The Chemistry behind Chocolate Production
Abstract
1. Introduction
- (1)
- The first phase is dominated by anaerobic yeasts and lasts for 24–36 h. In this phase, low content of oxygen and low pH (<4) are present.
- (2)
- The second phase is dominated by lactic acid bacteria. They are present from the beginning but become active between 48–96 h.
- (3)
- Third phase is dominated by acetic acid bacteria when aeration increases. During this last phase, an exothermic reaction (conversion of alcohol into acetic acid) occurs, responsible for a temperature rise (50 °C or higher).
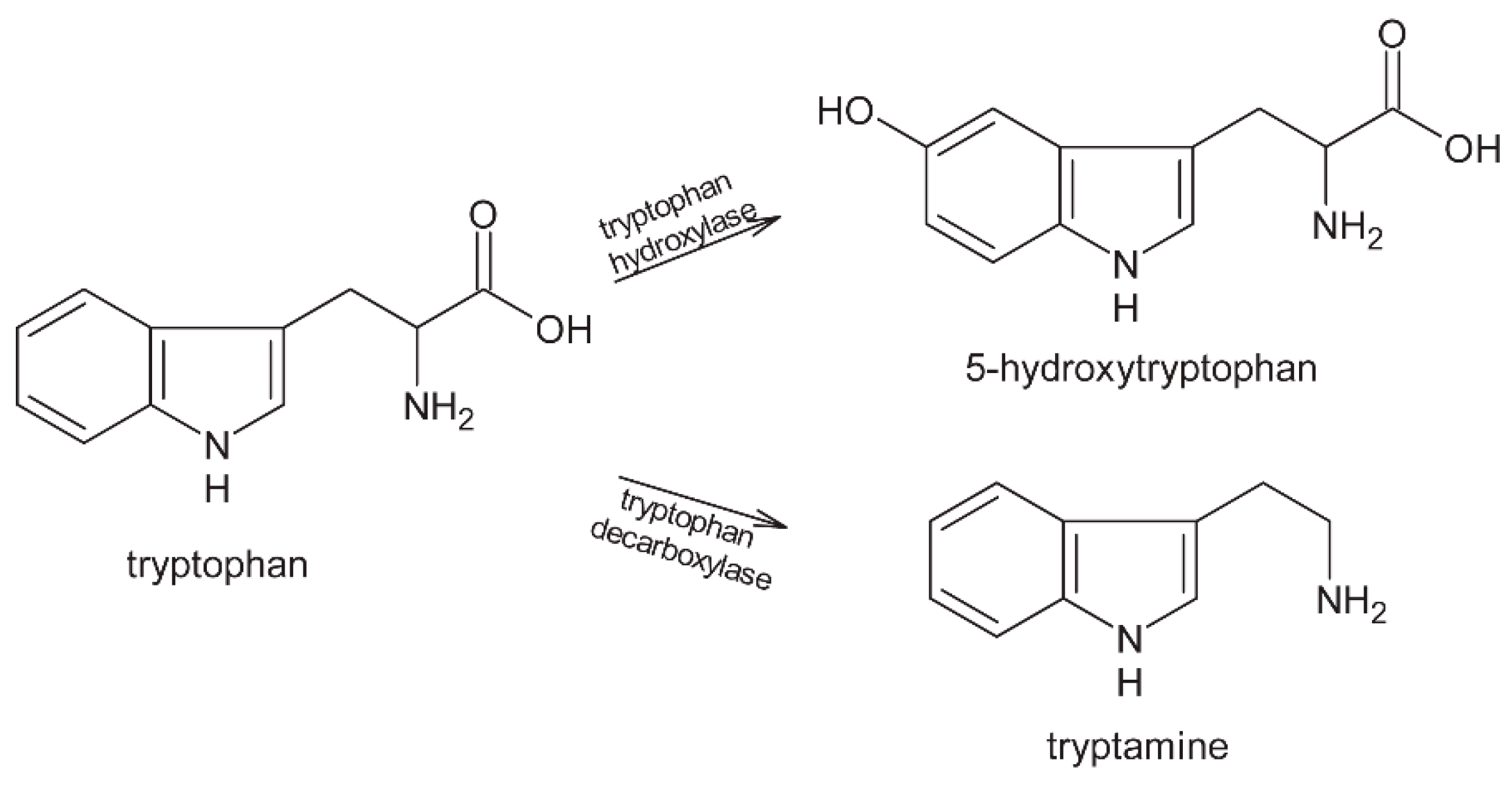
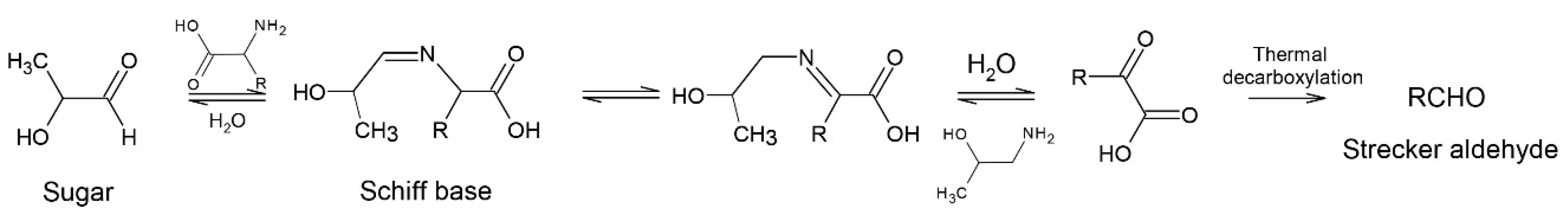
2. Proteins
2.1. Fermentation
2.2. Roasting
2.3. Other Processes
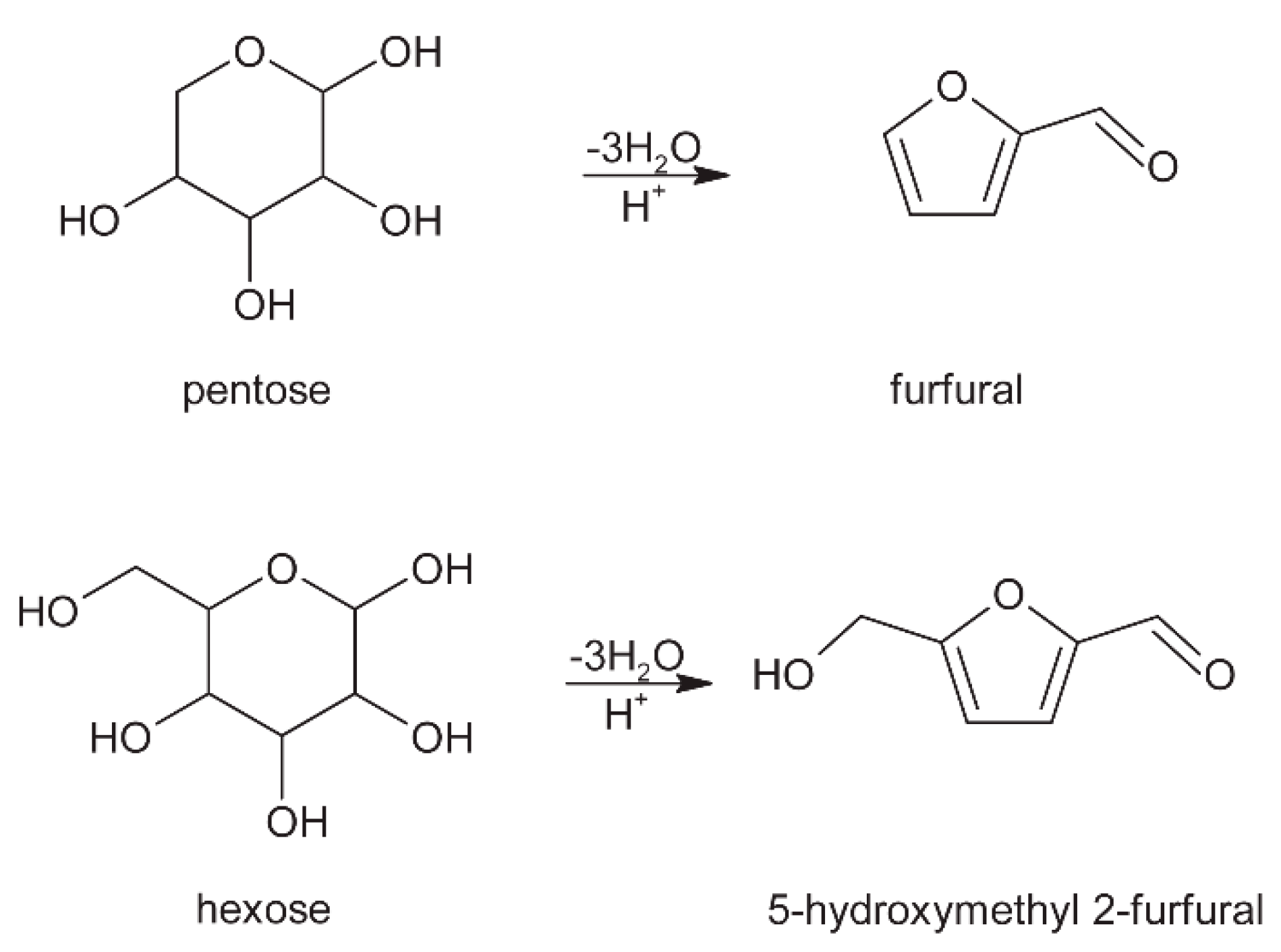
3. Carbohydrates
3.1. Fermentation
3.2. Drying
3.3. Roasting
4. Lipids
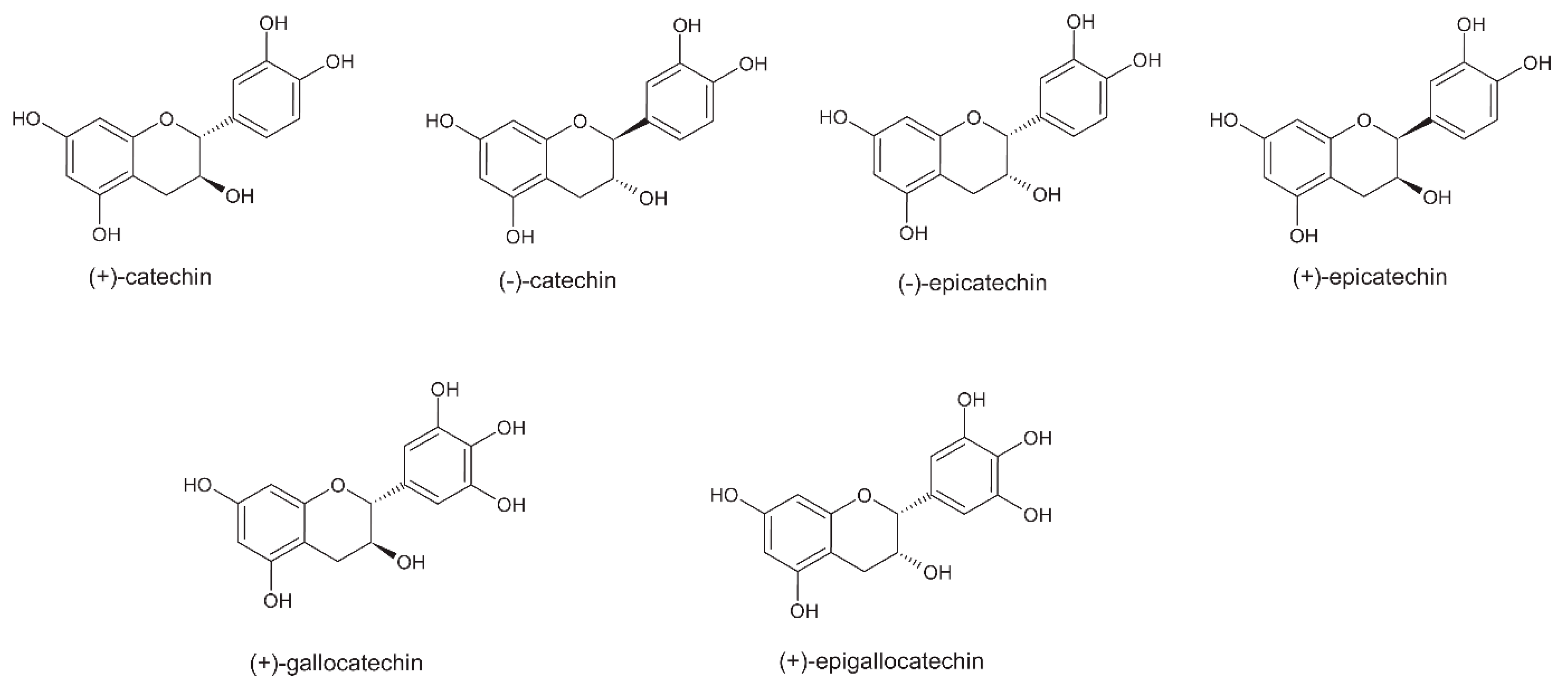
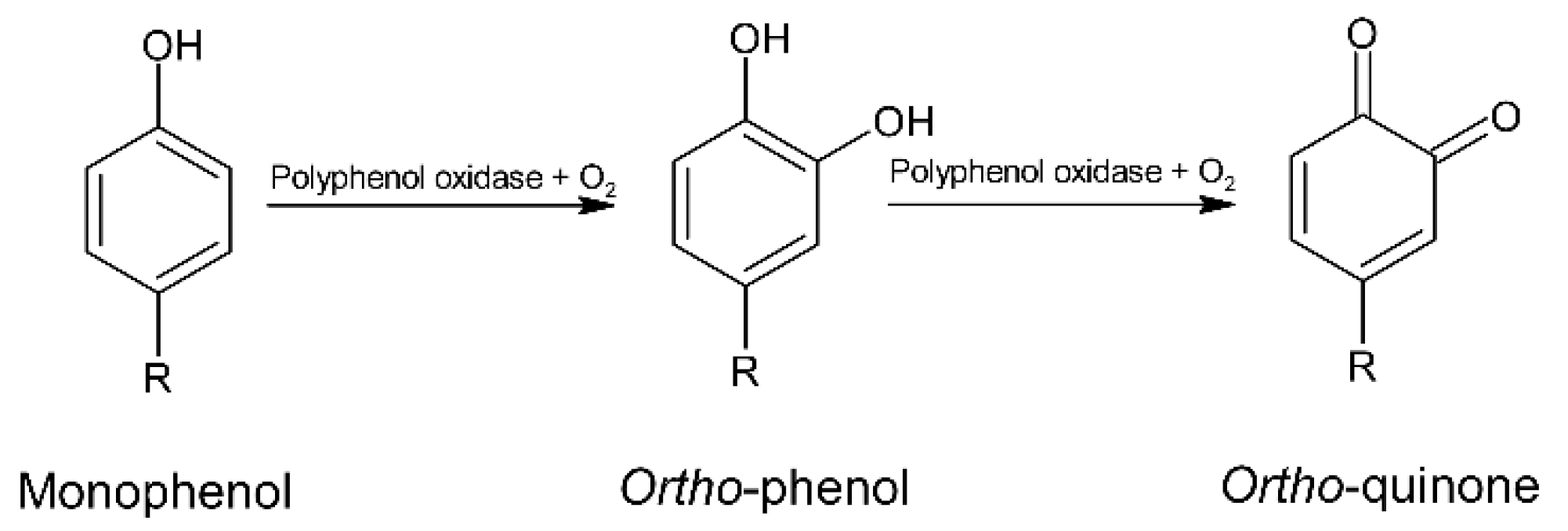
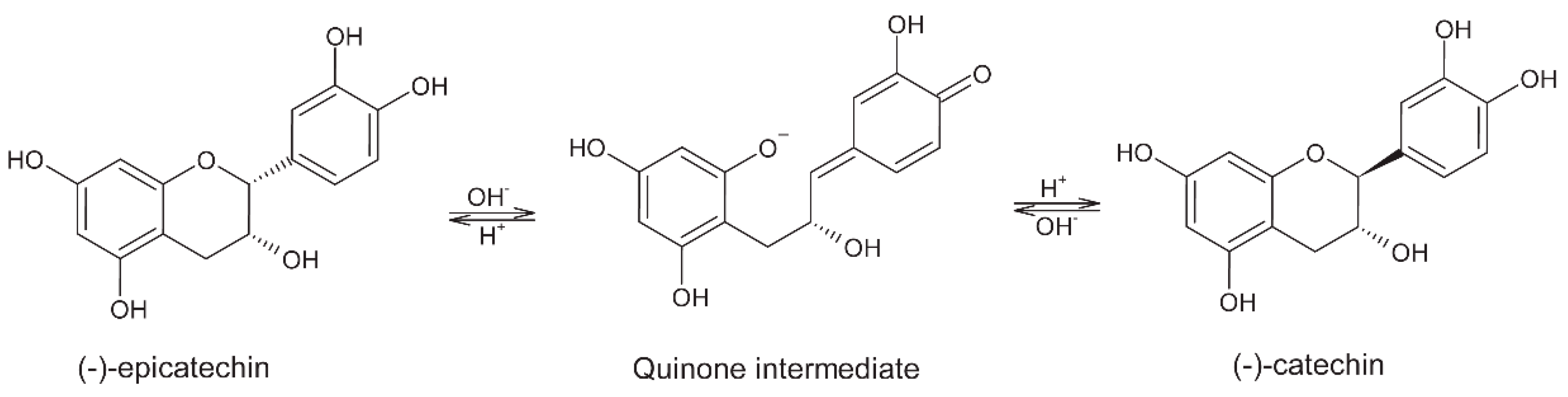
5. Polyphenols
5.1. Fermentation
5.2. Drying
5.3. Roasting
5.4. Conching
6. Other Components
6.1. Methylxanthines
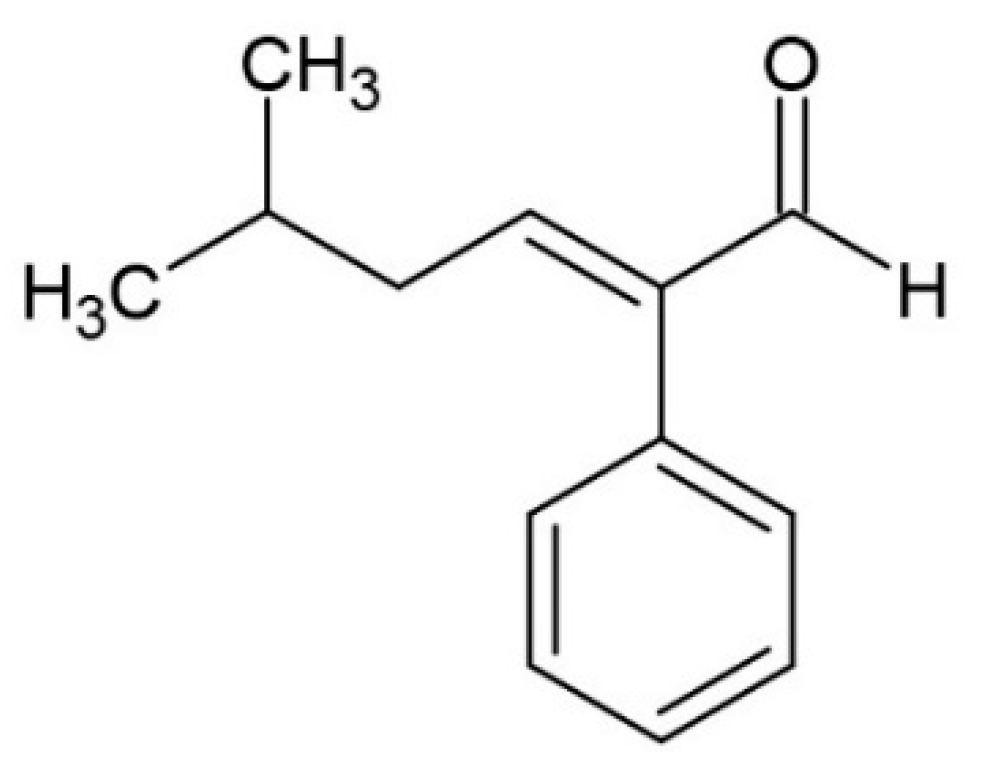
6.2. Aldehydes
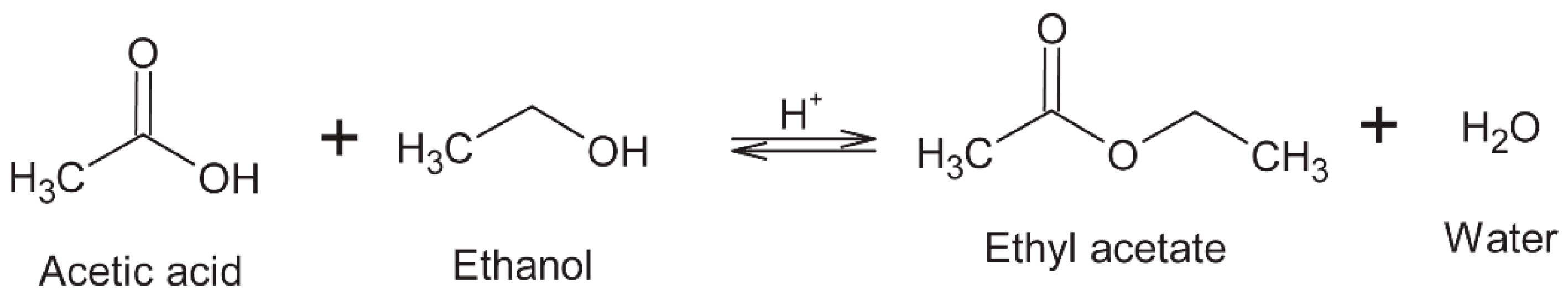
6.3. Esters
6.4. Ketones
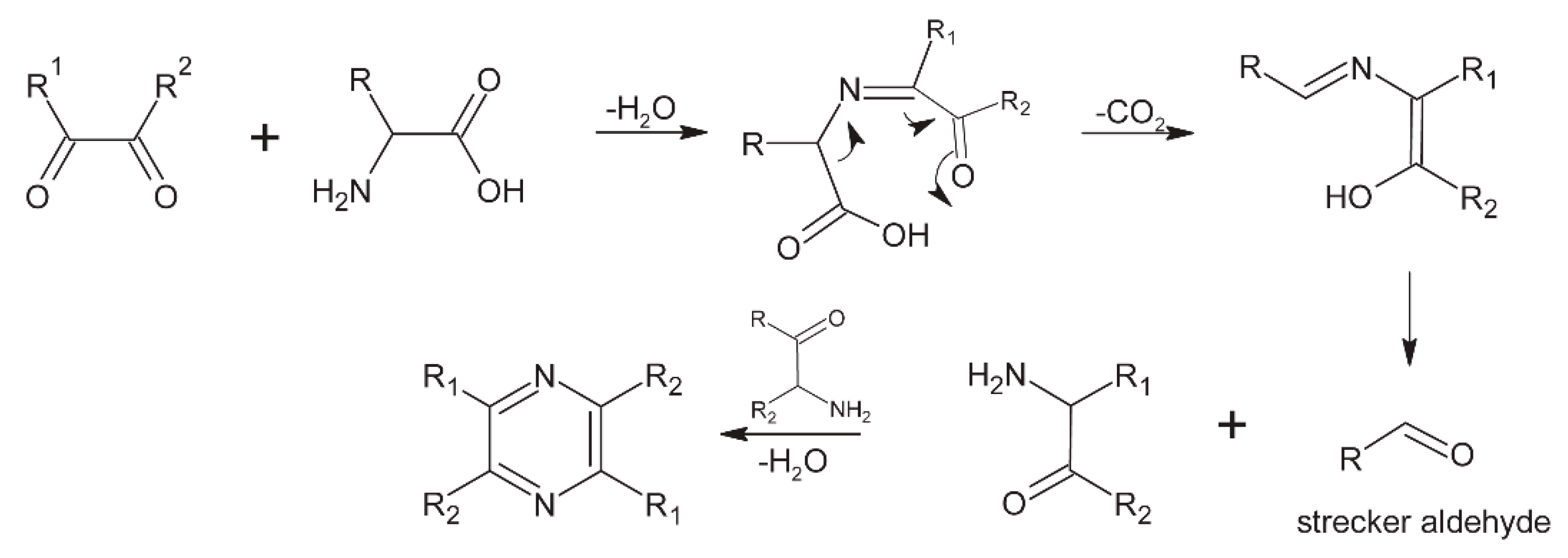
6.5. Pyrazines
6.6. Acids
6.7. Alcohols
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arunkumar, K.; Jegadeeswari, V. Evaluating the processed beans of different cocoa (Theobroma cacao L.) accessions for quality parameters. J. Phytol. 2019, 11, 1–4. [Google Scholar]
