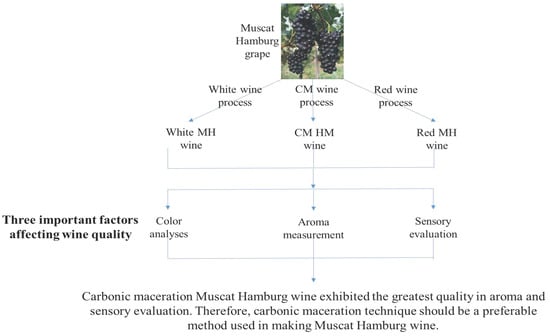
The Effect of Carbonic Maceration during Winemaking on the Color, Aroma and Sensory Properties of ‘Muscat Hamburg’ Wine
Abstract
1. Introduction
2. Results and Discussion
2.1. General Composition of Wine
2.2. Color Measurements
2.3. Aromatic Profile Analysis
2.4. Sensory Evaluation
3. Materials and Methods
3.1. Grape Sampling
3.2. Yeast Strains
3.3. Fermentation Experiments
3.4. General Enological Parameters
3.5. Color Measurements
3.6. Volatile Compounds Analysis
3.7. OAV Calculation
3.8. Sensory Evaluation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Antioxidant activity and phenolic profiles of Sauvignon Blanc wines made by various maceration techniques. Aust. J. Grape Wine Res. 2015, 21, 57–68. [Google Scholar] [CrossRef]
- Harrison, R. Practical interventions that influence the sensory attributes of red wines related to the phenolic composition of grapes: A review. Int. J. Food Sci. Technol. 2018, 53, 3–18. [Google Scholar] [CrossRef]
- Cuzmar, P.D.; Salgado, E.; Ribalta-Pizarro, C.; Olaeta, J.A.; López, E.; Pastenes, C.; Cáceres-Mella, A. Phenolic composition and sensory characteristics of Cabernet Sauvignon wines: effect of water stress and harvest date. Int. J. Food Sci. Technol. 2018, 53, 1726–1735. [Google Scholar] [CrossRef]
- Picariello, L.; Gambuti, A.; Petracca, F.; Rinaldi, A.; Moio, L. Enological tannins affect acetaldehyde evolution, colour stability and tannin reactivity during forced oxidation of red wine. Int. J. Food Sci. Technol. 2018, 53, 228–236. [Google Scholar] [CrossRef]
- Navarro, S.; Oliva, J.; Barba, A.; Navarro, G.; Garcia, M.A.; Zamorano, M. Evolution of chlorpyrifos, fenarimol, metalaxyl, penconazole, and vinclozolin in red wines elaborated by carbonic maceration of Monastrell grapes. J. Agric. Food Chem. 2000, 48, 3537–3541. [Google Scholar] [CrossRef] [PubMed]
- Etaio, I.; Meillon, S.; Pérez-Elortondo, F.J.; Schlich, P. Dynamic sensory description of Rioja Alavesa red wines made by different winemaking practices by using Temporal Dominance of Sensations. J. Sci. Food Agric. 2016, 96, 3492–3499. [Google Scholar] [CrossRef]
- Pace, C.; Giacosa, S.; Torchio, F.; Rio Seqade, S.; Caqnasso, E.; Rolle, L. Extraction kinetics of abthicyanins from skin to pulp during carbonic maceration of winegrape berries with different ripeness levels. Food Chem. 2014, 165, 77–84. [Google Scholar] [CrossRef]
- Fernández, M.J.; Oliva, J.; Barba, A.; Cámara, M.A. Fungicide dissipation curves in winemaking process with and without maceration step. J. Agric. Food Chem. 2005, 53, 804–811. [Google Scholar] [CrossRef]
- Gómez-Míquez, M.; Heredia, F.J. Effect of the maceration technique on the relationships between anthocyanin composition and objective color of Syrah wines. J. Agric. Food Chem. 2004, 52, 5117–5123. [Google Scholar] [CrossRef]
- Sun, B.; Spranger, I.; Roque-do-Vale, F.; Leandro, C.; Belchior, P. Effect of Different Winemaking Technologies on Phenolic Composition in Tinta Miúda Red Wines. J. Agric. Food Chem. 2001, 49, 5809–5816. [Google Scholar] [CrossRef]
