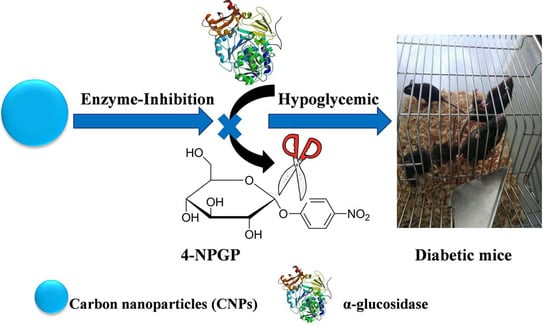
Carbon Nanoparticles Inhibit Α-Glucosidase Activity and Induce a Hypoglycemic Effect in Diabetic Mice
Abstract
1. Introduction
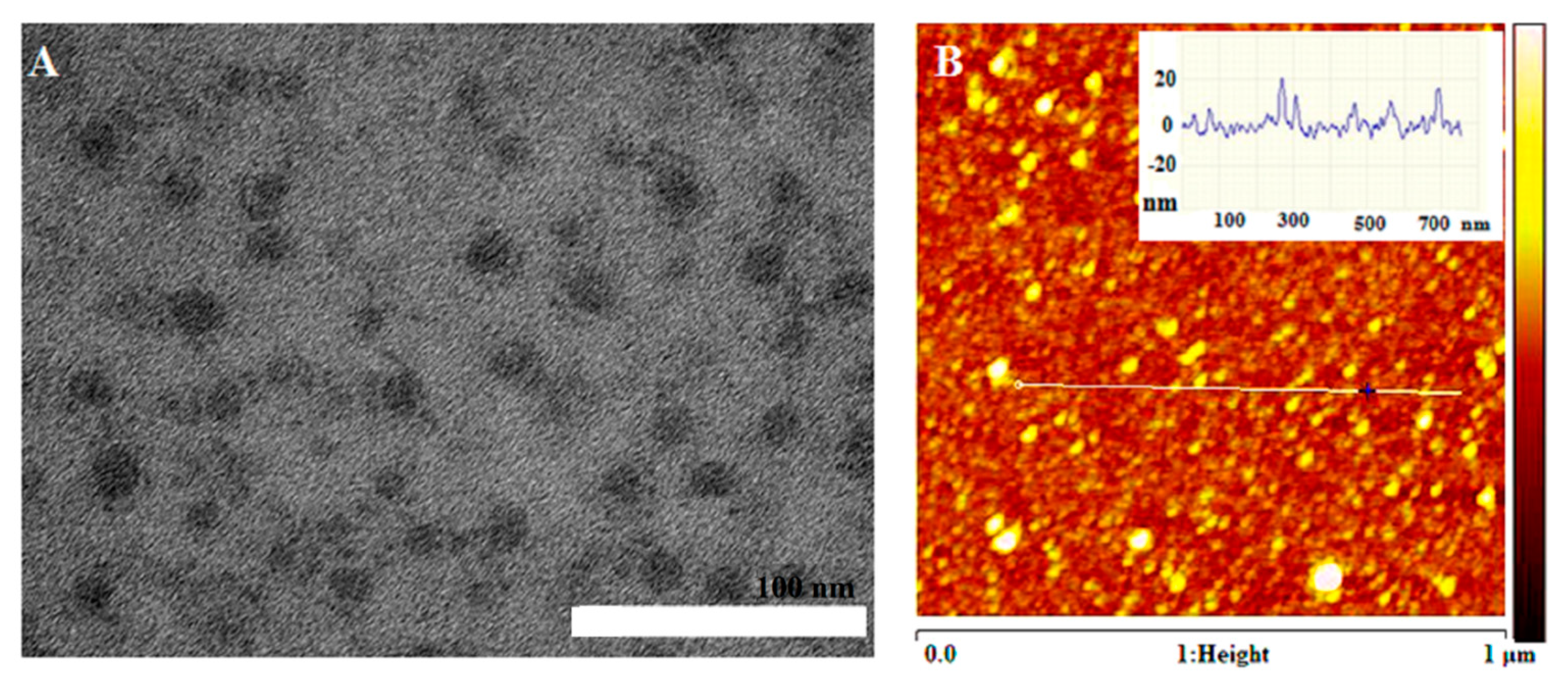
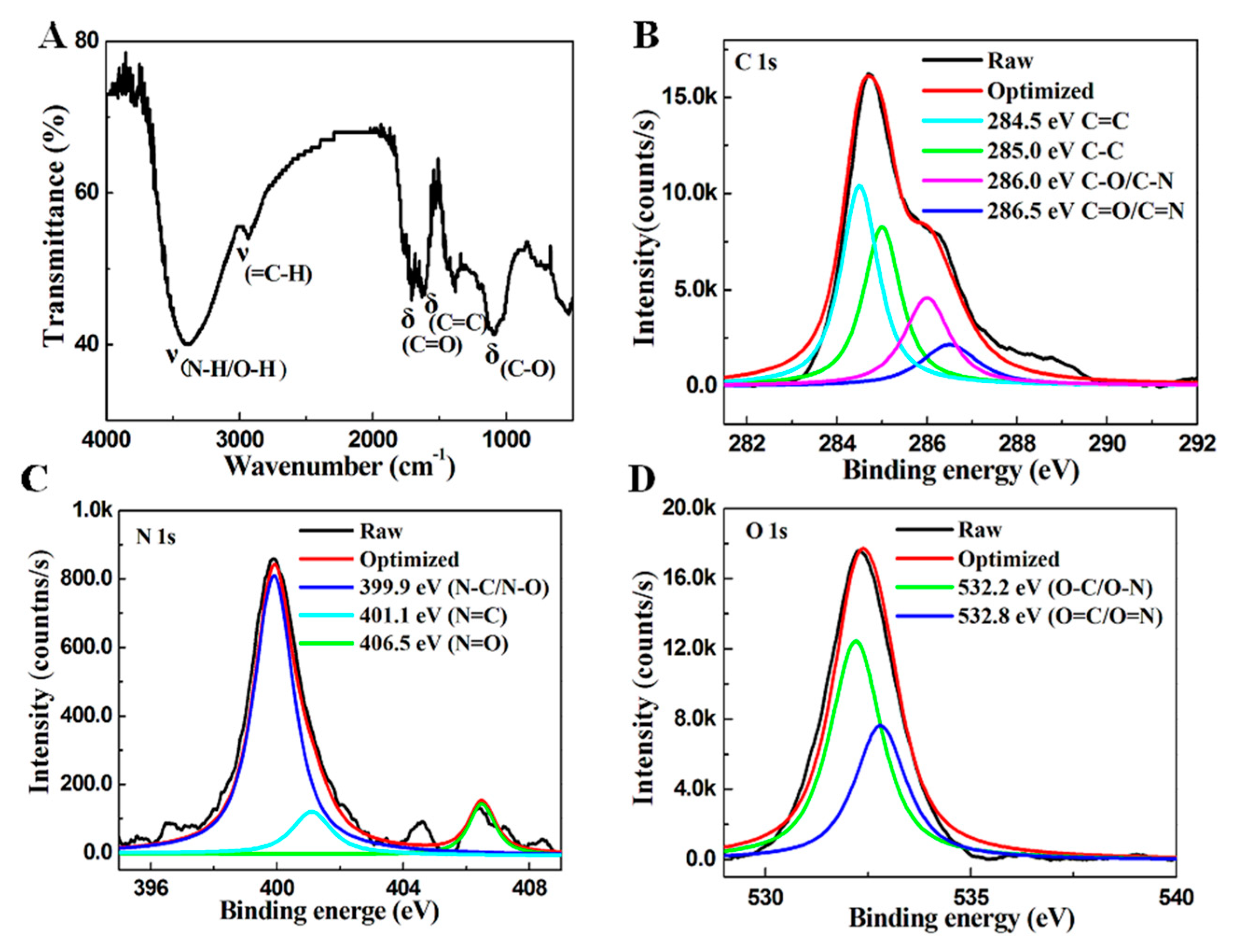
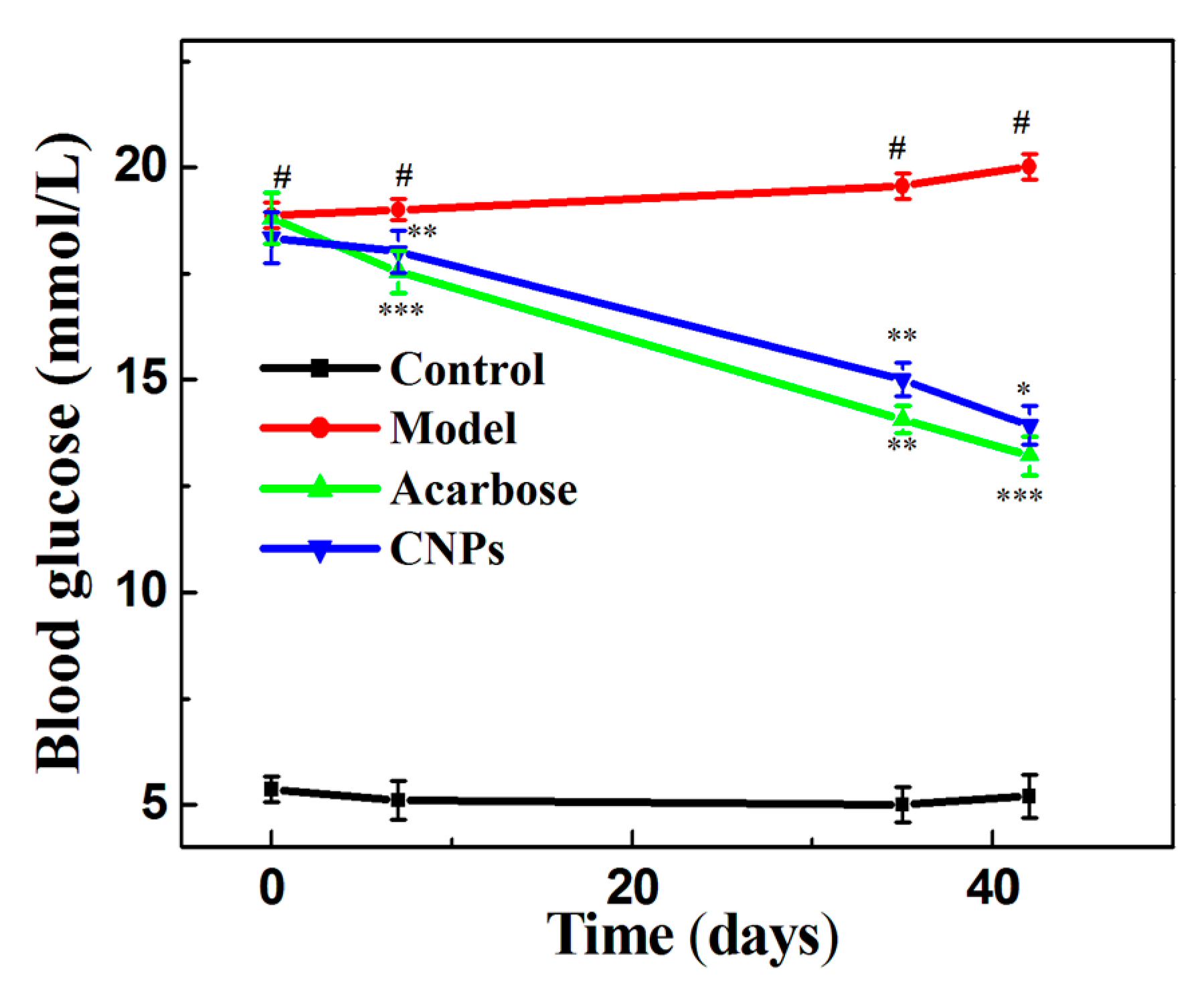
2. Results and Discussion
3. Materials and Methods
- Normal control group: Healthy mice that received a normal diet throughout the assay.
- Model group: Diabetic mice that were not treated. They received distilled water throughout the assay.
