Identification and Allelopathy of Green Garlic (Allium sativum L.) Volatiles on Scavenging of Cucumber (Cucumis sativus L.) Reactive Oxygen Species
Abstract
1. Introduction
2. Results
2.1. Identification of Volatile Allelochemicals in Green Garlic
2.2. Effect of Green Garlic Volatiles and DADS on Cucumber ROS and Antioxidant Activity
2.2.1. Effect of Green Garlic Volatiles and DADS on Cucumber ROS (O2•− and H2O2)
2.2.2. Effect of Green Garlic Volatiles and DADS on Cucumber Antioxidant Substances (MDA, GSH and ASA)
2.2.3. Effect of Green Garlic Volatiles and DADS on Cucumber Antioxidant Enzymes (SOD, CAT and POD)
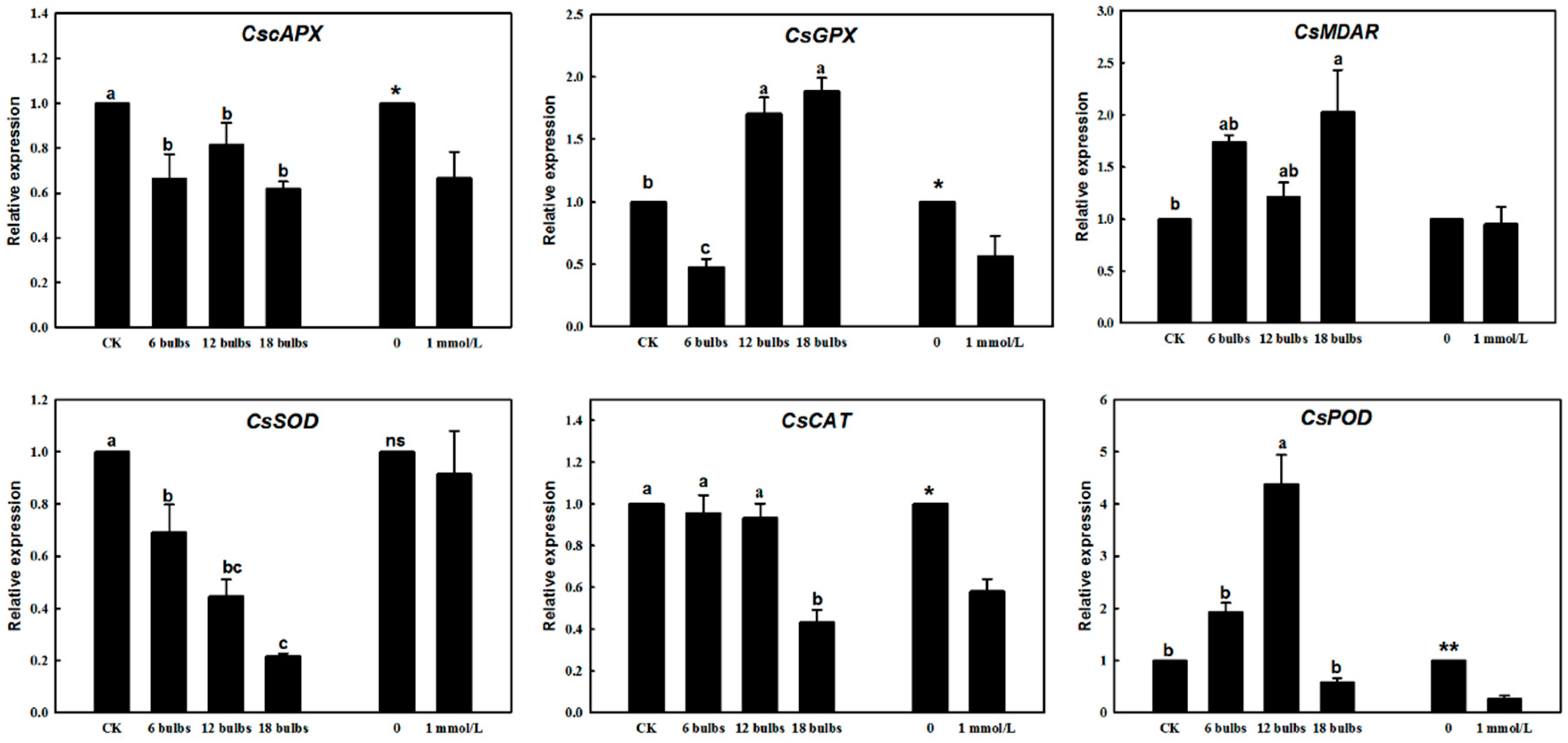
2.3. Gene Expression Changes in Response to Green Garlic Volatiles and DADS
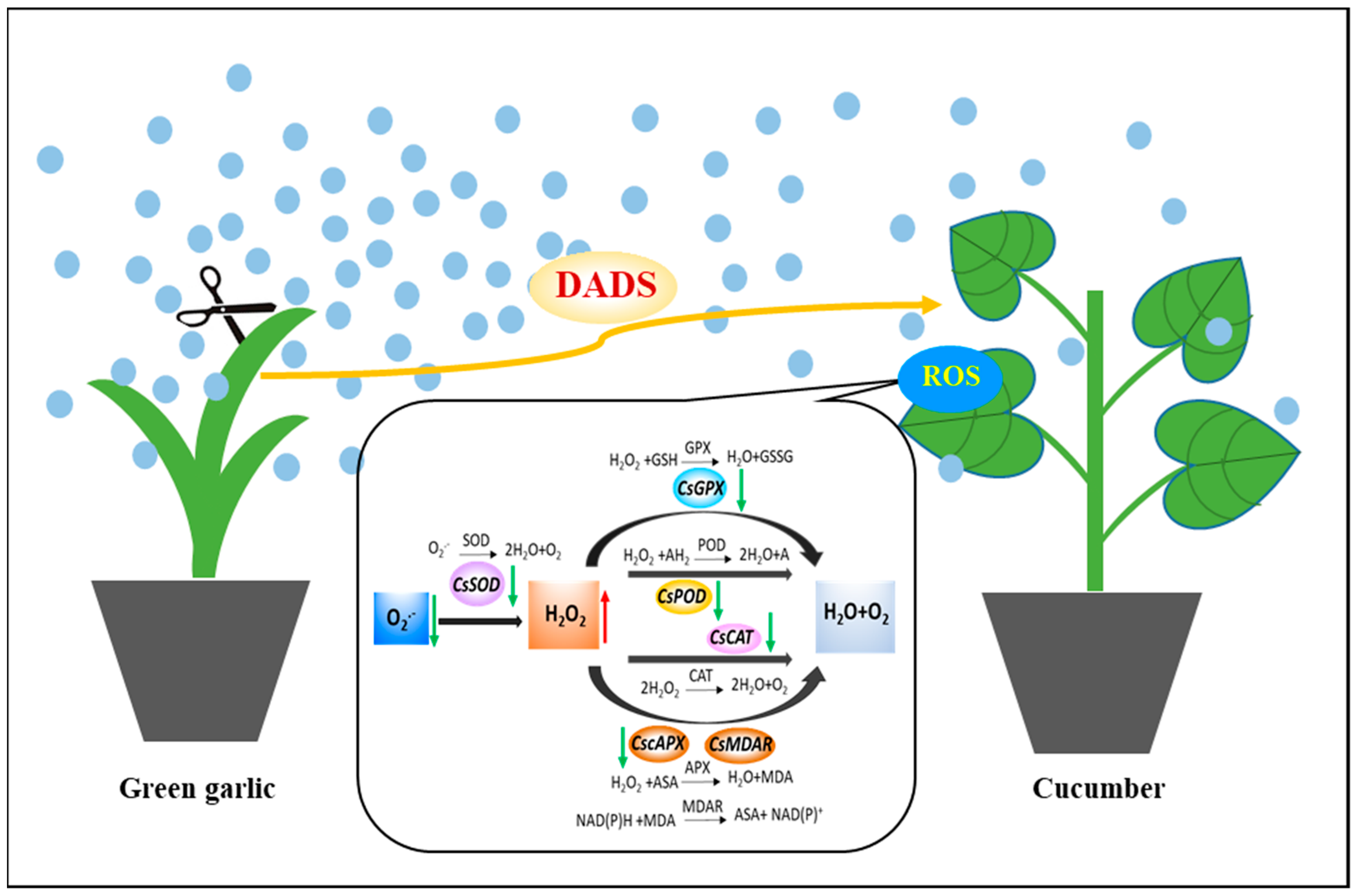
3. Discussion
4. Materials and Methods
4.1. Materials and Equipment
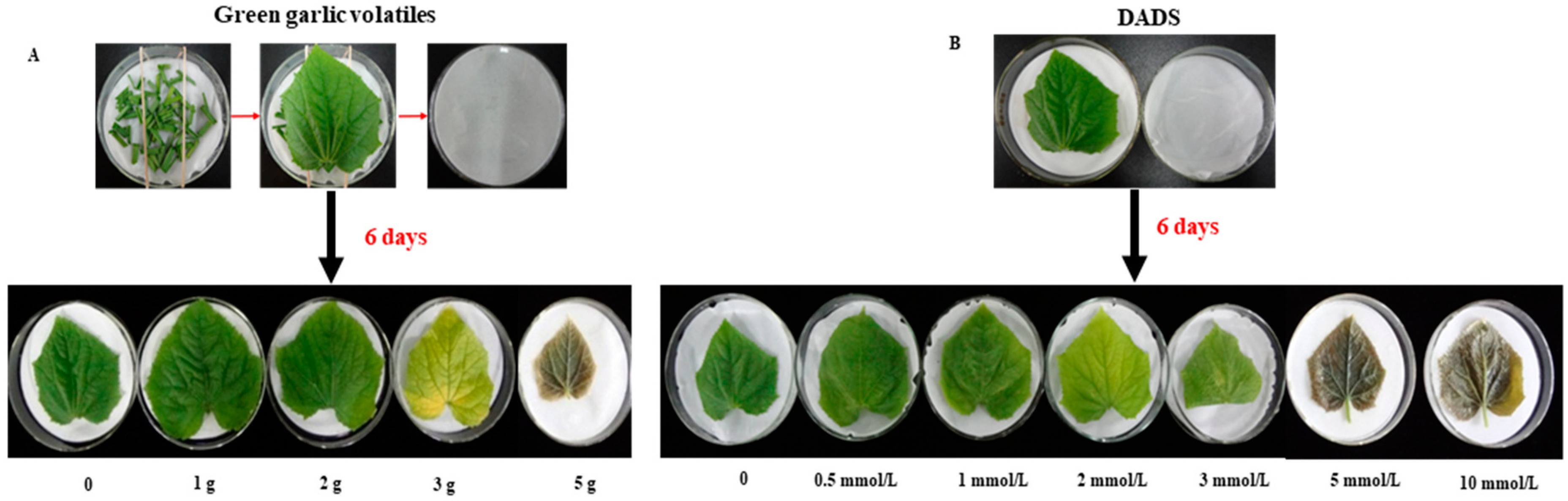
4.2. Plant Growth and Experimental Design
4.3. Identification of Green Garlic Allelochemicals by Fiber-HS-SPME-GC-MS
4.4. H2O2 Histological Analysis: 3,3-Diamino-Benzidine (DAB) Staining
4.5. ROS Assay
4.6. Antioxidative Enzyme and Antioxidant Substances Analysis
4.7. Total RNA Extraction and Antioxidant Enzyme Gene Expression Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefevre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant. Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jae, J.H.; Dong, S.J.; Mi, K.K.; Hoon, C.Y.; Ki Hyung, K.; Young Chul, P. Diallyl disulfide induces reversible G2/M phase arrest on a p53-independent mechanism in human colon cancer HCT-116 cells. Oncol. Rep. 2008, 19, 275–280. [Google Scholar]
