The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination
Abstract
1. Introduction
2. Results
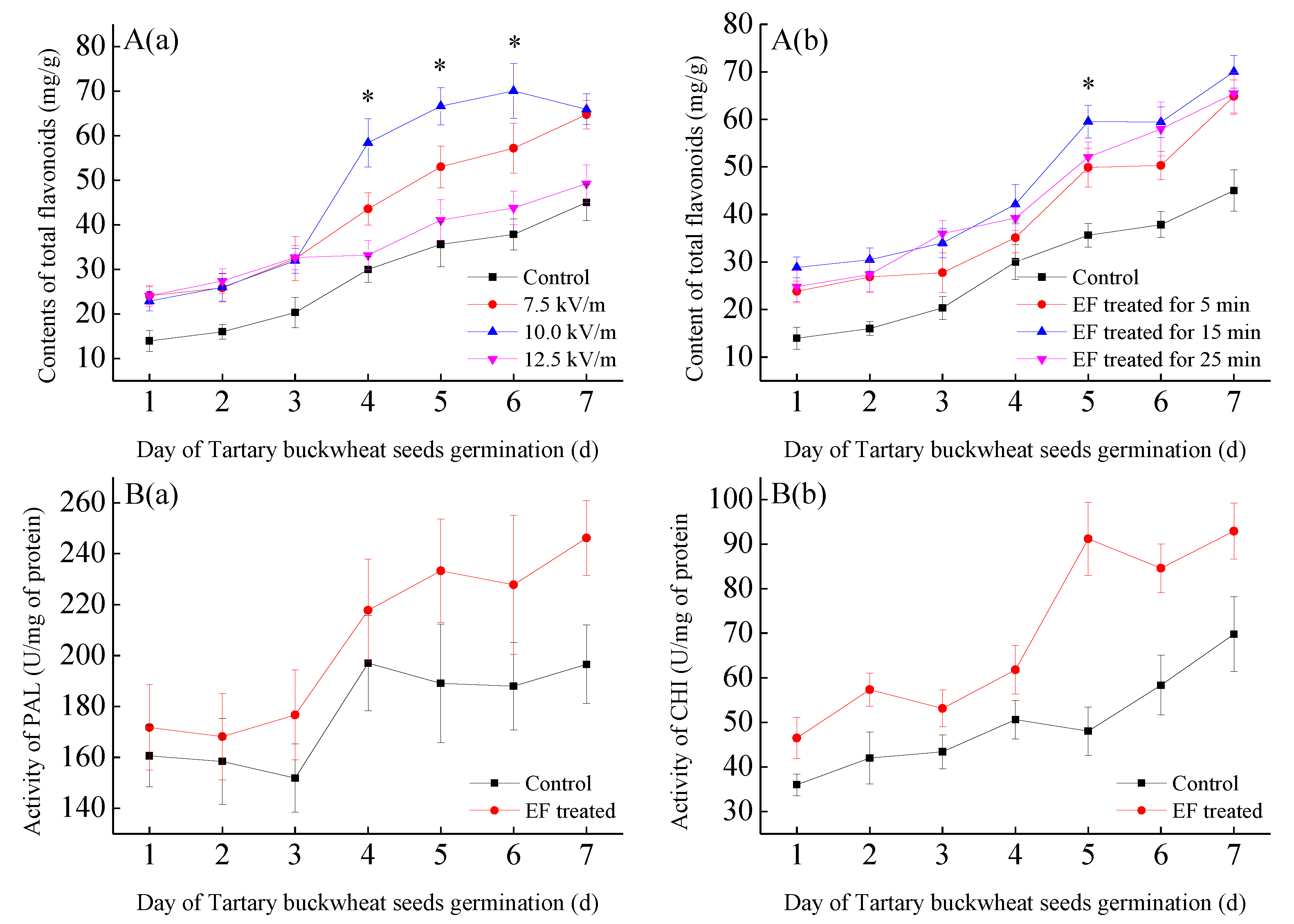
2.1. Effect of Electric Field on the Germination of Tartary Buckwheat Seeds
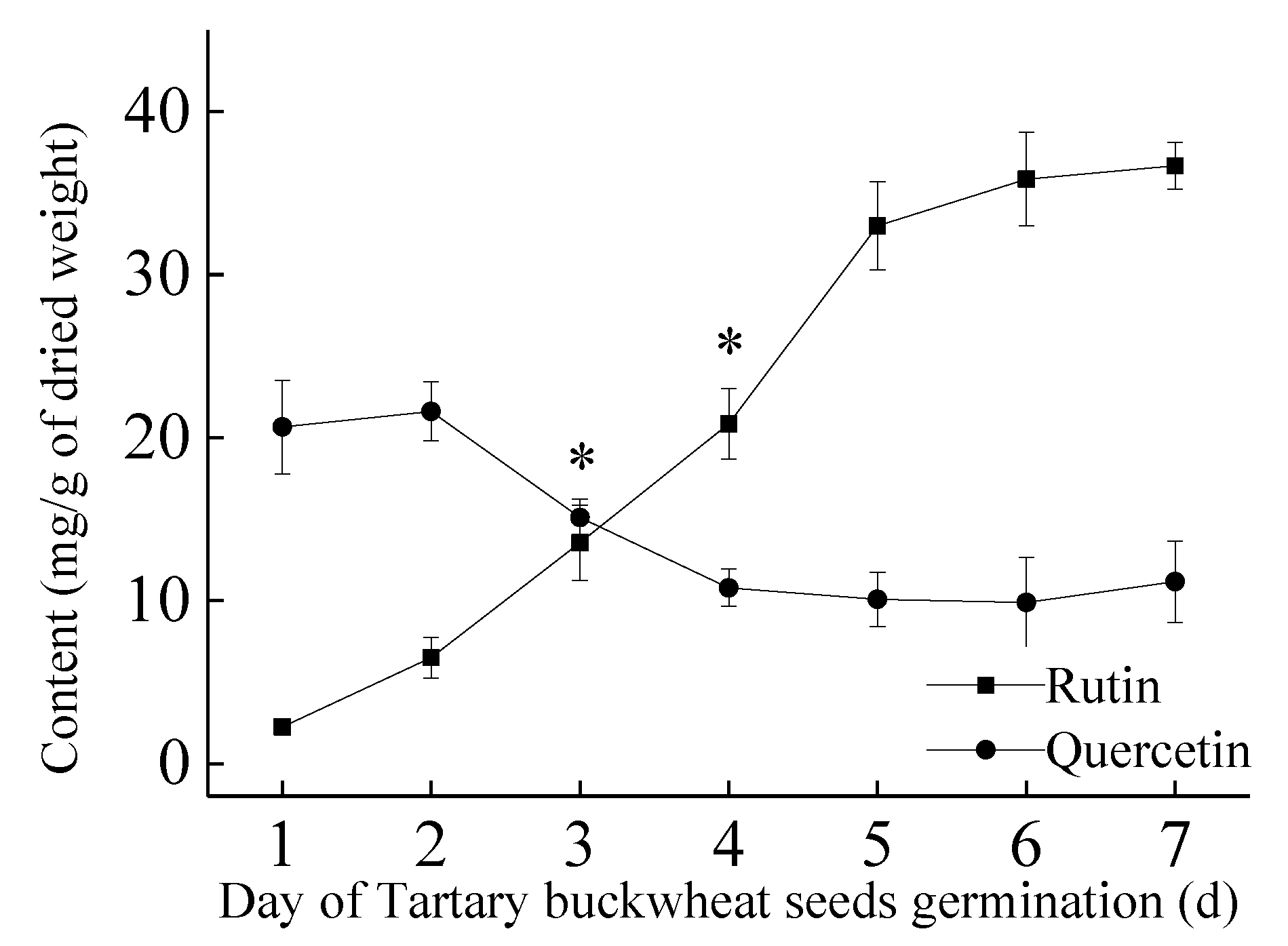
2.2. Quantitative Analysis of BWTFs
2.3. Anti-Tumor Activities of Buckwheat Flavonoids
2.4. Effects of Quercetin and Rutin on the Proliferation of MGC80-3 Cells
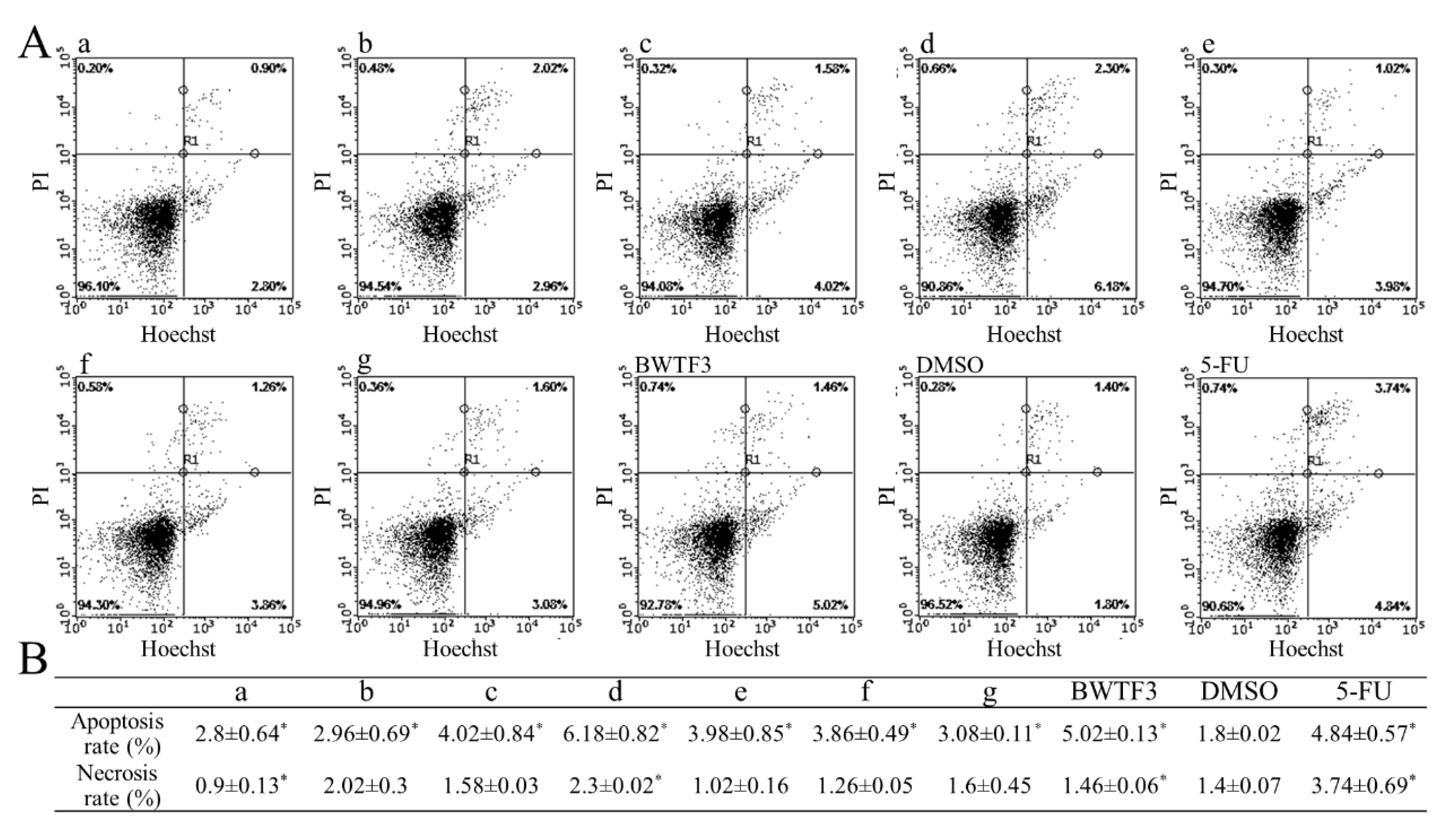
2.5. Apoptotic Assay
2.6. Protein Assay and Western Blotting
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Electrical Treatment and Germination of Buckwheat Seeds
4.3. Extraction of Total Flavonoids Extracts from Germinated Buckwheat Seeds
4.4. Enzyme Activity Assay
4.5. Cell Culture
4.6. Cell Proliferation Inhibitory Activity
4.7. UPLC-PDA Analysis Conditions
4.8. Apoptotic Assay
4.9. Protein Assay and Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fabjan, N.; Rode, J.; Kosir, I.J.; Wang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tartaricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Honda, Y.; Funatsuki, W.; Nakatsuka, K. Purification and characterization of flavonol 3-glucosidase, and its activity during ripening in tartary buckwheat seeds. Plant Sci. 2002, 163, 417–423. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Gupta, N.; Sharma, S.K.; Rana, J.C.; Chauhan, R.S. Expression of flavonoid biosynthesis genes vis-à-vis rutin content variation in different growth stages of Fagopyrum species. J. Plant Physiol. 2011, 168, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Babaoğlu, A.S.; Ozturk, S.; Aslım, B. Phenylalanine ammonia lyase (PAL) enzyme activity and antioxidant properties of some cyanobacteria isolates. Food Chem. 2013, 136, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Bowman, M.E.; Noel, J.P. Role of hydrogen bonds in the reaction mechanism of chalcone isomerase. Biochemistry 2002, 41, 5168–5176. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Yang, R.G.; Chen, H. Accumulation of γ-aminobutyric acid in germinated soybean (Glycine max L.) in relation to glutamate decarboxylase and diamine oxidase activity induced by additives under hypoxia. Eur. Food Res. Technol. 2012, 234, 679–687. [Google Scholar] [CrossRef]
- Imani, S.; Mehrjerdi, H.A.; Mohebbifar, M.R. The Effect of Electric Field on the Germination and Growth of Medicago Sativa Planet, as a native Iranian alfalfa seed. Acta Agriculturae Serbica 2012, 34, 105–115. [Google Scholar] [CrossRef]
- Leong, S.Y.; Burritt, D.J.; Oey, I. Electropriming of wheatgrass seeds using pulsed electric fields enhances antioxidant metabolism and the bioprotective capacity of wheatgrass shoots. Sci. Rep. 2016, 6, 25306. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, S.; Kautenburger, T.; Habermann, N.; Sauer, J.; Dietrich, H.; Will, F.; Pool-Zobel, B.L. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol. Carcinog. 2006, 45, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dou, H.; Li, H.; He, Z.; Wu, H. The citrus flavonoid nobiletin inhibits proliferation and induces apoptosis in human pancreatic cancer cells in vitro. Food Sci. Biotechnol. 2014, 23, 225–229. [Google Scholar] [CrossRef]
- Elkady, A.I.; Abu-Zinadah, O.A.; Hussein, R.A.E.H. Crude flavonoid extract of the Medicinal herb Nigella sativa inhibits proliferation and induces apoptosis in breast cancer cells. Biomater. Tissue Technol. 2017, 7, 1235–1249. [Google Scholar] [CrossRef]
- Kreft, S.; Knapp, M.; Kreft, L. Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. J. Aqric. Food Chem. 1999, 47, 4649–4652. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.A.D.; Nones, J.; Trentin, A.G.; Nones, J. Quercetin and Rutin Affect the Survival and Proliferation of Human Skin-Derived Multipotent Mesenchymal Stromal Cells. J. Pharm. Pharmacol. 2015, 3, 237–242. [Google Scholar] [CrossRef][Green Version]