- CNPs group: Diabetic mice that received 200 mg/kg of the CNPs daily during the assay.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Da, R.F.J.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Hou, Q.; Li, Y.; Li, L.; Cheng, G.; Sun, X.; Li, S.; Tian, H. The Metabolic Effects of Oats Intake in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 10369–10387. [Google Scholar] [CrossRef] [PubMed]
- Turkan, F.; Taslimi, P.; Saltan, F.Z. Tannic acid as a natural antioxidant compound: Discovery of a potent metabolic enzyme inhibitor for a new therapeutic approach in diabetes and Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2019, 23, e22340. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Gulçin, İ. Antidiabetic potential: In vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylaseenzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Aslan, H.E.; Demir, Y.; Oztaskin, N.; Maras, A.; Gulcin, İ.; Beydemir, S.; Goksu, S. Diarylmethanon, bromophenol and diarylmethane compounds: Discovery of potent aldose reductase, alpha-amylase and alpha-glycosidase inhibitors as new therapeutic approach in diabetes and functional hyperglycemia. Int. J. Biol. Macromol. 2018, 119, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Prada, A.L.; Amado, J.R.R.; Keita, H.; Zapata, E.P.; Carvalho, H.; Lima, E.S.; de Sousa, T.P.; Carvalho, J.C.T. Cassia grandis fruit extract reduces the blood glucose level in alloxan-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gulçin, İ.; Taslimi, P.; Aygün, A.; Sadeghian, N.; Bastem, E.; Kufrevioglu, O.I.; Turkan, F.; Şen, F. Antidiabetic and antiparasitic potentials: Inhibition effects of some natural antioxidant compounds on alpha-glycosidase, alpha-amylase and human glutathione S-transferase enzymes. Int. J. Biol. Macromol. 2018, 119, 741–746. [Google Scholar] [CrossRef]
- Burmaoglu, S.; Yilmaz, A.O.; Polat, M.F.; Kaya, R.; Gulcin, İ.; Algul, O. Synthesis and biological evaluation of novel tris-chalcones as potent carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibitors. Bioorg. Chem. 2019, 85, 191–197. [Google Scholar] [CrossRef]
- Kameda, Y.; Kawashima, K.; Takeuchi, M.; Ikeda, K.; Asano, N.; Matsui, K. Preparation and biological activity of manno- and galacto-validamines, new 5a-carba-glycosylamines as alpha-glycosidase inhibitors. Carbohydr. Res. 1997, 300, 259–264. [Google Scholar] [CrossRef]
- Dhameja, M.; Gupta, P. Synthetic heterocyclic candidates as promising α-glucosidase inhibitors: An overview. Eur. J. Med. Chem. 2019, 176, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Shahshahanipour, M.; Rezaei, B.; Ensafi, A.A.; Etemadifar, Z. An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug. Mater. Sci. Eng. C 2019, 98, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, J.H.; Li, R.S.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal. Chim. Acta 2019, 1063, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kong, W.; Zhao, M.; Lu, S.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. A fluorescence resonance energy transfer (FRET) based “Turn-On” nanofluorescence sensor using a nitrogen-doped carbon dot-hexagonal cobalt oxyhydroxide nanosheet architecture and application to alpha-glucosidase inhibitor screening. Biosens. Bioelectron. 2016, 79, 728–735. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Liu, Y.; Xiao, Q. Carbon dots doped with nitrogen and boron as ultrasensitive fluorescent probes for determination of alpha-glucosidase activity and its inhibitors in water samples and living cells. Microchim. Acta 2018, 185, 394–402. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, F.; Cheng, J.; Zhang, M.; Zhu, Y.; Zhang, Y.; Kong, H.; Qu, H.; Zhao, Y. Hypoglycemic Bioactivity of Novel Eco-Friendly Carbon Dots Derived from Traditional Chinese Medicine. J. Biomed. Nanotechnol. 2018, 14, 2146–2155. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Zhang, Z.; Xu, J.; Xie, Z.; Slavin, M.; Gao, X. In vitro and in vivo antioxidant activity of a fructan from the roots of Arctium lappa L. Int. J. Biol. Macromol. 2014, 446–453. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, P.; Guo, M.; Yu, W.; Chen, K. Burdock fructooligosaccharide induces fungal resistance in postharvest; Kyoho grapes by activating the salicylic acid-dependent pathway and; inhibiting browning. Food Chem. 2013, 138, 539–546. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Kuang, P.; Shi, X.; Wang, Z.; Guo, L. Regulation of lipid metabolism in diabetic rats by Arctium lappa L. polysaccharide through the PKC/NF-κB pathway. Int. J. Biol. Macromol. 2019, 136, 115–122. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Kan, J.; Wu, X.; Zhang, X.; Tang, S.; Sun, R.; Liu, J.; Qian, C.; Jin, C. In vivo and in vitro anti-inflammatory effects of water-soluble polysaccharide from Arctium lappa. Int. J. Biol. Macromol. 2019, 135, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.; Atchudan, R.; Shim, J.J.; Kalimuthu, S.; Ahn, B.C.; Lee, Y.R. Turn-off fluorescence sensor for the detection of ferric ion in water using green synthesized N-doped carbon dots and its bio-imaging. J. Photochem. Photobiol. B 2016, 158, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ning, G.; He, X.; Ma, X.; Yang, F.; Xu, Z.; Zhao, S.; Xu, C.; Li, Y. Carbon quantum dots derived by direct carbonization of carbonaceous microcrystals in mesophase pitch. Nanoscale 2018, 10, 21492–21498. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; He, H.; Fan, H.; Kang, X.; Song, X. A hydrothermal-carbonization process for simultaneously production of sugars, graphene quantum dots, and porous carbon from sugarcane bagasse. Bioresour. Technol. 2019, 282, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Song, Y.; Kwon, J.E.; Lee, S.H.; Park, K.S.; Kim, S.; Woo, J.; Kim, H. Food waste-driven N-doped carbon dots: Applications for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C 2019, 102, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, C.; Chen, Z. Exopolysaccharide-Derived Carbon Dots for Microbial Viability Assessment. Front. Microbiol. 2018, 9, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Zhang, P.; Tang, L.; Zhuo, S.; Zhu, C. Highly sensitive enzymatic determination of urea based on the pH-dependence of the fluorescence of grapheme quantum dots. Microchim. Acta 2015, 182, 1431–1437. [Google Scholar] [CrossRef]
- Shao, T.; Wang, G.; An, X.; Zhuo, S.; Xia, Y.; Zhu, C. A reformative oxidation strategy using high concentration nitric acid for enhancing the emission performance of graphene quantum dots. RSC Adv. 2014, 4, 47977–47981. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Wang, B.; Ma, Y.; Huang, H.; Liu, Y.; Shao, M.; Yao, B.; Kang, Z. Maltase Decorated by Chiral Carbon Dots with Inhibited Enzyme Activity for Glucose Level Control. Small 2019, 1901512–1901518. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, Characterization and Biological activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Fang, F.; Shao, T.; Li, P.; Hu, W.; Zhou, Y.; Wang, G.; Han, J.; Chen, K. Structure and Anti-Tumor Activities of Exopolysaccharides from Alternaria mali Roberts. Molecules 2019, 24, 1345. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Becerra-Jiménez, J.; Cárdenas-Vázquez, R. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J. Ethnopharmacol. 2008, 116, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Hamza, A.A. Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 2015, 141, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Fatani, A.J.; Alrejaie, S.S.; Abuohashish, H.M.; Alassaf, A.; Parmar, M.Y.; Ahmed, M.M. Lutein Dietary Supplementation Attenuates Streptozotocin-induced testicular damage and oxidative stress in diabetic rats. BMC Complement. Altern. Med. 2015, 15, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Dixit, Y.; Kar, A. Protective role of three vegetable peels in alloxan induced diabetes mellitus in male mice. Plant Foods Hum. Nutr. 2010, 65, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, S.; Prasad, K.B.; Peng, G.; Qian, L.G.; Yamahara, J.; Roufogalis, B.D. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J. Ethnopharmacol. 2005, 99, 239–244. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, T.; Yuan, P.; Zhu, L.; Xu, H.; Li, X.; He, S.; Li, P.; Wang, G.; Chen, K. Carbon Nanoparticles Inhibit Α-Glucosidase Activity and Induce a Hypoglycemic Effect in Diabetic Mice. Molecules 2019, 24, 3257. https://doi.org/10.3390/molecules24183257
Shao T, Yuan P, Zhu L, Xu H, Li X, He S, Li P, Wang G, Chen K. Carbon Nanoparticles Inhibit Α-Glucosidase Activity and Induce a Hypoglycemic Effect in Diabetic Mice. Molecules. 2019; 24(18):3257. https://doi.org/10.3390/molecules24183257
Chicago/Turabian StyleShao, Taili, Pingchuan Yuan, Lei Zhu, Honggang Xu, Xichen Li, Shuguang He, Ping Li, Guodong Wang, and Kaoshan Chen. 2019. "Carbon Nanoparticles Inhibit Α-Glucosidase Activity and Induce a Hypoglycemic Effect in Diabetic Mice" Molecules 24, no. 18: 3257. https://doi.org/10.3390/molecules24183257
APA StyleShao, T., Yuan, P., Zhu, L., Xu, H., Li, X., He, S., Li, P., Wang, G., & Chen, K. (2019). Carbon Nanoparticles Inhibit Α-Glucosidase Activity and Induce a Hypoglycemic Effect in Diabetic Mice. Molecules, 24(18), 3257. https://doi.org/10.3390/molecules24183257