- Gutiérrez, T.J. State-of-the-Art Chocolate Manufacture: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1313–1344. [Google Scholar] [CrossRef]
- Beckett, S.T.; Fowler, M.S.; Ziegler, G.R. Beckett’s Industrial Chocolate Manufacture and Use, 5th ed.; Wiley Blackwell: West Sussex, UK, 2017. [Google Scholar]
- Adeyeye, E.I.; Akinyeye, R.O.; Ogunlade, I.; Olaofe, O.; Boluwade, J.O. Effect of farm and industrial processing on the amino acid profile of cocoa beans. Food Chem. 2010, 118, 357–363. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Beckett, S.T. The Science of Chocolate, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2015, 15, 73–91. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Palla, G. Cocoa: Production, Chemistry, and Use. Encycl. Food Health 2016, 185–190. [Google Scholar] [CrossRef]
- Bertazzo, A.; Comai, S.; Brunato, I.; Zancato, M.; Costa, C.V.L. The content of protein and non-protein (free and protein-bound) tryptophan in Theobroma cacao beans. Food Chem. 2011, 124, 93–96. [Google Scholar] [CrossRef]
- Granvogl, M.; Bugan, S.; Schieberle, P. Formation of Amines and Aldehydes from Parent Amino Acids during Thermal Processing of Cocoa and Model Systems: New Insights into Pathways of the Strecker Reaction. J. Agric. Food Chem. 2006, 54, 1730–1739. [Google Scholar] [CrossRef]
- Bytof, G.; Biehl, B.; Heinrichs, H.; Voigt, J. Specificity and stability of the carboxypeptidase activity in ripe, ungerminated seeds of Theobroma cacao L. Food Chem. 1995, 54, 15–21. [Google Scholar] [CrossRef]
