Determination of Flavonoid Glycosides by UPLC-MS to Authenticate Commercial Lemonade
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Flavonoid Glycosides by UPLC-TOF/MS
2.2. The Selection of VA-DLLME Conditions
2.3. Determination of Flavonoid Glycosides by UPLC–QqQ/MS
2.4. Speculation on the Possible Counterfeiting Means of Lemonade
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Standard Solution
3.3. Sample Preparation by the VA-DLLME Procedure
3.4. UPLC–MS Analysis
3.4.1. Identification of Flavonoid Glycosides by UPLC-TOF/MS
3.4.2. Determination of Flavonoid Glycosides by UPLC-QqQ/MS
3.5. Analytical Figures of Merit
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q.V. Impact of different solvents on the recovery of bioactive compounds and antioxidant properties from lemon (Citrus limon L.) pomace waste. Food Sci. Biotechnol. 2016, 25, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Lemon (Citrus lemon L. Burm) as a source of unique bioactive compounds. Acta Hortic. 2014, 1040, 377–380. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Buren, L.v.; Wagner, E.; Wiseman, S.; Put, F.v.d.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Del Rı́o, J.A.; Fuster, M.D.; Gómez, P.; Porras, I.; Garcı́a-Lidón, A.; Ortuño, A. Citrus limon: A source of flavonoids of pharmaceutical interest. Food Chem. 2004, 84, 457–461. [Google Scholar] [CrossRef]
- Abad-García, B.; Garmón-Lobato, S.; Sánchez-Ilárduya, M.B.; Berrueta, L.A.; Gallo, B.; Vicente, F.; Alonso-Salces, R.M. Polyphenolic contents in Citrus fruit juices: Authenticity assessment. Eur. Food Res. Technol. 2014, 238, 803–818. [Google Scholar] [CrossRef]
- Abad-García, B.; Berrueta, L.A.; Garmón-Lobato, S.; Urkaregi, A.; Gallo, B.; Vicente, F. Chemometric characterization of fruit juices from spanish cultivars according to their phenolic compound contents: I. Citrus fruits. J. Agric. Food Chem. 2012, 60, 3635–3644. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.X.; Jia, H.F.; Wang, X. The research on detection techniques of adulteration about fruit juice in China. Food Res. Dev. 2016, 37, 205–208. [Google Scholar]