- Tesniere, F.; Flanzy, C. Carbonic maceration wines: Characteristics and winemaking process. Adv. Food Nutr. Res. 2011, 63, 1–15. [Google Scholar] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Elsevier-Academic Press: Oxford, UK, 2014; pp. 694–703. [Google Scholar]
- Gómez-Míguez, M.J.; Gómez-Míguez, M.; Vicario, I.M.; Heredia, F.J. Assessment of colour and aroma in white wines vinifications: Effects of grape maturity and soil type. J. Food Eng. 2007, 79, 758–764. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Katarína, F.; Katarína, M.; Katarína, Ď.; Ivan, Š.; Fedor, M. Influence of yeast strain on aromatic profile of Gewürztraminer wine. Lwt-Food Sci. Technol. 2014, 59, 1–7. [Google Scholar]
- Marín, J.; Ocete, R.; Pedroza, M.; Zalacain, A.; de Miguel, C.; López, M.A.; Salinas, M.R. Influence of the mite Carpoglyphus lactis (L) on the aroma of pale and dry wines aged under flor yeasts. J. Food Compos. Anal. 2009, 22, 745–750. [Google Scholar] [CrossRef]
- Lv, H.P.; Zhong, Q.S.; Lin, Z.; Wang, L.; Tan, J.F.; Guo, L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC–olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Manríquez, D.A.; Muñoz-Robredo, P.; Gudenschwager, O.; Robledo, P.; Defilippi, B.G. Development of flavor-related metabolites in cherimoya (Annona cherimola Mill.) fruit and their relationship with ripening physiology. Postharvest Biol. Technol. 2014, 94, 58–65. [Google Scholar]
- Mencarelli, F.; Bellincontro, A. Recent advances in the postharvest technology of wine grape to improve the wine aroma. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odorants. Anal. Chim. Acta 2004, 513, 254–262. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. Fems. Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Daniela, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360. [Google Scholar] [CrossRef]
- King, A.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- Bitteur, S.; Tesnière, C.; Fauconnet, A.; Beyonove, C.; Franzy, C. Carbonic anaerobiosis of Muscat grape, 2. Changes in the distribution of free and bound terpenols. Sci. Aliment. 1996, 16, 37–48. [Google Scholar]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wine (Review). J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Antonelli, A.; Castellari, L.; Zambonelli, C.; Carnacini, A. Yeast influence on volatile composition of wines. J. Agric. Food Chem. 1999, 47, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Schreirer, P. Flavor composition of wines: a review. Crit. Rev. Food Sci. 1979, 12, 59–111. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Karagiannis, S.; Economou, A.; Lanaridis, P. Phenolic and volatile composition of wines made from Vitis vinifera Cv. Muscat Lefko grapes from the isoland of Samos. J. Sci. Food Agric. 2000, 48, 5369–5375. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Sarni-Manchado, P. Wine taste and mouthfeel. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Oxford, UK, 2010; pp. 43–72. [Google Scholar]
- Du, G.; Zhan, J.; Li, J.; You, Y.; Zhao, Y.; Huang, W. Effect of Grapevine Age on the Aroma Compounds in ‘Beihong’ Wine. S. Afr. J. Enol. Vitic. 2012, 33, 7–13. [Google Scholar] [CrossRef][Green Version]