- Xiao, X.M.; Cheng, Z.H.; Meng, H.W.; Khan, M.A.; Li, H.Z. Intercropping with garlic alleviated continuous cropping obstacle of cucumber in plastic tunnel. Acta. Agr. Scand. B-S P. 2012, 62, 696–705. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.H.; Meng, H.W.; Tang, X.W. The garlic allelochemical diallyl disulfide affects tomato root growth by influencing cell division, phytohormone balance and expansin gene expression. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Wu, C.N.; Cheng, Z.H.; Meng, H.W.; Zhang, M.R.; Zhang, H.J. Soil chemical property changes in eggplant/garlic relay intercropping systems under continuous cropping. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Ding, H.Y.; Cheng, Z.H.; Liu, M.L.; Hayat, S.; Feng, H. Garlic exerts allelopathic effects on pepper physiology in a hydroponic co-culture system. Biol. Open. 2016, 5, 631–637. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.H. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant. Sci. 2015, 6. [Google Scholar] [CrossRef]
- Xiao, X.M.; Cheng, Z.H.; Meng, H.W.; Liu, L.H.; Li, H.Z.; Dong, Y.X. Intercropping of green garlic (Allium sativum L.) induces nutrient concentration changes in the soil and plants in continuously cropped cucumber (Cucumis sativus L.) in a plastic tunnel. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Su, Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem. Toxicol. 2013, 57, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Yamane, Y.; Toriu, K.; Kawazu, K.; Higuchi, T.; Nishimura, S. Identification of active substances in garlic responsible for breaking bud dormancy in grapevines. J. Jpn. Soc. Hortic. Sci. 1999, 68, 1111–1117. [Google Scholar] [CrossRef][Green Version]