- National Standards of China. General Analytical Methods for Beverage; National Standards of China: GB/T 12143-2008; Standards Press of China: Beijin, China, 2009.
- Salazar, M.O.; Pisano, P.L.; González Sierra, M.; Furlan, R.L.E. NMR and multivariate data analysis to assess traceability of argentine citrus. Microchem. J. 2018, 141, 264–270. [Google Scholar] [CrossRef]
- Guyon, F.; Auberger, P.; Gaillard, L.; Loublanches, C.; Viateau, M.; Sabathié, N.; Salagoïty, M.H.; Médina, B. 13C/12C isotope ratios of organic acids, glucose and fructose determined by HPLC-co-IRMS for lemon juices authenticity. Food Chem. 2014, 146, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Desiderio, C.; De Rossi, A.; Sinibaldi, M. Analysis of flavanone-7-O-glycosides in citrus juices by short-end capillary electrochromatography. J. Chromatogr. A 2005, 1081, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Schreiber, A.; Lacina, O.; Cajka, T.; Hajslova, J. Liquid chromatography–mass spectrometry-based metabolomics for authenticity assessment of fruit juices. Metabolomics 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Qing, L.S.; Xiong, J.; Xue, Y.; Liu, Y.M.; Guang, B.; Ding, L.S.; Liao, X. Using baicalin-functionalized magnetic nanoparticles for selectively extracting flavonoids from Rosa chinensis. J. Sep. Sci. 2011, 34, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.S.; Xue, Y.; Liu, Y.M.; Liang, J.; Xie, J.; Liao, X. Rapid magnetic solid-phase extraction for the selective determination of isoflavones in soymilk using baicalin-functionalized magnetic nanoparticles. J. Agric. Food Chem. 2013, 61, 8072–8078. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.S.; Xue, Y.; Zhang, J.G.; Zhang, Z.F.; Liang, J.; Jiang, Y.; Liu, Y.M.; Liao, X. Identification of flavonoid glycosides in Rosa chinensis flowers by liquid chromatography–tandem mass spectrometry in combination with 13C nuclear magnetic resonance. J. Chromatogr. A 2012, 1249, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review. part II. TrAC Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and opportunities of experimental design for the dispersive liquid–liquid microextraction method—A review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Alhooshani, K. Dispersive liquid-liquid microextraction based binary extraction techniques prior to chromatographic analysis: A review. TrAC Trends Anal. Chem. 2018, 108, 167–182. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Homem, V.; Alves, A.; Alves, A.; Santos, L. Ultrasound-assisted dispersive liquid–liquid microextraction for the determination of synthetic musk fragrances in aqueous matrices by gas chromatography–mass spectrometry. Talanta 2016, 148, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xu, X.S.; Yong, L.; Hu, B.; Li, X.D.; Zhong, S.H.; Li, Y.; Xie, J.; Qing, L.S. Optimization of vortex-assisted dispersive liquid-liquid microextraction for the simultaneous quantitation of eleven non-anthocyanin polyphenols in commercial blueberry using the multi-objective response surface methodology and desirability function approach. Molecules 2018, 23, 2921. [Google Scholar]
- Li, G.; Row, K.H. Air assisted dispersive liquid–liquid microextraction (AA-DLLME) using hydrophilic–hydrophobic deep eutectic solvents for the isolation of monosaccharides and amino acids from kelp. Anal. Lett. 2019, 1–15. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Mohtadinia, J.; Mousavi, M.M.; Ansarin, M.; Nemati, M. Application of the microwave-assisted extraction and dispersive liquid–liquid microextraction for the analysis of PAHs in smoked rice. Food Anal. Methods 2017, 10, 277–286. [Google Scholar] [CrossRef]