- Du, G.; Zhan, J.; Li, J.; You, Y.; Zhao, Y.; Huang, W. Effect of Fermentation Temperature and Culture Medium on Glycerol and Ethanol during Wine Fermentation. Am. J. Enol. Viticult. 2012, 63, 1–7. [Google Scholar] [CrossRef]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL medium to profile native flora fermentations. Am. J. Enol. Viticult. 2001, 52, 198–203. [Google Scholar]
- OIV. Résolution OIV/OENO 379/2009; Organisation Internationale de la Vigne et du Vin: Paris, France, 2009; pp. 21–23. [Google Scholar]
- Zhang, M.X.; Xu, Q.F.; Duan, C.Q.; Qu, W.Q.; Wu, Y.W. Comparative study of aromatic compounds in young red wines from Cabernet Sauvignon, Cabernet Franc, and Cabernet Gernischet Varieties in China. J. Food Sci. 2007, 72, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.L.; Mike, J.H.; Riesen, R. Validation of a solid-phase microextraction method for headspace analysis of wine aroma components. Am. J. Enol. Viticult. 2005, 56, 37–45. [Google Scholar]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Agric. Food Chem. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Falqué, E.; Fernández, E.; Dubourdieu, D. Differentiation of white wines by their aromatic index. Talanta 2001, 54, 271–281. [Google Scholar] [CrossRef]
- Tomasino, E.; Harrison, R.; Sedcole, R.; Frost, A. Regional differentiation of New Zealand Pinot noir wine by professionals using canonical variate analysis. Am. J. Enol. Viticul. 2013, 64, 357–363. [Google Scholar] [CrossRef]
- Xi, Z.; Tao, Y.; Zhang, L.; Li, H. Impact of cover crops in vineyard on the aroma compounds of Vitis vinifera L. cv Cabernet Sauvignon wine. Food Chem. 2011, 127, 516–522. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |

| Composition of Wine | Type of Wine | Significance b | ||
|---|---|---|---|---|
| W | R | CM | ||
| Alcoholicity (v/v, %) | 12.05 ± 0.13 | 11.85 ± 0.16 | 11.75 ± 0.17 | — |
| Reducing sugar (g/L) | 2.67 ± 0.08 | 2.98 ± 0.11 | 3.11 ± 0.15 | — |
| Free SO2 (mg/L) | 5.42 ± 0.21 | 4.08 ± 0.25 | 5.33 ± 0.15 | — |
| Total acidity a (g/L) | 6.25 ± 0.15 | 6.45 ± 0.25 | 5.85 ± 0.16 | — |
| pH value | 3.41 ± 0.16 | 3.35 ± 0.24 | 3.65 ± 0.21 | * |
| Dry extract (g/L) | 25.35 ± 1.52 | 26.53 ± 2.16 | 26.15 ± 1.56 | — |
| Color | Type of Wine | Significance * | |
|---|---|---|---|
| R | CM | ||
| L* | 96.41 ± 0.34 | 96.79 ± 0.42 | — |
| C* | 35.67 ± 0.28 | 41.26 ± 0.45 | ** |
| h | 23.68 ± 0.12 | 24.14 ± 0.25 | — |
| a* | 37.65 ± 0.59 | 43.25 ± 0.45 | ** |
| b* | 0.75 ± 0.12 | 0.84 ± 0.11 | ** |
| Aroma Compound | Retention Index a | Ion | Type of Wine (µg/L) | Odor Descriptors c | Odorant Series | Threshold (µg/L) | Significance d | ||
| m/zb | W | R | CM | ||||||
| Alcohols | |||||||||
| 3-Methyl-1-butanol | 1203 | 55 | 31.47 ± 1.23 * | 42.74 ± 3.15 * | 58.52 ± 1.47 * | Floral | 2 | 30 | *** |
| 2-Nonanol | 1517 | 45 | 4.17 ± 0.27 * | 4.56 ± 0.21 * | 2.29 ± 0.08 * | Cucumber | 4 | 58 | *** |
| Subtotal (µg/L) | 35.64 | 47.3 | 60.81 | ||||||
| Subtotal (w/w, %) | 0.84 | 1.31 | 0.95 | ||||||