- Caparosa, M.H.; Hartel, R.W. Structure and Properties of Chocolate. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2018. [Google Scholar]
- Rottiers, H.; Tzompa Sosa, D.A.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of volatile compounds and flavor precursors during spontaneous fermentation of fine flavor Trinitario cocoa beans. Eur. Food Res. Technol. 2019. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 50, 1–10. [Google Scholar] [CrossRef]
- Do Carmo Brito, B.N.; Campos Chisté, R.; da Silva Pena, R.; Abreu Gloria, M.B.; Santos Lopes, A. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer Verlag: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Oracz, J.; Nebesny, E. Effect of roasting parameters on physicochemical characteristics of high-molecular-weight Maillard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food Res. Technol. 2018, 245, 111. [Google Scholar] [CrossRef]
- Pontillon, J. La fabrication du chocolat. In Pour la Science; Editions Belin: Paris, France, 1995; pp. 118–126. [Google Scholar]
- Counet, C.; Callemien, D.; Ouwerx, C.; Collin, S. Use of Gas Chromatography−Olfactometry to Identify Key Odorant Compounds in Dark Chocolate. Comparison of Samples before and after Conching. J. Agric. Food Chem. 2002, 50, 2385–2391. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Hansen, C.E. Isolation and characterization of cell wall polysaccharides from cocoa (Theobroma cacao L.) beans. Planta 2000, 210, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, O.E. Chocolate Science and Technology, 2nd ed.; Wiley Blakewll: Chichester, UK, 2016. [Google Scholar]