- Chen, C.; Xue, Y.; Li, Q.M.; Wu, Y.; Liang, J.; Qing, L.S. Neutral loss scan - based strategy for integrated identification of amorfrutin derivatives, new peroxisome proliferator-activated receptor gamma agonists, from Amorpha Fruticosa by UPLC-QqQ-MS/MS and UPLC-Q-TOF-MS. J. Am. Soc. Mass Sp. 2018, 29, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.L. Accurate masses for structure confirmation. J. Am. Soc. Mass Sp. 1994, 5, 57. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Liang, J.; Luo, P.; Qing, L.S.; Ding, L.S. Determination of contents of catechins in oolong teas by quantitative analysis of multi-components via a single marker (QAMS) method. Food Anal. Methods 2017, 10, 363–368. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Moghaddam, G.; Mousavi, S.; Sadeghi, N.; Oveisi, M.; Jannat, B. Total antioxidant activity, and hesperidin, diosmin, eriocitrin and quercetin contents of various lemon juices. Trop. J. Pharm. Res. 2014, 13, 951–956. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, L.Y.; Wang, J.R.; Ding, G.; Xu, J. Study on quality evaluation of Flos Sophorae Immaturus. Nat. Prod. Res. Dev. 2018, 30, 138–146+174. [Google Scholar]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.I.; Gabrieli, C.; Kokkalou, E.; Georgarakis, M.; Niopas, I. Simultaneous reversed-phase high-performance liquid chromatographic method for the determination of diosmin, hesperidin and naringin in different citrus fruit juices and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2003, 33, 243–249. [Google Scholar] [CrossRef]
- Belajová, E.; Suhaj, M. Determination of phenolic constituents in citrus juices: Method of high performance liquid chromatography. Food Chem. 2004, 86, 339–343. [Google Scholar] [CrossRef]
- Tu, X.; Yang, S.; Wu, Z.; Wu, C.; Zhang, L.; Lü, X. Simultaneous determination of eleven flavonoids in different lemon (Citrus limon) varieties by HPLC. J. Hunan Agric. Univ. 2016, 42, 543–548. [Google Scholar]
- Zheng, J.; Zhao, Q.Y.; Zhang, Y.H.; Jiao, B.N. Simultaneous determination of main flavonoids and phenolic acids in citrus fruit by ultra performance liquid chromatography. Sci. Agric. Sin. 2014, 47, 4706–4717. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