| Aldehydes and Ketones | |||||||||
| (E)-2-Nonenal | 1539 | 43 | 119.65 ± 9.23 * | 70.34 ± 5.35 * | 198.46 ± 16.32 * | Fatty | 5 | 600 | *** |
| Octanal | 1292 | 43 | 3.41 ± 0.13 * | — | 4.41 ± 0.24 * | Fatty | 5 | 15 | — |
| Nonanal | 1394 | 57 | — | 5.45 ± 0.25 * | 4.47 ± 0.42 * | Green | 4 | 1 | — |
| Decanal | 1499 | 43 | 11.86 ± 0.73 * | 15.25 ± 1.07 * | 12.54 ± 1.02 * | Grass | 4 | 1000 | *** |
| β-Damascenone | 1833 | 69 | 4.69 ± 0.26 * | 6.39 ± 0.43 * | 6.11 ± 0.41 * | Flowers, Apple, Rose, Honey | 2, 3, 6 | 0.05 | *** |
| Subtotal (µg/L) | 139.61 | 97.43 | 225.99 | ||||||
| Subtotal (w/w, %) | 3.28 | 2.7 | 3.54 | ||||||
| Terpenes | |||||||||
| (−)-Rose oxide | 1356 | 139 | 17.02 ± 0.71 * | 6.86 ± 0.24 * | 19.54 ± 0.83 * | Rose, Lychee | 2, 6 | 0.2 | *** |
| (±)-β-Citronellol | 1770 | 69 | 258.04 ± 21.59 * | 40.18 ± 1.72 * | 230.14 ± 11.8 * | Floral, Rose | 2 | 18 | *** |
| 4-Terpinenol | 1575 | 81 | 1.78 ± 0.096 * | 76.12 ± 7.19 * | 2.25 ± 0.25 * | Sweet, Green, Citrus, Floral, | 2, 3, 4, 6 | 250 | *** |
| Linalool | 1547 | 71 | 179.21 ± 8.16 * | 216.11 ± 8.31 * | 269.45 ± 15.36 * | Flowery, Fruity | 2, 6 | 15 | *** |
| Nerol | 1805 | 69 | 11.6 ± 1.05 * | 4.04 ± 0.16 * | 18.04 ±1.62 * | Rose, Lime | 2, 6 | 400 | *** |
| Limonene | 1191 | 68 | 91.68 ± 6.52 * | 26.68 ± 1.92 * | 111.9 ± 5.19 * | Citrus-like, Fruity, Green | 4, 6 | 10 | *** |
| Citral | 1748 | 69 | 6.78 ± 0.52 * | — | 2.68 ± 0.15 * | Floral, Lemon | 2, 6 | 41 | — |
| Caryophyllene | 1581 | 93 | — | 9.18 ± 0.65 * | 8.05 ± 0.58 * | Flowery | 2 | 64000 | — |
| Geraniol | 1855 | 69 | 81.18 ± 5.32 * | 13.76 ± 0.89 * | 77.38 ± 6.32 * | Floral, Rose | 2 | 30 | *** |
| α-Terpineol | 1703 | 59 | 31.67 ± 2.14 * | 12.17 ± 0.58 * | 48.47 ± 2.86 * | Floral, Sweet | 2, 3 | 1000 | *** |
| Subtotal (µg/L) | 678.96 | 405.1 | 787.9 | ||||||
| Subtotal (w/w, %) | 15.97 | 11.2 | 12.34 | ||||||
| Acids | |||||||||
| Hexanoic acid | 1860 | 60 | 52.16 ± 2.62 * | 42.35 ± 2.15 * | 35.85 ± 1.21 * | Cheese, Fatty, Grass, Fruity | 5, 6 | 140 | *** |
| n–Decanoic acid | 2292 | 60 | 67.38 ± 3.15 * | 53.24 ± 1.56 * | 53.16 ± 1.36 * | Fatty | 5 | 15,000 | *** |
| Subtotal (µg/L) | 119.54 | 95.59 | 89.01 | ||||||
| Subtotal (w/w, %) | 2.81 | 2.64 | 1.39 | ||||||
| Esters | |||||||||
| (Z)-3-Hexenyl acetate | 1331 | 43 | 10.08 ± 0.69 * | 3.83 ± 0.19 * | 13.15 ± 0.73 * | Green, Apple, Grassy | 4, 6 | 8 | *** |
| Ethyl butyrate | 1047 | 43 | 21.25 ± 0.86 * | 23.14 ± 1.34 * | 24.23 ± 0.97 * | Fruity | 6 | 20 | *** |
| Isoamyl acetate | 1122 | 70 | 654.65 ± 5.35 * | 581.24 ± 4.19 * | 952.64 ± 8.17 * | Banana, Fruity, Sweet | 3, 6 | 30 | *** |
| Ethyl caproate | 1227 | 88 | 6.15 ± 0.27 * | 5.01 ± 0.37 * | 6.98 ± 0.19 * | Fruity, Banana | 6 | 5 | *** |
| Ethyl hexanoate | 1232 | 88 | 914.41 ± 5.32 * | 785.63 ± 4.53 * | 1077.56 ± 6.92 * | Green apple, Banana | 6 | 14 | *** |
| Hexenyl acetate | 1007 | 43 | 8.77 ± 0.75 * | 10.16 ± 0.45 * | 7.58 ± 0.34 * | Fruity | 6 | 2 | * |
| Ethyl heptanoate | 1334 | 88 | 2.84 ± 0.06 * | 3.91 ± 0.09 * | — | Pineapple, Green | 4, 6 | 14 | — |