- Cheng, F.; Cheng, Z.H.; Meng, H.W. Transcriptomic insights into the allelopathic effects of the garlic allelochemical diallyl disulfide on tomato roots. Sci Rep.-Uk. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.L.; Hayat, S.; Qi, X.F.; Liu, T.; Cheng, Z.H. The garlic allelochemical DADS influences cucumber root growth involved in regulating hormone levels and modulating cell cycling. J. Plant. Physiol. 2018, 230, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N.; Kim, N.S.; Lee, D.S. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal Chem. 2003, 377, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A. Pappas CS, Polissiou MG: Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum L.). Ultrason. Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.M.; Parkinson, D.R.; Pawliszyn, J. Assessment of thiol compounds from garlic by automated headspace derivatized in-needle-NTD-GC-MS and derivatized in-fiber-SPME-GC-MS. J. Agr. Food Chem. 2013, 61, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Godinez-Vidal, D.; Rocha-Sosa, M.; Sepulveda-Garcia, E.B.; Lara-Reyna, J.; Rojas-Martinez, R.; Zavaleta-Mejia, E. Phenylalanine ammonia lyase activity in chilli CM-334 infected by Phytophthora capsici and Nacobbus aberrans. Eur. J. Plant. Pathol. 2008, 120, 299–303. [Google Scholar] [CrossRef]
- Rio D, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D. Allelopathic effects of Lantana camara L. on Lathyrus sativus L.: Oxidative imbalance and cytogenetic consequences. Allelopath. J. 2013, 31, 71–90. [Google Scholar]
