Carbon Dots-Modified Nanoporous Membrane and Fe3O4@Au Magnet Nanocomposites-Based FRET Assay for Ultrasensitive Histamine Detection
Abstract
1. Introduction
2. Results
2.1. Mechanism of Histamine Detection by the FRET System
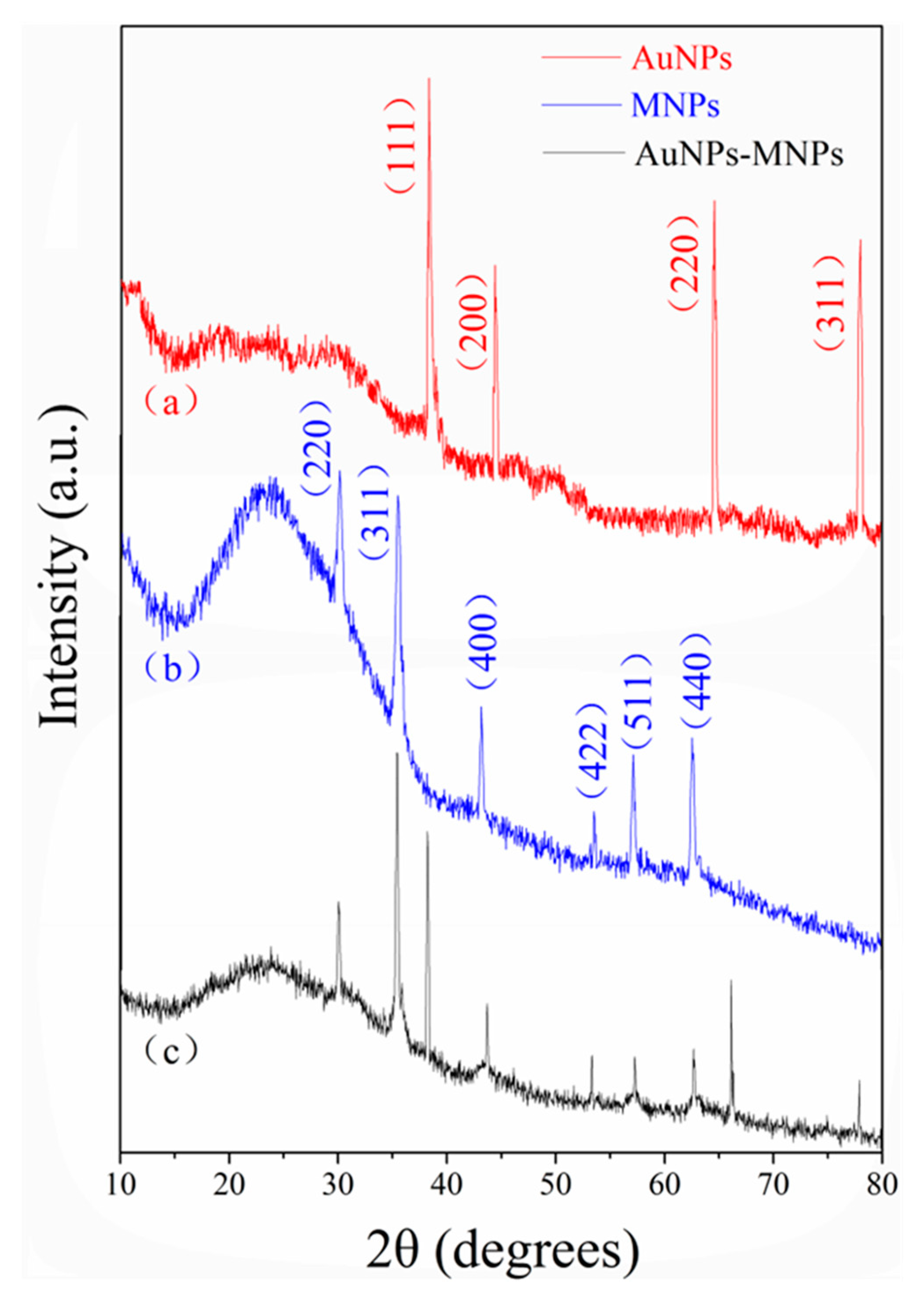
2.2. Characterization of Magnetic Nanoparticles and Nanoporous Alumina Membranes
2.3. Histamine Determination
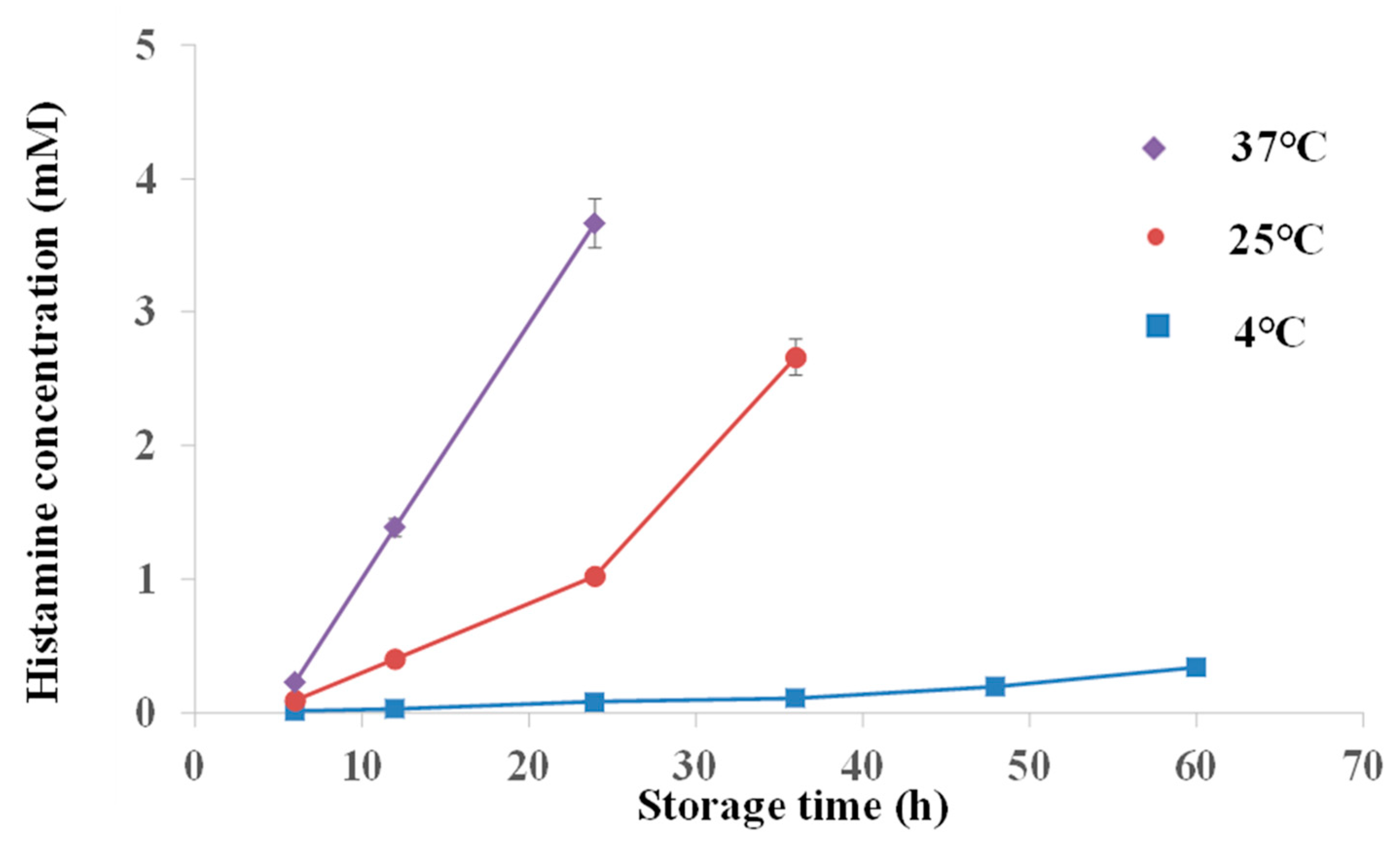
2.4. Histamine Detection in Mackerel Fish
3. Materials and Methods
3.1. Materials
3.2. CDs Conjugated on Nanoporous Alumina Membranes
3.3. Fe3O4@Au Magnetic Nanocomposites Preparation and Functionalization
3.4. Characterization
3.5. Histamine Extraction from Mackerel Fish
3.6. Histamine Concentration and Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peng, J.; Fang, K.; Xie, D.; Ding, B.; Yin, J.; Cui, X.; Zhang, Y.; Liu, J. Development of an automated on-line-pre-column derivatization procedure for sensitive determination of histamine in food with high-performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2008, 1209, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, Y.; Pan, M.; Kong, L.; Wang, S. Development and application of quartz crystal microbalance sensor based on novel molecularly imprinted sol-gel polymer for rapid detection of histamine in foods. J. Agric. Food Chem. 2014, 62, 5269–5274. [Google Scholar] [CrossRef] [PubMed]
- Keow, C.M.; Bakar, F.A.; Salleh, A.B.; Heng, L.Y.; Wagiran, R.; Bean, L.S. An amperometric biosensor for the rapid assessment of histamine level in tiger prawn (Penaeus monodon) spoilage. Food Chem. 2007, 105, 1636–1641. [Google Scholar] [CrossRef]
- Hungerford, J.M.; Lewis, R.J.; Poli, M. Scombroid poisoning: a review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Janči, T.; Valinger, D.; Gajdoš, K.J.; Mikac, L.; Vidaček, S.; Ivanda, M. Determination of histamine in fish by Surface Enhanced Raman Spectroscopy using silver colloid SERS substrates. Food Chem. 2017, 224, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, J.; Busch, I.; Brückner, H. Determination of biogenic amines in food by automated pre-column derivatization with 2-naphthyloxycarbonyl chloride (NOC-CI). Chromatographia 1997, 45, 263–268. [Google Scholar] [CrossRef]