| Methyl octanoate | 1390 | 74 | 3.49 ± 0.21 * | 4.16 ± 0.13 * | 5.42 ± 0.17 * | Fruity, Green | 4, 6 | 200 | *** |
| Ethyl octanoate | 1437 | 88 | 724.43 ± 6.17 * | 830.21 ± 7.21 * | 1540.84 ± 11.17 * | Floral, Fruity, Banana, Pear | 2, 6 | 5 | *** |
| Ethyl decanoate | 1639 | 88 | 494.4 ± 4.13 * | 293.25 ± 3.34 * | 824.07 ± 6.24 * | Fruity | 6 | 200 | *** |
| Ethyl 9-decenoate | 1697 | 88 | 53.45 ± 2.36 * | 31.26 ± 1.21 * | 48.15 ± 2.17 * | Fruity, Fatty | 5, 6 | 100 | *** |
| Phenylethyl acetate | 1830 | 104 | 301.09 ± 2.07 * | 341.08 ± 2.15 * | 467.62 ± 1.67 * | Floral | 2 | 250 | *** |
| Ethyl laurate | 1848 | 88 | 82.89 ± 1.43 * | 56.36 ± 0.32 * | 253.02 ± 3.43 * | Fruity | 6 | 1,500 | *** |
| Subtotal (µg/L) | 3277.9 | 2969.24 | 5221.26 | ||||||
| Subtotal (w/w, %) | 77.1 | 82.15 | 81.78 | ||||||
| Total (µg/L) | 4251.65 | 3614.66 | 6384.97 | ||||||
| Attributes | Class | Type of Wine | Significance b | ||
|---|---|---|---|---|---|
| W | R | CM | |||
| Visual analysis | Clarity (5) | 4.5 ± 0.15 | 4.4 ± 0.15 | 4.6 ± 0.1 | — |
| Appearance (10) | 8.5 ± 0.15 | 8.1 ± 0.2 | 8.2 ± 0.1 | ** | |
| Aroma analysis | Aroma purity (6) | 5.3 ± 0.1 | 5.1 ± 0.15 | 5.5 ± 0.15 | *** |
| Aroma intensity (8) | 6.8 ± 0.15 | 5.8 ± 0.15 | 7.3 ± 0.2 | *** | |
| Aroma quality (16) | 12.1 ± 0.15 | 11.5 ± 0.15 | 13.5 ± 0.2 | *** | |
| Taste analysis | Taste purity (6) | 5.1 ± 0.1 | 4.6 ± 0.2 | 5.3 ± 0.2 | *** |
| Taste intensity (8) | 6.8 ± 0.1 | 6.7 ± 0.15 | 6.9 ± 0.2 | — | |
| Taste prolongation (8) | 6.6 ± 0.15 | 6.7 ± 0.1 | 7.1 ± 0.2 | * | |
| Taste quality (22) | 16.9 ± 0.1 | 16.1 ± 0.2 | 18.2 ± 0.2 | *** | |
| Global evaluation | Harmony (11) | 9.5 ± 0.15 | 8.6 ± 0.1 | 10.2 ± 0.15 | *** |
| Total a | 100 | 82.1 ± 0.3 | 77.6 ± 0.2 | 86.8 ± 0.4 | *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-S.; Du, G.; Gao, Y.-T.; Wang, L.-W.; Meng, D.; Li, B.-J.; Brennan, C.; Wang, M.-Y.; Zhao, H.; Wang, S.-Y.; et al. The Effect of Carbonic Maceration during Winemaking on the Color, Aroma and Sensory Properties of ‘Muscat Hamburg’ Wine. Molecules 2019, 24, 3120. https://doi.org/10.3390/molecules24173120
Zhang Y-S, Du G, Gao Y-T, Wang L-W, Meng D, Li B-J, Brennan C, Wang M-Y, Zhao H, Wang S-Y, et al. The Effect of Carbonic Maceration during Winemaking on the Color, Aroma and Sensory Properties of ‘Muscat Hamburg’ Wine. Molecules. 2019; 24(17):3120. https://doi.org/10.3390/molecules24173120
Chicago/Turabian StyleZhang, Yu-Shu, Gang Du, Yu-Ting Gao, Li-Wen Wang, Dan Meng, Bing-Juan Li, Charles Brennan, Mei-Yan Wang, Hui Zhao, Su-Ying Wang, and et al. 2019. "The Effect of Carbonic Maceration during Winemaking on the Color, Aroma and Sensory Properties of ‘Muscat Hamburg’ Wine" Molecules 24, no. 17: 3120. https://doi.org/10.3390/molecules24173120
APA StyleZhang, Y.-S., Du, G., Gao, Y.-T., Wang, L.-W., Meng, D., Li, B.-J., Brennan, C., Wang, M.-Y., Zhao, H., Wang, S.-Y., & Guan, W.-Q. (2019). The Effect of Carbonic Maceration during Winemaking on the Color, Aroma and Sensory Properties of ‘Muscat Hamburg’ Wine. Molecules, 24(17), 3120. https://doi.org/10.3390/molecules24173120