- Zhao, L.J.; Hu, Q.R.; Huang, Y.X.; Fulton, A.N.; Hannah-Bick, C.; Adeleye, A.S.; Keller, A.A. Activation of antioxidant and detoxification gene expression in cucumber plants exposed to a Cu(OH)2 nanopesticide. Env. Sci-Nano. 2017, 4, 1750–1760. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.C.; Mau, J.L. Release of volatile compounds from microwave heating of garlic juice with 2,4-decadienals. Food Chem. 1999, 64, 531–535. [Google Scholar] [CrossRef]
- Kim, N.Y.; Park, M.H.; Jang, E.Y.; Lee, J. Volatile distribution in garlic (Allium sativum L.) by solid phase microextraction (SPME) with different processing conditions. Food Sci. Biotechnol. 2011, 20, 775–782. [Google Scholar] [CrossRef]
- Biagini, D.; Lomonaco, T.; Ghimenti, S.; Bellagambi, F.G.; Onor, M.; Scali, M.C.; Barletta, V.; Marzilli, M.; Salvo, P.; Trivella, M.G. Determination of volatile organic compounds in exhaled breath of heart failure patients by needle trap micro-extraction coupled with gas chromatography-tandem mass spectrometry. J. Breath Res. 2017, 11. [Google Scholar] [CrossRef]
- Ghimenti, S.; Lomonaco, T.; Bellagambi, F.G.; Tabucchi, S.; Onor, M.; Trivella, M.G.; Ceccarini, A.; Fuoco, R.; Francesco, F.D. Comparison of sampling bags for the analysis of volatile organic compounds in breath. J. Breath Res. 2015, 9. [Google Scholar] [CrossRef]
- Roxas, V.P.; Lodhi, S.A.; Garrett, D.K.; Mahan, J.R.; Allen, R.D. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant. Cell Physiol. 2000, 41, 1229–1234. [Google Scholar] [CrossRef]
- Liu, J.G.; Zhang, X.L.; Sun, Y.H.; Lin, W. Antioxidative capacity and enzyme activity in Haematococcus pluvialis cells exposed to superoxide free radicals. Chin. J. Oceanol. Limn. 2010, 28, 1–9. [Google Scholar] [CrossRef]