- Marcobal, A.; Polo, M.C.; MartiN-Álvarez, P.J.; Moreno-Arribas, M.V. Biogenic amine content of red Spanish wines: comparison of a direct ELISA and an HPLC method for the determination of histamine in wines. Food Res. Int. 2005, 38, 387–394. [Google Scholar] [CrossRef]
- Vitali, L.; Valese, A.C.; Azevedo, M.S.; Gonzaga, L.V.; Costa, A.C.O.; Piovezan, M.; Vistuba, J.P.; Micke, G.A. Development of a fast and selective separation method to determine histamine in tuna fish samples using capillary zone electrophoresis. Talanta 2013, 106, 181–185. [Google Scholar] [CrossRef]
- Ali, M.; Ramirez, P.; Duznovic, I.; Nasir, S.; Mafe, S.; Ensinger, W. Label-free histamine detection with nanofluidic diodes through metal ion displacement mechanism. Colloids Surf. B. 2017, 150, 201–208. [Google Scholar] [CrossRef]
- Ausländer, D.; Eggerschwiler, B.; Kemmer, C.; Geering, B.; Ausländer, S.; Fussenegger, M. A designer cell-based histamine-specific human allergy profiler. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Tsang, M.K.; Ye, W.W.; Wang, G.; Li, J.M.; Yang, M.; Hao, J.H. Ultrasensitive detection of Ebola virus oligonucleotide based on upconversion nanoprobe/nanoporous membrane system. ACS Nano 2016, 10, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yeh, H.C.; Kuroki, M.T.; Wang, T.H. Single-quantum-dot-based DNA nanosensor. Nat.Mater. 2005, 4, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.W.; Tsang, M.K.; Liu, X.; Yang, M.; Hao, J.H. Upconversion luminescence resonance energy transfer (LRET)-based biosensor for rapid and ultrasensitive detection of avian influenza virus H7 subtype. Small 2014, 10, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 125, 4045–4049. [Google Scholar] [CrossRef]
- Wei, W.; Xu, C.; Ren, J.; Xu, B.; Qu, X. Sensing metal ions with ion selectivity of a crown ether and fluorescence resonance energy transfer between carbon dots and graphene. Chem. Commun. 2012, 48, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonance energy transfer analysis: beyond traditional donor–acceptor combinations. Angew. Chem. Int. Ed. 2006, 45, 4562–4589. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Zhu, J.; Zhang, L.; Lin, H. A FRET-based carbon dot-MnO2 nanosheet architecture for glutathione sensing in human whole blood samples. Chem. Commun. 2015, 51, 12748–12751. [Google Scholar] [CrossRef] [PubMed]