- Yu, L.; Alva, A.; Su, H.; Dutt, P.; Freundt, E.; Welsh, S.; Baehrecke, E.H.; Lenardo, M.J. Regulation of an ATG7-beclin 1 Program of Autophagic Cell Death by Caspase-8. Science 2004, 304, 1500–1502. [Google Scholar] [CrossRef]
- Li, H.L.; Zhu, H.; Xu, C.J.; Yuan, J.Y. Cleavage of BID by Caspase 8 Mediates the Mitochondrial Damage in the Fas Pathway of Apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Kim, S.J.; Zaidul, I.S.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef]
- Thiyagarajan, K.; Vitali, F.; Tolaini, V.; Galeffi, P.; Cantale, C.; Vikram, P.; Singh, S.; De Rossi, P.; Nobili, C.; Procacci, S.; et al. Genomic Characterization of Phenylalanine Ammonia Lyase Gene in Buckwheat. PLoS ONE 2016, 11, e0151187. [Google Scholar] [CrossRef]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; Ric De Vos, C.H.; van Tunen, A.J.; Verhoeyen, M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef]
- Calabrese, J.C.; Jordan, D.B.; Boodhoo, A.; Sariaslani, S.; Vannelli, T. Crystal structure of phenylalanine ammonia lyase: Multiple helix dipoles implicated in catalysis. Biochemistry 2004, 43, 11403–11416. [Google Scholar] [CrossRef]
- Funk, R.H.; Monsees, T.; Ozkucur, N. Electromagnetic effects-From cell biology to medicine. Prog. Histochem. Cytochem. 2009, 43, 177–264. [Google Scholar] [CrossRef]
- Li, Y.; Gao, F.; Gao, F.; Shan, F.; Bian, J.; Zhao, C. Study on the interaction between 3 flavonoid compounds and alpha-amylase by fluorescence spectroscopy and enzymatic kinetics. J. Food Sci. 2009, 74, C199–C203. [Google Scholar] [CrossRef]
