Phytochemical Study of Stem and Leaf of Clausena lansium
Abstract
:1. Introduction
2. Results and Discussion
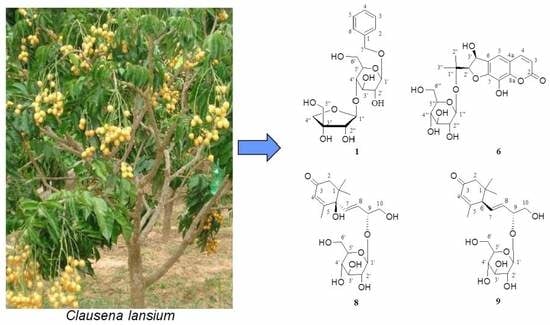
2.1. Elucidation of Chemical Structures of Four New Compounds 1,6,8,9
2.2. Structural Identification and Function of the Known Compounds 2–5,7,10–12
2.3. Characterization of Compounds 1–12
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Determination of Absolute Configurations of Sugars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1997; Volume 43, p. 126. [Google Scholar]
- Arbab, I.A.; Abdul, A.B.; Aspollah, M.; Abdullah, R.; Abdelwahab, S.I.; Ali, M.L.Z. A review of traditional uses, phytochemical and pharmacological aspects of selected members of Clausena genus (Rutaceae). J. Med. Plants Res. 2012, 6, 5107–5118. [Google Scholar]
- Liu, Y.P.; Guo, J.M.; Liu, Y.Y.; Hu, S.; Yan, G.; Qiang, L.; Fu, Y.H. Carbazole Alkaloids with Potential Neuroprotective Activities from the Fruits of Clausena lansium. J. Agric. Food Chem. 2019, 67, 5764. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, C.J.; Yang, J.Z.; Ning, N.; Si, Y.K.; Li, L.; Chen, N.H.; Zhao, Q.; Zhang, D.M. Carbazole alkaloids from the stems of Clausena lansium. J. Nat. Prod. 2012, 75, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, F.; Li, C.J.; Yang, J.Z.; Li, L.; Chen, N.H.; Zhang, D.M. Bioactive furanocoumarins from stems of Clausena lansium. Phytochemistry 2014, 107, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.Y.; Chao, C.H.; Chan, H.H.; Huang, G.J.; Hwang, T.L.; Lai, C.Y.; Lee, K.H.; Thang, T.D.; Wu, T.S. Bioactiveconstituents of Clausena lansium and a method for discrimination ofaldose enantiomers. Phytochemistry 2012, 82, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Song, W.W.; Zeng, G.Z.; Peng, W.W.; Chen, K.X.; Tan, N.H. Cytotoxic amides and quinolones from Clausena lansium. Helv. Chim. Acta 2014, 97, 298–305. [Google Scholar] [CrossRef]
- Du, Y.Q.; Liu, H.; Li, C.J.; Ma, J.; Zhang, D.; Li, L.; Sun, H.; Bao, X.Q.; Zhang, D.M. Bioactive carbazole alkaloids from the stemsof Clausena lansium. Fitoterapia 2015, 103, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.Y.; Chan, Y.Y.; Hwang, T.L.; Juang, S.H.; Huang, S.C.; Kuo, P.C.; Thang, T.D.; Lee, E.J.; Damu, A.G.; Wu, T.S. Constituents of the roots of Clausena lansium and their potential antiinflammatory activity. J. Nat. Prod. 2014, 77, 1215–1223. [Google Scholar] [CrossRef]
- Deng, H.D.; Mei, W.L.; Wang, H.; Guo, Z.K.; Dong, W.H.; Wang, H.; Li, S.P.; Dai, H.F. Carbazole alkaloids from the peels of Clausena lansium. J. Asian Nat. Prod. Res. 2014, 16, 1024–1028. [Google Scholar] [CrossRef]