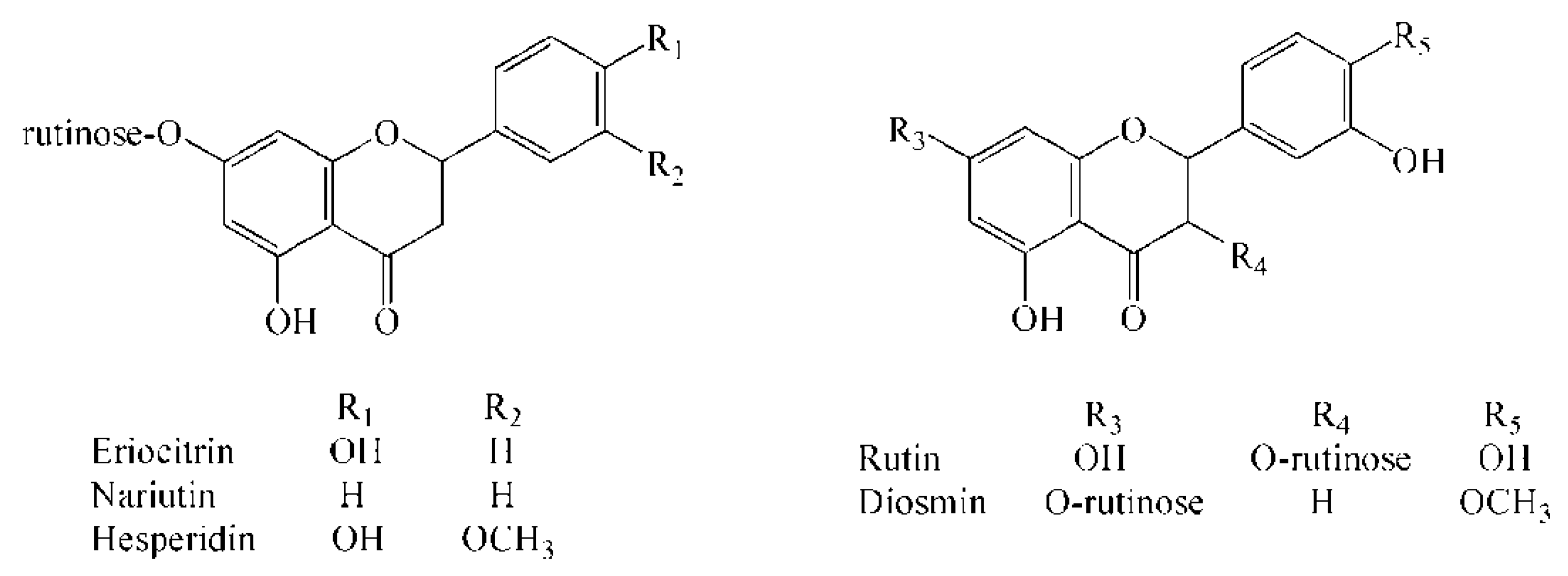
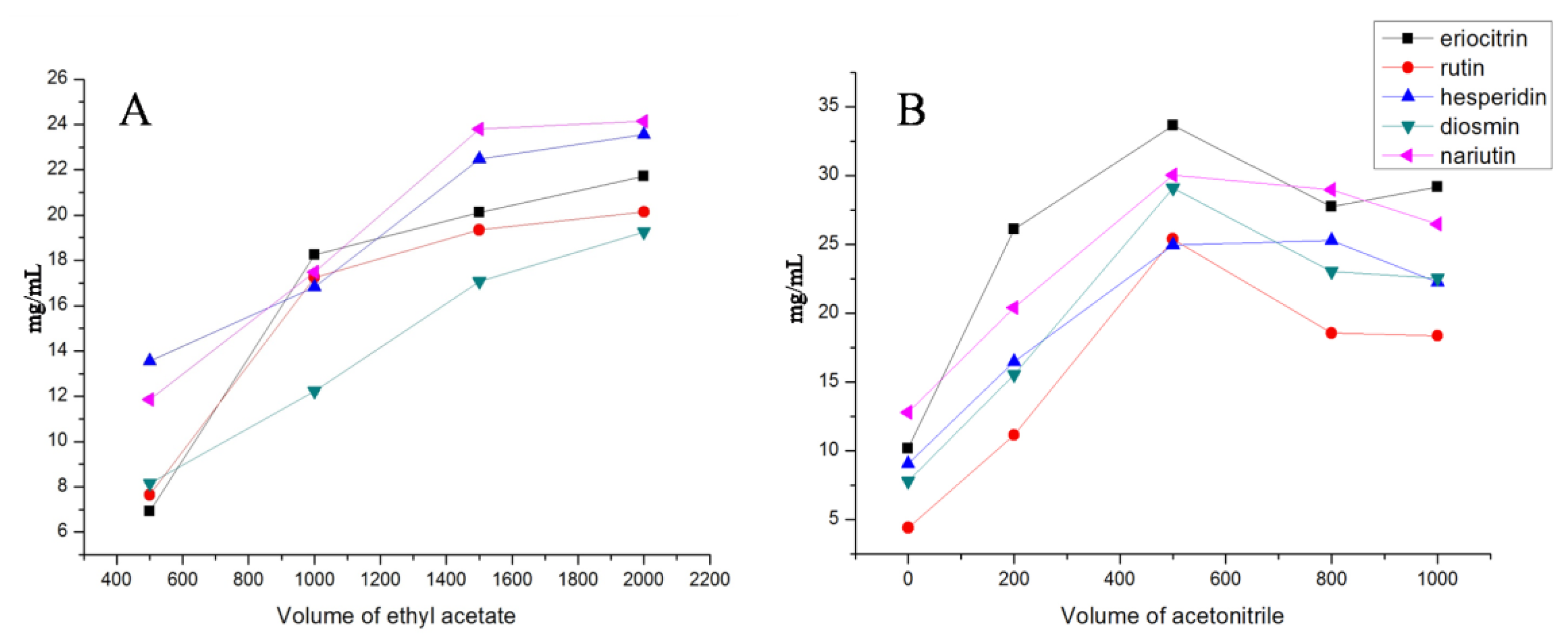
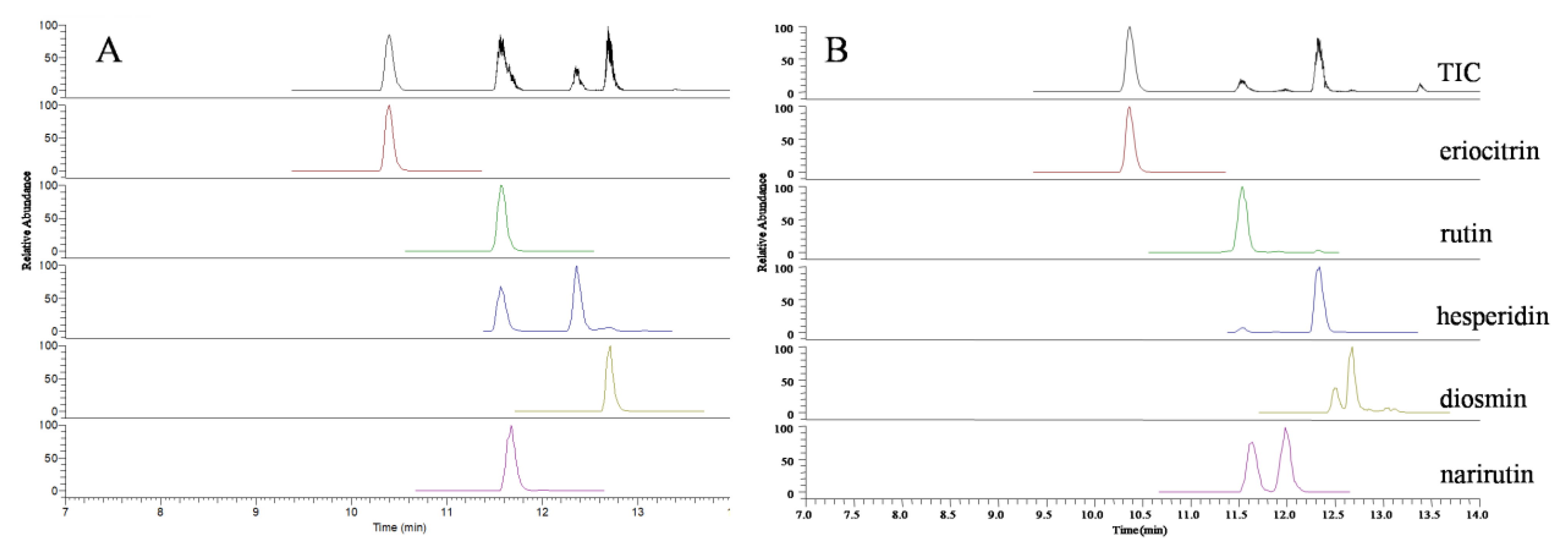
| Analyte | TOF/MS | QqQ/MS | |||
|---|---|---|---|---|---|
| Quasi-Molecular Ion (m/z) | Error (ppm) | Product Ion (m/z) | Parent Ion (m/z) | Product Ion (m/z) | |
| eriocitrin | 595.16788 | 1.7 | 287.0586, 151.0065 | 595 | 287 *, 151 # |
| narirutin | 579.17238 | 0.8 | 271.0612 | 579 | 271 *, 151 # |
| hesperidin | 609.18386 | 2.2 | 301.0737 | 609 | 301 *, 286 # |
| rutin | 609.14689 | 1.3 | 301.0383, 300.0281 | 609 | 300 *, 271 # |
| diosmin | 607.16784 | 1.0 | 299.0582, 284.0345 | 607 | 299 *, 284 # |
| Sample No. | Eriocitrin | Rutin | Hesperidin | Diosmin | Narirutin | Total |
|---|---|---|---|---|---|---|
| S1 | 0.04 | 0.27 | 1.00 | nd | nd | 1.31 |
| S2 | 0.92 | 0.34 | 3.82 | 0.66 | nd | 5.75 |
| S3 | 1.73 | 0.29 | 16.33 | 2.95 | nd | 21.30 |
| S4 | 28.96 | 6.01 | 28.30 | 0.28 | 0.74 | 49.07 |
| S5 | 2.66 | 191.54 | nd | 0.64 | nd | 194.83 |
| S6 | 0.04 | 243.71 | nd | nd | nd | 243.75 |
| S7 | 0.05 | 264.24 | nd | nd | nd | 264.29 |
| S8 | 0.35 | 470.00 | nd | nd | nd | 470.35 |
| Analyte | Regression Equation | Linear Range (ng/mL) | LOD | LOQ | Precision (RSD, n = 6) | Repeatability (n = 6) | Recovery (n = 6) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (y = ax + b, r2) | (ng/mL) | (ng/mL) | Intra-Day | Inter-Day | Mean (µg/100 mL) | RSD | Mean | RSD | ||
| eriocitrin | y = 523.81x − 82.61, 0.996 | 2.01–50.3 | 0.70 | 2.01 | 1.36% | 3.53% | 28.9 | 3.22% | 88.5% | 3.93% |
| rutin | y = 1034.77x − 1160.22, 0.996 | 2.44–60.9 | 0.81 | 2.44 | 2.51% | 3.79% | 6.01 | 4.62% | 89.9% | 4.51% |
| hesperidin | y = 513.03x + 252.28, 0.998 | 2.01–50.4 | 0.70 | 2.10 | 1.97% | 2.58% | 28.3 | 3.47% | 88.7% | 5.30% |
| diosmin | y = 769.76x + 84.74, 0.995 | 2.14–53.4 | 0.71 | 2.14 | 2.02% | 3.17% | 0.28 | 5.47% | 102% | 2.61% |
| narirutin | y = 556.25x + 148.69, 0.997 | 2.02–51.2 | 0.70 | 2.02 | 1.48% | 3.69% | 0.74 | 4.86% | 92.8% | 4.47% |