- Megías-Pérez, R.; Ruiz-Matute, A.I.; Corno, M.; Kuhnert, N. Analysis of minor low molecular weight carbohydrates in cocoa beans by chromatographic techniques coupled to mass spectrometry. J. Chromatogr. A 2018, 1584, 135–143. [Google Scholar] [CrossRef]
- Noor-Soffalina, S.S.; Jinap, S.; Nazamid, S.; Nazimah, S.A.H. Effect of polyphenol and pH on cocoa Maillard-related flavour precursors in a lipidic model system. Int. J. Food Sci. Technol. 2009, 44, 168–180. [Google Scholar] [CrossRef]
- Amoako, D.; Awika, J.M. Polyphenol interaction with food carbohydrates and consequences on availability of dietary glucose. Curr. Opin. Food Sci. 2016, 8, 14–18. [Google Scholar] [CrossRef]
- Sirbu, D.; Grimbs, A.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Variation of triacylglycerol profiles in unfermented and dried fermented cocoa beans of different origins. Food Res. Int. 2018, 111, 361–370. [Google Scholar] [CrossRef]
- Sirbu, D.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Characterization of triacylglycerols in unfermented cocoa beans by HPLC-ESI mass spectrometry. Food Chem. 2018, 254, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Masuchi Buscato, H.M.; Hara, L.M.; Bonomi, É.C.; Calligaris, G.A.; Cardoso, L.P.; Grimaldi, R.; Kieckbusch, T.G. Delaying fat bloom formation in dark chocolate by adding sorbitan monostearate