- Xu, X.Y.; Xie, H.H.; Wei, X.Y. Jasmonoid glucosides, sesquiterpenes and coumarins from the fruit of Clausena lansium. LWT-Food Sci. Technol. 2014, 59, 65–69. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Xu, Y.S.; Feng, Z.L.; Meng, F.C.; Zhang, Q.W.; Gan, L.S.; Lin, L.G. Clausoxamine, an alkaloid possessing a 1,3-oxazine-4-one ring from the seeds of Clausena lansium and the antiobesity effect of lansiumamide B. RSC Adv. 2017, 7, 46900–46905. [Google Scholar] [CrossRef]
- Fan, Y.J.; Chen, H.Q.; Mei, W.L.; Kong, F.D.; Li, F.X.; Chen, P.W.; Cai, C.H.; Huang, M.J.; Dai, H.F. Nematicidal amidealkaloids from the seeds of Clausena lansium. Fitoterapia 2018, 128, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xiong, Z.; Xie, N.; Liu, S.Z.; Zhang, L.L.; Xu, F.; Guo, W.H.; Feng, J.T. Bioassay-guided isolation of antifungal amidesagainst sclerotinia sclerotiorum from the seeds of Clausena lansium. Ind. Crops Prod. 2018, 121, 352–359. [Google Scholar] [CrossRef]
- Deng, H.D.; Mei, W.L.; Guo, Z.K.; Liu, S.; Zuo, W.J.; Dong, W.H.; Li, S.P.; Dai, H.F. Monoterpenoid coumarins from the peels of Clausena lansium. Planta Med. 2014, 80, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.W.; Zheng, L.X.; Ji, C.J.; Shi, X.G.; Xiong, Z.H.; Shangguan, X.C. Carbazole alkaloids isolated from the branch and leaf extracts of Clausena lansium. Chin. J. Nat. Med. 2018, 16, 509–512. [Google Scholar] [CrossRef]
- Peng, W.W.; Zheng, L.X.; Huo, G.H. A new furan-coumarin from Clausena lansium. Chem. Nat. Compd. 2019, 55, 440–442. [Google Scholar] [CrossRef]
- Wang, M.F.; Li, J.G.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.C.; Ho, C.T. Antioxidative phenolic compounds from Sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4872. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashina, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Kim, A.; Choi, J.; Htwe, K.M.; Chin, Y.W.; Kim, J.; Yoon, K.D. Flavonoid glycosides from the aerial parts of Acacia pennata in Myanmar. Phytochemistry 2015, 118, 17–22. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Yokosuka, A.; Sano, T.; Hashimoto, K.; Sakgami, H.; Mimaki, Y. Triterpene glycosides from the whole plant of anemone hupehensis var. japonica and their cytotoxic activity. Chem. Pharm. Bull. 2009, 57, 1425–1430. [Google Scholar] [CrossRef]
- Kasai, R.; Suzuo, M.; Asakawa, J.I.; Tanaka, O. Carbon-13 chemical shifts of isoprenoid-β-d-glucopyranosides and -β-d-mannopyranosides. Stereochemical influences of aglycone alcohols. Tetrahedron Lett. 1977, 2, 175–178. [Google Scholar] [CrossRef]
- Lemmich, J.; Havelund, S.; Thastrup, O. Dihydrofurocoumarin glucosides from angelica archangelica and angelica szlvestris. Phytochemistry 1983, 22, 553–555. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Li, L.; Masahiko, T.; Kimiye, B. Glucosides from pleurospermum rivulorum. Acta Pharm. Sin. 2001, 36, 519–522. [Google Scholar]