- Basozabal, I.; Guerreiro, A.; Gomezcaballero, A.; Aranzazu, G.M.; Barrio, R.J. Direct potentiometric quantification of histamine using solid-phase imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2014, 58, 138–144. [Google Scholar] [CrossRef]
- Henao-Escobar, W.; Román, T.D.; Domínguez-Renedo, O.; Alonso-Lomillo, M.A.; Arcos-Martínez, M.J. Dual enzymatic biosensor for simultaneous amperometric determination of histamine and putrescine. Food Chem. 2016, 190, 818–823. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetische nanopartikel: synthese, stabilisierung, funktionalisierung und anwendung. Angew. Chem. Int. Ed. 2007, 119, 1242–1266. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, supramolecular chemistry, quantum-Size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Sung, Y.J.; Suk, H.J.; Sung, H.Y.; Li, T.; Poo, H.; Kim, M.G. Novel antibody/gold nanoparticle/magnetic nanoparticle nanocomposites for immunomagnetic separation and rapid colorimetric detection of Staphylococcus aureus, in milk. Biosens. Bioelectron. 2013, 43, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Y.; Zhen, R.; Wang, B.; Feng, Y.J.; Chen, P.; Hao, J. Fe3O4/Au core/shell nanoparticles modified with Ni2+−nitrilotriacetic acid specific to histidine-tagged proteins. J. Phys. Chem. C 2010, 114, 4825–4830. [Google Scholar] [CrossRef]
- Yu, H.; Min, C.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Nurmikko, A.V.; Sun, S. Enhanced magnetooptical response in dumbbell-like Ag-CoFe2O4 nanoparticle pairs. Nano Lett. 2005, 5, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Uddin, I.; Poddar, P.; Phogat, N. Novel green hemoglobin-mediated biosynthesis of gold nanoparticles. Mater. Focus 2013, 2, 80–85. [Google Scholar] [CrossRef]
- Duan, S.; Xu, X.; Liu, X.; Wang, Y.; Hayat, T.; Alsaedi, A.; Meng, Y.; Li, J. Highly enhanced adsorption performance of U(VI) by non-thermal plasma modified magnetic Fe3O4 nanoparticles. J. Colloid Interface Sci. 2018, 513, 92–103. [Google Scholar] [CrossRef]