or cocoa butter stearin. Food Chem. 2018, 256, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.K.; Hurst, W.J. Chocolate and Health—Chemistry, Nutrition and Therapy; The Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Hurst, W.J.; Tarka, S.M.; Dobson, G.; Reid, C. M Determination of Conjugated Linoleic Acid (CLA) Concentrations on Milk Chocolate. J. Agric. Food Chem. 2001, 49, 1264–1265. [Google Scholar] [CrossRef] [PubMed]
- Cambell, W.; Drake, M.A.; Larick, D.K. The Impact of Fortification with Conjugated Linoleic Acid (CLA) on the Quality of Fluid Milk. J. Dairy Sci. 2003, 86, 43–51. [Google Scholar] [CrossRef]
- Sonwai, S.; Rousseau, D. Controlling fat bloom formation in chocolate—Impact of milk fat on microstructure and fat phase crystallisation. Food Chem. 2010, 119, 286–297. [Google Scholar] [CrossRef]
- Jalil, A.; Ismail, A. Polyphenols in Cocoa and Cocoa Products: Is there a Link between Antioxidant Properties and Health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef] [PubMed]
- Zyzelewicz, D.; Krysiak, W.; Oracz, J.; Sosnowska, D.; Budryn, G.; Nebesny, E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res. Int. 2016, 89, 918–929. [Google Scholar] [CrossRef]
- De Taeye, C.; Caullet, G.; Eyamo Evina, V.J.; Collin, S. Procyanidin A2 and Its Degradation Products in Raw, Fermented, and Roasted Cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef]
