 | 0.6156 | ||
| r2 | 0.984 | 0.899 | ||
| Σ(q-qcal)2 | 595 | 316 | ||
| Pseudo-second order [23] | ||||
| q = 0 at t = 0 and q = q at t = t | qe | 161.2 | 56.31 | |
| k2 | 2.246 × 10−4 | 0.02031 | ||
| r2 | 0.992 | 0.934 | ||
| Σ(q-qcal)2 | 295 | 207 | ||
| q = qi at t = 0 and q = q at t = t | qe | 162.5 | 56.32 | |
| qi | 6.616 | 0.2600 | ||
| k2 | 2.008 × 10−4 | 0.02026 | ||
| r2 | 0.994 | 0.934 | ||
| Σ(q-qcal)2 | 229 | 207 | ||
| Elovich [45] | ||||
| q = 0 at t = 0 and q = q at t = t | a | 15.66 | 1.026 × 106 | |
| b | 0.03659 | 0.32098 | ||
| r2 | 0.982 | 0.987 | ||
| Σ(q-qcal)2 | 666 | 39 | ||
| q = qi at t = 0 and q = q at t = t | a | 15.81 | 1.026 × 106 | |
| b | 0.03667 | 0.3210 | ||
| qi | −1.210 | 7.136 × 10−9 | ||
| r2 | 0.982 | 0.987 | ||
| Σ(q-qcal)2 | 665 | 39 | ||
| Model | Equation | Parameter | Biomass Preparation | |
|---|---|---|---|---|
| VMSM | M3 | |||
| Langmuir [46] | qm | 403.4 | 96.05 | |
| B | 9.252 × 10−3 | 0.03575 | ||
| r2 | 0.992 | 0.988 | ||
| Σ(q-qcal)2 | 549 | 75 | ||
| Freundlich [47] | KF | 14.485 | 15.34 | |
| N | 1.874 | 3.202 | ||
| r2 | 0.963 | 0.915 | ||
| Σ(q-qcal)2 | 2473 | 517 | ||
| Sips [48] | Ks | 1.219 | 2.316 | |
| as | 3.640 × 10−3 | 2.502 × 10−2 | ||
| N | 0.7541 | 0.8776 | ||
| r2 | 0.996 | 0.989 | ||
| Σ(q-qcal)2 | 267 | 68 | ||
| Redlich-Peterson [49] | KRP | 2.922 | 2.910 | |
| aRP | 3.625 × 10−4 | 1.782 × 10−2 | ||
| Β | 1.513 | 1.092 | ||
| r2 | 0.998 | 0.990 | ||
| Σ(q-qcal)2 | 153 | 60 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.J.; Espínola, F.; Ruiz, E.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Castro, E. Assessment of By-Product from Botryosphaeria rhodina MAMB-05 as an Effective Biosorbent of Pb(II). Molecules 2019, 24, 3306. https://doi.org/10.3390/molecules24183306
Muñoz AJ, Espínola F, Ruiz E, Barbosa-Dekker AM, Dekker RFH, Castro E. Assessment of By-Product from Botryosphaeria rhodina MAMB-05 as an Effective Biosorbent of Pb(II). Molecules. 2019; 24(18):3306. https://doi.org/10.3390/molecules24183306
Chicago/Turabian StyleMuñoz, Antonio J., Francisco Espínola, Encarnación Ruiz, Aneli M. Barbosa-Dekker, Robert F. H. Dekker, and Eulogio Castro. 2019. "Assessment of By-Product from Botryosphaeria rhodina MAMB-05 as an Effective Biosorbent of Pb(II)" Molecules 24, no. 18: 3306. https://doi.org/10.3390/molecules24183306
APA StyleMuñoz, A. J., Espínola, F., Ruiz, E., Barbosa-Dekker, A. M., Dekker, R. F. H., & Castro, E. (2019). Assessment of By-Product from Botryosphaeria rhodina MAMB-05 as an Effective Biosorbent of Pb(II). Molecules, 24(18), 3306. https://doi.org/10.3390/molecules24183306