| Method | Analyte | Linea Range | LOD | LOQ | Recovery |
|---|---|---|---|---|---|
| CEC [13] | eriocitrin, narirutin, hesperidin | 5–200 μg/mL | 2.5 μg/mL | 5 μg/mL | 71–112% |
| HPLC/UV [33] | narirutin, hesperidin, diosmin | 0.25–20 μg/mL | - | 0.1 μg/mL | - |
| HPLC/UV [34] | narirutin | 2–50 mg/L | 1.25 mg/L | 2.5 mg/L | 83% |
| hesperidin | 2–50 mg/L | 1.0 mg/L | 2.5 mg/L | 74% | |
| HPLC/UV [35] | eriocitrin | 1.01–50.50 μg/mL | 0.02 μg/mL | 0.065 μg/mL | 103.10% |
| narirutin | 0.505–10.10 μg/mL | 0.024 μg/mL | 0.18 μg/mL | 99.14% | |
| hesperidin | 5.00–100.00 μg/mL | 0.04 μg/mL | 0.132 μg/mL | 99% | |
| rutin | 0.101–10.100 μg/mL | 0.079 μg/mL | 0.263 μg/mL | 98.37% | |
| UPLC/UV [36] | eriocitrin | 0.5–130 mg/L | 6 μg/kg | - | 90.50% |
| narirutin | 0.05–300 mg/L | 5 μg/kg | - | 87.40% | |
| hesperidin | 0.05–500 mg/L | 8 μg/kg | - | 92.70% | |
| rutin | 0.05–310 mg/L | 5 μg/kg | - | 88.40% | |
| diosmin | 0.01–200 mg/L | 8 μg/kg | - | 100.80% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Qing, L.-S.; Yong, L.; Xu, X.-S.; Hu, B.; Tang, M.-Q.; Xie, J. Determination of Flavonoid Glycosides by UPLC-MS to Authenticate Commercial Lemonade. Molecules 2019, 24, 3016. https://doi.org/10.3390/molecules24163016
Xue Y, Qing L-S, Yong L, Xu X-S, Hu B, Tang M-Q, Xie J. Determination of Flavonoid Glycosides by UPLC-MS to Authenticate Commercial Lemonade. Molecules. 2019; 24(16):3016. https://doi.org/10.3390/molecules24163016
Chicago/Turabian StyleXue, Ying, Lin-Sen Qing, Li Yong, Xian-Shun Xu, Bin Hu, Ming-Qing Tang, and Jing Xie. 2019. "Determination of Flavonoid Glycosides by UPLC-MS to Authenticate Commercial Lemonade" Molecules 24, no. 16: 3016. https://doi.org/10.3390/molecules24163016
APA StyleXue, Y., Qing, L.-S., Yong, L., Xu, X.-S., Hu, B., Tang, M.-Q., & Xie, J. (2019). Determination of Flavonoid Glycosides by UPLC-MS to Authenticate Commercial Lemonade. Molecules, 24(16), 3016. https://doi.org/10.3390/molecules24163016