- Bonvehí, J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur. Food Res. Technol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Emmanuel, O.A.; Jennifer, Q.; Agnes, S.B.; Jemmy, K.S.T.; Firibu, K.S. Influence of pulp-preconditioning and fermentation on fermentative quality and appearance of Ghanaian cocoa (Theobroma cacao) beans. Int. Food Res. J. 2012, 19, 127–133. [Google Scholar]
- Kothe, L.; Zimmermann, B.F.; Galensa, R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013, 141, 3656–3663. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Reyes, C.N.; Fogliano, V. Design cocoa processing towards healthy cocoa products: The role of phenolics and melanoidins. J. Funct. Foods 2018, 45, 480–490. [Google Scholar] [CrossRef]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015, 174, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.H.; Van Buiten, C.B.; Baker, S.A.; Elias, R.J.; Anantheswaran, R.C.; Lambert, J.D. Impact of roasting on the flavan-3-ol composition, sensory-related chemistry, and in vitro pancreatic lipase inhibitory activity of cocoa beans. Food Chem. 2018, 255, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Hurst, W.J.; Krake, S.H.; Bergmeier, S.C.; Payne, M.J.; Miller, K.B.; Stuart, D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Dimick, P.S.; Hoskin, J.C. The Chemistry of Flavour Development in Chocolate. In Industrial Chocolate Manufacture and Use, 3rd ed.; Beckett, S.T., Ed.; Blackwell Science: Oxford, UK, 1999; pp. 137–152. [Google Scholar]