- Babu, T.M.C.; Rammohan, A.; Bhaskar, B.V.; Savita, D.; Duvvuru, G.; Wudayagiri, R. Development of novel HER2 inhibitors against gastric cancer derived from flavonoid source of Syzygium alternifolium through molecular dynamics and pharmacophore-based screening. Drug Des. Devel. Ther. 2016, 10, 3611–3632. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Harima, Y.; Nagata, K.; Harima, K.; Oka, A.; Ostapenko, V.V.; Shikata, N.; Ohnishi, T.; Tanaka, Y. Bax and Bcl-2 protein expression following radiation therapy versus radiation plus thermoradiotherapy in stage IIIB cervical carcinoma. Cancer 2000, 88, 132–138. [Google Scholar] [CrossRef]
- Delbridge, A.R.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.J.; Jiang, W.; Qian, J.Y. Effect of pulsed electric field on functional and structural properties of canola protein by pretreating seeds to elevate oil yield. LWT Food Sci. Technol. 2017, 84, 73–81. [Google Scholar] [CrossRef]
- Watanabe, M.; Ohshita, Y.; Tsushida, T. Antioxidant compounds from buckwheat (Fagopyrum esculentum Möench) hulls. J. Agric. Food Chem. 1997, 45, 1039–1044. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R.L. Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J. Sci. Food Agric. 1996, 71, 313–320. [Google Scholar] [CrossRef]
- McCallum, J.A.; Walker, J.R.L. Phenolic biosynthesis during grain development in wheat: Changes in phenylalanine Ammonia-lyase activity and soluble phenolic content. J. Cereal Sci. 1990, 11, 35–49. [Google Scholar] [CrossRef]
- Hu, Y.M.; Hu, X.P.; Zhang, L.; Zhang, S.S.; Xu, J.M. Uptake characteristics of levofloxacin for the eradication of Helicobacter pylori by GES-1 and MGC80-3 cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 486–490. [Google Scholar]
- Hongrapipat, J.; Kopecková, P.; Liu, J.; Prakongpan, S.; Kopecek, J. Combination Chemotherapy and Photodynamic Therapy with Fab’ Fragment Targeted HPMA Copolymer Conjugates in Human Ovarian Carcinoma Cells. Mol. Pharm. 2008, 5, 696–709. [Google Scholar] [CrossRef]