- Chakthong, S.; Weaaryee, P.; Puangphet, P.; Mahabusarakam, W.; Patimaporn, P.; Voravuthikunchai, S.P.; Kanjana-Opas, A. Alkaloid and coumarins from the green fruits of Aegle marmelos. Phytochemistry 2012, 75, 108–113. [Google Scholar] [CrossRef]
- Yamano, Y.; Ito, M. Synthesis of optically active vomifoliol and roseoside stereoisomers. Chem. Pharm. Bull. 2005, 53, 541–546. [Google Scholar] [CrossRef]
- Pabst, A.; Barron, D.; Sbmons, E.; Schreier, P. Two diastereomeric 3-OXO-α-ionol β-d-glucosides from raspberry fruit. Phytochemistry 1992, 31, 1649–1652. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Han, C.K.; Jin, C.; Lee, Y.S. Secondary metabolites from Opuntia ficus-indica var. saboten. Phytochemistry 2006, 67, 1390–1394. [Google Scholar] [CrossRef]
- Kil, H.W.; Rho, T.; Yoon, K.D. Phytochemical study of aerial parts of Leea asiatica. Molecules 2019, 24, 1733. [Google Scholar] [CrossRef]
- Da, Y.; Satoh, Y.; Ohtwka, M.; Nagasao, M.; Shoji, J. Phenolic constituents of phellodezvdron amurense Bark. Phyrochmisrry 1994, 35, 209–215. [Google Scholar]
- Chassagne, D.; Crouzet, J.; Bayonove, C.L.; Baumes, R.L. Glycosidically bound eugenol and methyl salicylate in the fruit of edible Passiflora species. J. Agric. Food Chem. 1997, 45, 2685–2689. [Google Scholar] [CrossRef]
- Greger, H.; Pacher, T.; Brem, B.; Bacher, M.; Hofer, O. Insecticidal flavaglines and other compounds from Fijian Aglaia species. Phytochemistry 2001, 57, 57–64. [Google Scholar] [CrossRef]
- Breimaier, E.; Voelter, W. Konfigurations-und konformations-untersuchungen von adenosinanalogen mit 13C-resonanz. Tetrahedron 1973, 29, 227–232. [Google Scholar] [CrossRef]
- Peng, W.W.; Song, W.W.; Huang, M.B.; Zeng, G.Z.; Tan, N.H. Twelve benzene derivatives from Clausena excavate. Acta Pharm. Sin. 2014, 49, 1689–1693. [Google Scholar]
- Peng, W.W.; Song, W.W.; Huang, M.B.; Tan, N.H. Monoterpenes and sesquiterpenes from Clausena excavata. Chin. J. Chin. Mater. Med. 2014, 39, 1620–1624. [Google Scholar]
- Alkhatib, R.; Hennebelle, T.; Roumy, V.; Sahpaz, S.; Suzge, S.; Akalın, E.; Meriçl, A.H.; Bailleul, F. Coumarins, caffeoyl derivatives and a monoterpenoid glycoside from Ferulago asparagifolia. Biochem. Syst. Ecol. 2009, 37, 230–233. [Google Scholar] [CrossRef]
- Xu, K.; Jiangb, S.; Sunc, H.; Zhou, Y.; Xub, X.; Penga, S.; Dinga, L. New alkaloids from the seeds of Notopterygium incisum. Nat. Prod. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef]
- Ishikawa, T.; Sega, Y.; Kitajima, J. Water-Soluble Constituents of Glehnia littoralis Fruit. Chem. Pharm. Bull. 2001, 49, 584–588. [Google Scholar] [CrossRef]
- He, R.J.; Zhang, Y.J.; Wu, L.D.; Nie, H.; Huang, Y.; Liu, B.M.; Deng, S.P.; Yang, R.Y.; Huang, S.; Nong, Z.J.; et al. Benzofuran glycosides and coumarins from the bark of Streblus indicus (Bur.) Corner. Phytochemistry 2017, 138, 170–177. [Google Scholar] [CrossRef]