- Todorovic, V.; Redovnikovic, I.R.; Todorovic, Z.; Jankovic, G.; Dodevska, M.; Sobajic, S. Polyphenols, methylxanthines, and antioxidant capacity of chocolates produced in Serbia. J. Food Compost. Anal. 2015, 41, 137–143. [Google Scholar] [CrossRef]
- Barišić, V.; Flanjak, I.; Križić, I.; Jozinović, A.; Šubarić, D.; Babić, J.; Miličević, B.; Ačkar, Đ. Impact of high-voltage electric discharge treatment on cocoa shell phenolic components and methylxanthines. J. Food Process. Eng. 2019, in press. [Google Scholar]
- Williams, O.B. Advances in Food Research; Mark, E.M., Stewart, G.F., Eds.; Academic Press: New York, NY, USA, 1958; Volume VIII. [Google Scholar]
- Rodriguez-Campos, J.; Escalona-Buend’ıa, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Janek, K.; Textoris-Taube, K.; Niewienda, A.; Wöstemeyer, J. Partial purification and characterisation of the peptide precursors of the cocoa-specific aroma components. Food Chem. 2016, 192, 706–713. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of turning, pod storage and fermentation time on microbial ecology and volatile composition of cocoa beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef]
- Moreira, I.M.V.; Vilela, L.F.; Santos, C.; Lima, N.; Schwan, R.F. Volatile compounds and protein profiles analyses of fermented cocoa beans and chocolates from different hybrids cultivated in Brazil. Food Res. Int. 2018, 109, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Batista, N.N.; Ramos, C.; Silva, A.R.A.; Efraim, P.; Pinheiro, A.C.M.; Schwan, R.F. Investigation of chocolate produced from four different Brazilian varieties of cocoa (Theobroma cacao L.) inoculated with Saccharomyces cerevisiae. Food Res. Int. 2016, 81, 83–90. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 5th ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ramli, N.; Hassan, O.; Said, M.; Samsudin, W.; Idris, N.A. Influence of roasting conditions on volatile flavor of roasted Malaysian cocoa beans. J. Food Process Press 2006, 30, 280–298. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Song, H.; Guo, J.; Wang, Y.; Yang, H.; Su, X. A comparative study of aroma-active compounds between dark and milk chocolate: Relationship to sensory perception. J. Sci. Food Agric. 2014, 95, 1362–1372. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. https://doi.org/10.3390/molecules24173163
Barišić V, Kopjar M, Jozinović A, Flanjak I, Ačkar Đ, Miličević B, Šubarić D, Jokić S, Babić J. The Chemistry behind Chocolate Production. Molecules. 2019; 24(17):3163. https://doi.org/10.3390/molecules24173163
Chicago/Turabian StyleBarišić, Veronika, Mirela Kopjar, Antun Jozinović, Ivana Flanjak, Đurđica Ačkar, Borislav Miličević, Drago Šubarić, Stela Jokić, and Jurislav Babić. 2019. "The Chemistry behind Chocolate Production" Molecules 24, no. 17: 3163. https://doi.org/10.3390/molecules24173163
APA StyleBarišić, V., Kopjar, M., Jozinović, A., Flanjak, I., Ačkar, Đ., Miličević, B., Šubarić, D., Jokić, S., & Babić, J. (2019). The Chemistry behind Chocolate Production. Molecules, 24(17), 3163. https://doi.org/10.3390/molecules24173163