- Tanaka, T.; Izawa, S.; Inoue, Y. GPX2, encoding a phospholipid hydroperoxide glutathione peroxidase homologue, codes for an atypical 2-Cys peroxiredoxin in Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 42078–42087. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant. Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Komatsu, S. Contribution of proteomic studies towards understanding plant heavy metal stress response. Front. Plant. Sci. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zho, Y.J.; Su, J.; Shi, L.; Liao, Q.J.; Su, Q. DADS downregulates the Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling pathway, inhibiting cell migration and invasion. Oncol. Rep. 2013, 29, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Wang, C.F.; Cheng, Y.L.; Chen, X.M.; Han, Q.M.; Huang, L.L.; Wei, G.R.; Kang, Z.S. Histological and cytological characterization of adult plant resistance to wheat stripe rust. Plant. Cell Rep. 2012, 31, 2121–2137. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.J.; Randall, D.P.; Flowers, T.J. Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant. Cell Env. 2006, 29, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, S.J. Pre-storage cold acclimation maintained quality of cold-stored cucumber through differentially and orderly activating ROS scavengers. Postharvest Biol. Tec. 2017, 129, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Lai, F.; Wu, H. Germinating peanut (Arachis hypogaea L.) seedlings attenuated selenite-induced toxicity by activating the antioxidant enzymes and mediating the ascorbate-glutathione cycle. J. Agric. Food Chem. 2016, 64, 1298–1308. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
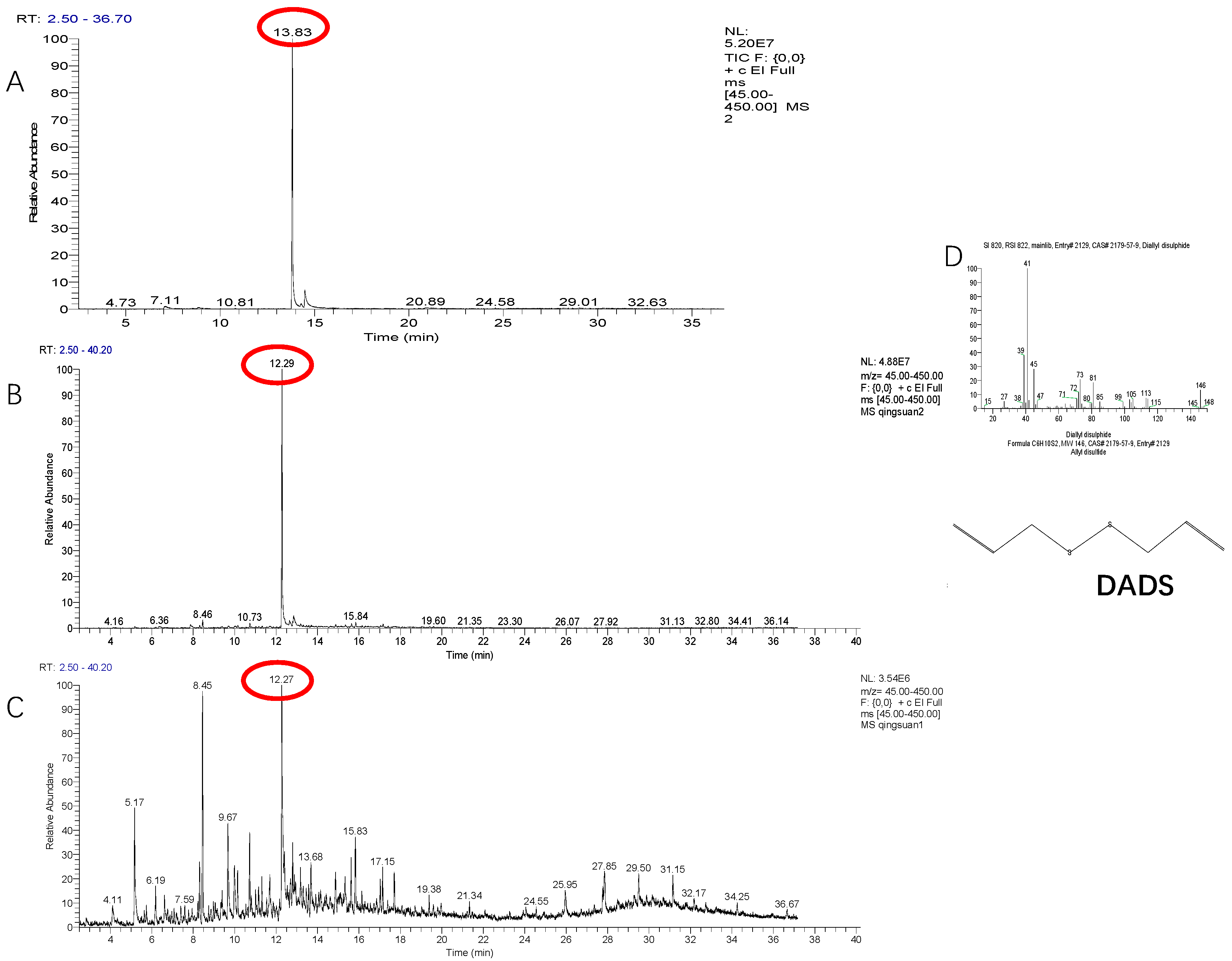