- Uchiyama, T.; Hara, S.; Makino, M.; Fujimoto, Y. seco-Adianane-type triterpenoids from Dorstenia brasiliensis (Moraceae). Phytochemistry 2002, 60, 761–764. [Google Scholar] [CrossRef]
- Peng, W.W.; Song, W.W.; Liu, X.Y.; Tan, N.H. Reseach progress on carbazole alkaloids from plants of Clausena Burmf. Chin. Tradi. Herbal Drugs 2017, 48, 2761. [Google Scholar]
- Peng, W.W.; Song, W.W.; Tan, N.H. Reseach progress on from Clausena and their pharmacological activities. Nat. Prod. Res. Dev. 2017, 29, 1428. [Google Scholar]
- Ito, C.; Itoigawa, M.; Katsuno, S.; Omura, M.; Tokuda, H.; Nishino, H.; Furukawa, H. Chemical constituents of Clausena excavata: Isolation and structure elucidation of novel furanone-coumarins with inhibitory effects for tumor-promotion. J. Nat. Prod. 2000, 63, 1218–1224. [Google Scholar] [CrossRef]
- Chaichantipyuth, C.; Pummangera, S.; Naowsaran, K.; Thanyavuthi, D.; Anderson, J.; McLaughlin, J.L. Two new bioactive carbazole alkaloids from Clausena harmandiana. J. Nat. Prod. 1988, 51, 1285–1288. [Google Scholar] [CrossRef]
- Wu, T.S.; Huang, S.C.; Wu, P.L.; Lee, K.D. Structure and synthesis of clausenaquinone A. A novel carbazolequinone alkaloid and bioactive principle from Clausena excavata. Bioorg. Med. Chem. Lett. 1994, 4, 2395–2398. [Google Scholar] [CrossRef]
- Feng, Z.J.; Zhangb, X.H.; Zhanga, J.P.; Shanga, X.H.; Gao, Y.; Lu, X.L.; Liu, X.Y.; Jiao, B.H. A new aromatic glycoside from Glehnia littoralis. Nat. Prod. Res. 2014, 28, 551–554. [Google Scholar] [CrossRef]
- He, D.H.; Slebodnick, C.; Rakotondraibe, L.H. Bioactive drimane sesquiterpenoids and aromatic glycosides from Cinnamosma fragrans. Bioorg. Med. Chem. Lett. 2017, 27, 1754–1759. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, W.; Lu, Z.K.; Guo, R.; Xia, Y.G.; Lv, S.W.; Yang, B.Y.; Kuang, H.X. New sesquiterpenoids from the stems of Datura metel L. Fitoterapia 2019, 134, 417–421. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 137.6 (s) | 4′ | 3.84 (m) | 76.6 (d) | |
| 2 | 7.31 (d, 7.2) | 127.8 (d) | 5′ | 3.51 (m) | 77.2 (d) |
| 3 | 7.23 (m) | 127.9 (d) | 6′ | 3.78 (m) | 61.4 (t) |
| 4 | 7.18 (d, 7.3) | 127.3 (d) | 3.60 (m) | ||
| 5 | 7.23 (m) | 127.9 (d) | 1″ | 5.27 (d, 2.1) | 109.3 (d) |
| 6 | 7.31 (d, 7.2) | 127.8 (d) | 2″ | 3.31 (m) | 77.6 (d) |
| 7 | 4.56 (d,11.6) | 70.4 (t) | 3″ | 79.2 (s) | |
| 4.80 (d,11.6) | 4″ | 3.81 (m) | 73.9 (t) | ||
| 1′ | 4.30 (d,7.5) | 100.8 (d) | 3.55 (m) | ||
| 2′ | 3.16 (m) | 76.5 (d) | 5″ | 3.45 (d, 12.2) | 64.6 (t) |
| 3′ | 3.38 (m) | 70.3 (d) | 3.41 (d, 12.2) |
| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 2 | 161.3 (s) | 1″ | 77.5 (s) | ||
| 3 | 6.25 (d, 9.5) | 110.7 (d) | 2″ | 1.64 (3H, s) | 23.3 (q) |