- Prasad, C.; Sreenivasulu, K.; Gangadhara, S.; Venkateswarlu, P. Bio inspired green synthesis of Ni/Fe3O4 magnetic nanoparticles using Moringa oleiferaleaves extract: A magnetically recoverable catalyst for organic dye degradation in aqueous solution. J. Alloy. Compd. 2017, 700, 252–258. [Google Scholar] [CrossRef]
- Luo, S.; Liu, Y.; Rao, H.; Wang, Y.; Wang, X. Fluorescence and magnetic nanocomposite Fe3O4@SiO2@Au MNPs as peroxidase mimetics for glucose detection. Anal. Biochem. 2017, 538, 26–33. [Google Scholar] [CrossRef]
- Mohammadi, S.; Salimi, A.; Hamd-Ghadareha, S.; Fathi, F.; Soleimani, F. A FRET immunosensor for sensitive detection of CA 15-3 tumor marker in human serum sample and breast cancer cells using antibody functionalized luminescent carbon-dots and AuNPs-dendrimer aptamer as donor-acceptor pair. Anal. Biochem. 2018, 557, 18–26. [Google Scholar] [CrossRef]
- Mukherjee, V.; Yadav, T. Conformational study of neutral histamine monomer and their vibrational spectra. Spectrochim. Acta A 2016, 165, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.; Srivastava, A.K.; Bajpai, A. Vibrational spectra, HOMO, LUMO, MESP surfaces and reactivity descriptors of amylamine and its isomers: A DFT study. Spectrochim. Acta A 2015, 149, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.Q.; Zhao, F.L.; Yin, B.C.; Ye, B.C. A novel, colorimetric method for biogenic amine detection based on arylalkylamine N-acetyltransferase. Chem. Commun. 2015, 51, 8712–8714. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.X.; Yang, J.Y.; Luo, L.; Zhang, Y.F.; Mao, C.B.; Sun, Y.M.; Lei, H.T.; Shen, Y.D.; Beier, R.C.; Xu, Z.L. Portable amperometric immunosensor for histamine detection using Prussian blue-chitosan-gold nanoparticle nanocomposite films. Biosens. Bioelectron. 2017, 98, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Peng, Y.; Ning, B.; Bai, J.; Liu, Y.; Zhang, N.; Gao, Z. Surface plasmon resonance sensor based on molecularly imprinted polymer film for detection of histamine. Sens. Actuators B Chem. 2015, 221, 15–21. [Google Scholar] [CrossRef]