- Shi, Z.; Liang, Y.J.; Chen, Z.S.; Wang, X.W.; Wang, X.H.; Ding, Y.; Chen, L.M.; Yang, X.P.; Fu, L.W. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol. Ther. 2006, 5, 39–47. [Google Scholar] [CrossRef]
- Ratra, G.S.; Kamita, S.G.; Casida, J.E. Role of human GABAA receptor β3 subunit in insecticide toxicity. Toxicol. Appl. Pharmacol. 2001, 172, 233–240. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, L.; Hu, H.; Yang, Y.; Zhang, X.; Peng, Y.; Xiao, P. DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE. Molecules 2017, 22, 1622. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Yi, T.; Liu, J.; Zhao, Z.Z.; Chen, H.B. Quercetin Induces Apoptosis via the Mitochondrial Pathway in KB and KBv200 Cells. J. Agric. Food Chem. 2013, 61, 2188–2195. [Google Scholar] [CrossRef]
- Santos, B.L.; Silva, A.R.; Pitanga, B.P.; Sousa, C.S.; Grangeiro, M.S.; Fragomeni, B.O.; Coelho, P.L.; Oliveira, M.N.; Menezes-Filho, N.J.; Costa, M.F.; et al. Antiproliferative, proapoptotic and morphogenic effects of the flavonoid rutin on human glioblastoma cells. Food Chem. 2011, 127, 404–411. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.-L.; Chen, Z.-D.; Zhou, Y.-M.; Shi, R.-H.; Li, Z.-J. The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination. Molecules 2019, 24, 3092. https://doi.org/10.3390/molecules24173092
Zhou X-L, Chen Z-D, Zhou Y-M, Shi R-H, Li Z-J. The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination. Molecules. 2019; 24(17):3092. https://doi.org/10.3390/molecules24173092
Chicago/Turabian StyleZhou, Xiao-Li, Zhi-Dong Chen, Yi-Ming Zhou, Rong-Hua Shi, and Zong-Jie Li. 2019. "The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination" Molecules 24, no. 17: 3092. https://doi.org/10.3390/molecules24173092
APA StyleZhou, X.-L., Chen, Z.-D., Zhou, Y.-M., Shi, R.-H., & Li, Z.-J. (2019). The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination. Molecules, 24(17), 3092. https://doi.org/10.3390/molecules24173092