| 4 | 7.91 (d, 9.5) | 144.9 (d) | 3″ | 1.63 (3H, s) | 22.2 (q) |
| 4a | 113.8 (s) | 1‴ | 4.85 (d, 7.5) | 97.2 (d) | |
| 5 | 7.21 (s) | 114.3 (d) | 2‴ | 3.16 (m) | 73.1 (d) |
| 6 | 127.8 (s) | 3‴ | 3.41 (m) | 76.1 (d) | |
| 7 | 150.1 (s) | 4‴ | 3.40 (m) | 69.3 (d) | |
| 8 | 129.3 (s) | 5‴ | 3.19 (m) | 75.6 (d) | |
| 8a | 144.1 (s) | 6‴ | 3.50 (m) | 59.9 (t) | |
| 2′ | 4.57 (d, 6.5) | 91.5 (d) | 3.17 (m) | ||
| 3′ | 5.35 (d, 6.5) | 71.1 (d) |
| Position | 8 | Position | 9 | ||
|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J inHz) | δC | ||
| 1 | 40.5 (s) | 1 | 36.8 (s) | ||
| 2 | 2.61 (d, 16.6) | 48.8 (t) | 2 | 2.48 (d, 15.8) | 48.4 (t) |
| 2.16 (d, 16.6) | 2.11 (d, 15.8) | ||||
| 3 | 199.5 (s) | 3 | 200.8 (s) | ||
| 4 | 5.85 (s) | 125.3 (d) | 4 | 5.79 (br s) | 124.9 (d) |
| 5 | 165.3 (s) | 5 | 164.3 (s) | ||
| 6 | 78.4 (s) | 6 | 2.72 (d, 9.8) | 57.1 (d) | |
| 7 | 6.92 (dd, 15.6, 6.3) | 127.0 (d) | 7 | 5.82 (dd, 15.4, 9.8) | 130.7 (d) |
| 8 | 5.79 (dd, 15.6, 7.4) | 133.9 (d) | 8 | 5.55 (dd, 15.4, 7.1) | 132.1 (d) |
| 9 | 4.45 (m) | 77.6 (d) | 9 | 4.30 (m) | 78.2 (d) |
| 10 | 3.60 (dd, 11.8, 4.2) | 64.1 (d) | 10 | 3.61 (dd, 11.6, 4.1) | 64.6 (t) |
| 3.57 (dd, 11.8, 4.2) | 3.57 (dd, 11.6, 4.1) | ||||
| 11 | 0.95 (3H, s), | 21.6 (q) | 11 | 0.93 (3H, s), | 26.7 (q) |
| 12 | 0.93 (3H, s), | 22.9 (q) | 12 | 0.88 (3H, s), | 26.2 (q) |
| 13 | 1.91 (3H, s), | 17.7 (q) | 13 | 2.04 (3H, s), | 22.6 (q) |
| 1′ | 4.26 (d, 7.6) | 99.5 (d) | 1′ | 4.21 (d, 7.3) | 99.9 (d) |
| 2′ | 3.25 (m) | 73.0 (d) | 2′ | 3.32 (m) | 73.5 (d) |
| 3′ | 3.24 (m) | 76.2 (d) | 3′ | 3.25 (m) | 76.7 (d) |
| 4′ | 3.23 (m) | 69.6 (d) | 4′ | 3.22 (m) | 70.2 (d) |
| 5′ | 3.13 (m) | 76.2 (d) | 5′ | 3.14 (m) | 76.7 (d) |
| 6′ | 3.81 (dd, 11.2, 2.1) | 60.8 (t) | 6′ | 3.73 (dd, 12.1, 2.2) | 61.3 (d) |
| 3.64 (dd, 11.2, 2.1) | 3.68 (dd, 12.1, 2.2) | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Fu, X.; Li, Y.; Xiong, Z.; Shi, X.; Zhang, F.; Huo, G.; Li, B. Phytochemical Study of Stem and Leaf of Clausena lansium. Molecules 2019, 24, 3124. https://doi.org/10.3390/molecules24173124
Peng W, Fu X, Li Y, Xiong Z, Shi X, Zhang F, Huo G, Li B. Phytochemical Study of Stem and Leaf of Clausena lansium. Molecules. 2019; 24(17):3124. https://doi.org/10.3390/molecules24173124
Chicago/Turabian StylePeng, Wenwen, Xiaoxiang Fu, Yuyan Li, Zhonghua Xiong, Xugen Shi, Fang Zhang, Guanghua Huo, and Baotong Li. 2019. "Phytochemical Study of Stem and Leaf of Clausena lansium" Molecules 24, no. 17: 3124. https://doi.org/10.3390/molecules24173124
APA StylePeng, W., Fu, X., Li, Y., Xiong, Z., Shi, X., Zhang, F., Huo, G., & Li, B. (2019). Phytochemical Study of Stem and Leaf of Clausena lansium. Molecules, 24(17), 3124. https://doi.org/10.3390/molecules24173124