- Chan, K.Y.; Ye, W.W.; Zhang, Y.; Xiao, L.D.; Leung, P.H.; Li, Y.; Yang, M. Ultrasensitive detection of E. coli O157:H7 with biofunctional magnetic bead concentration via nanoporous membrane based electrochemical immunosensor. Biosens.Bioelectron. 2013, 41, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.W.; Shi, J.Y.; Chan, C.Y.; Zhang, Y.; Yang, M. A nanoporous membrane based impedance sensing platform for DNA sensing with gold nanoparticle amplification. Sens. Actuators B Chem. 2014, 193, 877–882. [Google Scholar] [CrossRef]
- Ye, W.W.; Chen, T.; Mao, Y.J.; Tian, F.; Sun, P.L.; Yang, M. The effect of pore size in an ultrasensitive DNA sandwich-hybridization assay for the Escherichia coli, O157:H7 gene based on the use of a nanoporous alumina membrane. Microchim. Acta 2017, 184, 4835–4844. [Google Scholar] [CrossRef]
- Fan, H.L.; Li, L.; Zhou, S.F.; Liu, Y.Z. Continuous preparation of Fe3O4, nanoparticles combined with surface modification by l-cysteine and their application in heavy metal adsorption. Ceram. Int. 2016, 42, 4228–4237. [Google Scholar] [CrossRef]
- Ye, W.W.; Xu, Y.F.; Zheng, L.H.; Zhang, Y.; Yang, M.; Sun, P.L. A Nanoporous alumina membrane based electrochemical biosensor for histamine determination with biofunctionalized magnetic nanoparticles concentration and signal amplification. Sensors 2016, 16, 1767. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds carbon dots modified nanoporous membranes are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Zhang, Y.; Hu, W.; Ye, W. Carbon Dots-Modified Nanoporous Membrane and Fe3O4@Au Magnet Nanocomposites-Based FRET Assay for Ultrasensitive Histamine Detection. Molecules 2019, 24, 3039. https://doi.org/10.3390/molecules24173039
Mao Y, Zhang Y, Hu W, Ye W. Carbon Dots-Modified Nanoporous Membrane and Fe3O4@Au Magnet Nanocomposites-Based FRET Assay for Ultrasensitive Histamine Detection. Molecules. 2019; 24(17):3039. https://doi.org/10.3390/molecules24173039
Chicago/Turabian StyleMao, Yijie, Yu Zhang, Wei Hu, and Weiwei Ye. 2019. "Carbon Dots-Modified Nanoporous Membrane and Fe3O4@Au Magnet Nanocomposites-Based FRET Assay for Ultrasensitive Histamine Detection" Molecules 24, no. 17: 3039. https://doi.org/10.3390/molecules24173039
APA StyleMao, Y., Zhang, Y., Hu, W., & Ye, W. (2019). Carbon Dots-Modified Nanoporous Membrane and Fe3O4@Au Magnet Nanocomposites-Based FRET Assay for Ultrasensitive Histamine Detection. Molecules, 24(17), 3039. https://doi.org/10.3390/molecules24173039