| Treatment | Total Sulfur Compounds | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | Area% | RT | Compound Name | Area% | RT | Compound Name | Area% | RT | ||
| Cut (SPME vial) |  2 2 | Diallyl disulphide | 99.52 | 13.83 | Methyl propenyl disulfide | 0 | – | Diallyl sulfide | 0.48 | 7.04 |
| Cut (sealed desiccator) |  3 3 | Diallyl disulphide | 86.33 | 12.29 | Methyl propenyl disulfide | 1.53 | 7.87 | Diallyl sulfide | 0.35 | 6.36 |
| Whole (sealed desiccator) |  1 1 | Diallyl disulphide | 15.30 | 12.27 | Methyl propenyl disulfide | 0 | – | Diallyl sulfide | 0 | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Liu, X.; Wang, H.; Deng, R.; Yu, H.; Cheng, Z. Identification and Allelopathy of Green Garlic (Allium sativum L.) Volatiles on Scavenging of Cucumber (Cucumis sativus L.) Reactive Oxygen Species. Molecules 2019, 24, 3263. https://doi.org/10.3390/molecules24183263
Yang F, Liu X, Wang H, Deng R, Yu H, Cheng Z. Identification and Allelopathy of Green Garlic (Allium sativum L.) Volatiles on Scavenging of Cucumber (Cucumis sativus L.) Reactive Oxygen Species. Molecules. 2019; 24(18):3263. https://doi.org/10.3390/molecules24183263
Chicago/Turabian StyleYang, Fan, Xiaoxue Liu, Hui Wang, Rui Deng, Hanhan Yu, and Zhihui Cheng. 2019. "Identification and Allelopathy of Green Garlic (Allium sativum L.) Volatiles on Scavenging of Cucumber (Cucumis sativus L.) Reactive Oxygen Species" Molecules 24, no. 18: 3263. https://doi.org/10.3390/molecules24183263
APA StyleYang, F., Liu, X., Wang, H., Deng, R., Yu, H., & Cheng, Z. (2019). Identification and Allelopathy of Green Garlic (Allium sativum L.) Volatiles on Scavenging of Cucumber (Cucumis sativus L.) Reactive Oxygen Species. Molecules, 24(18), 3263. https://doi.org/10.3390/molecules24183263






