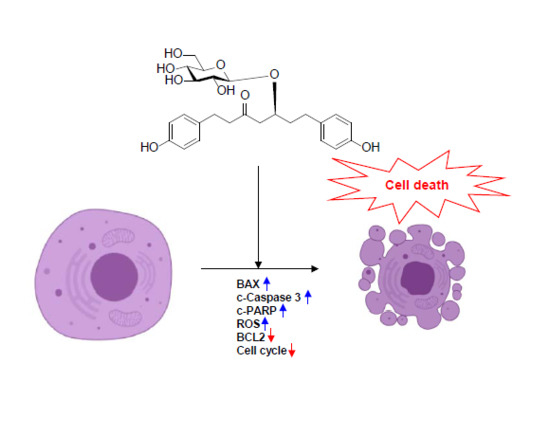
Platyphylloside Isolated from Betula platyphylla is Antiproliferative and Induces Apoptosis in Colon Cancer and Leukemic Cells
Abstract
:1. Introduction
2. Results and Discussion
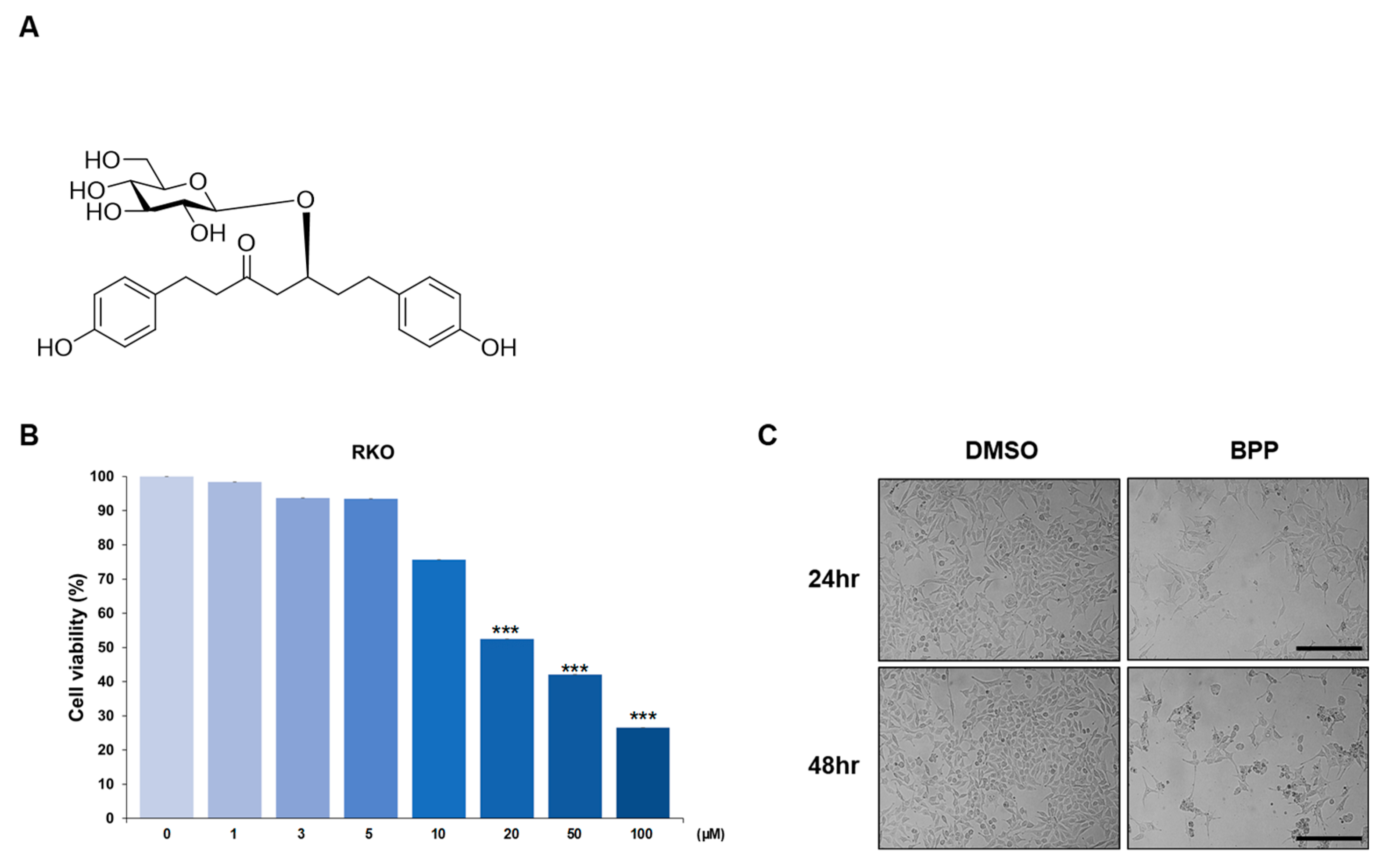
2.1. BPP-Induced Antiproliferative Effects in Colon Cancer Cells
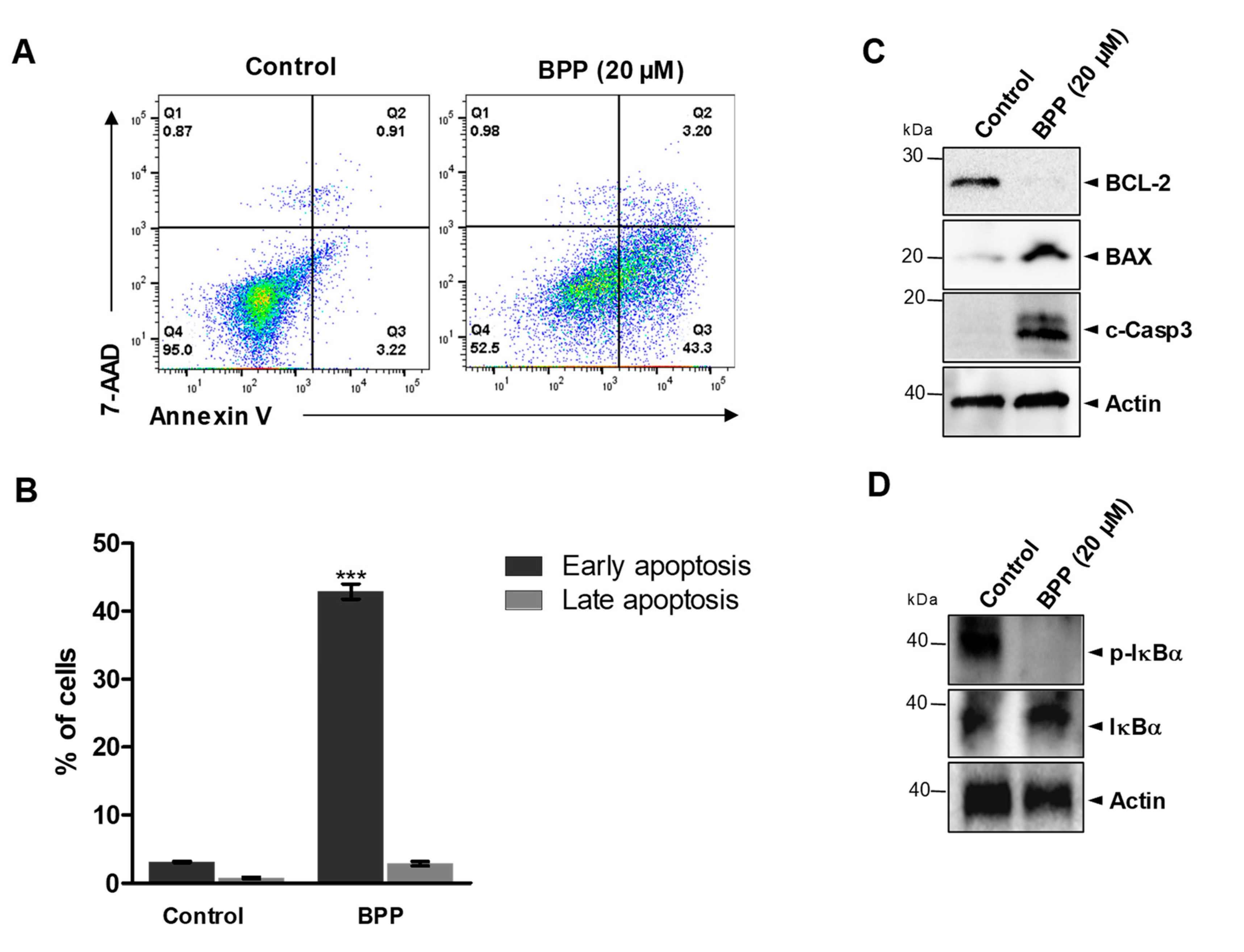
2.2. BPP-Induced Apoptosis in RKO Cells
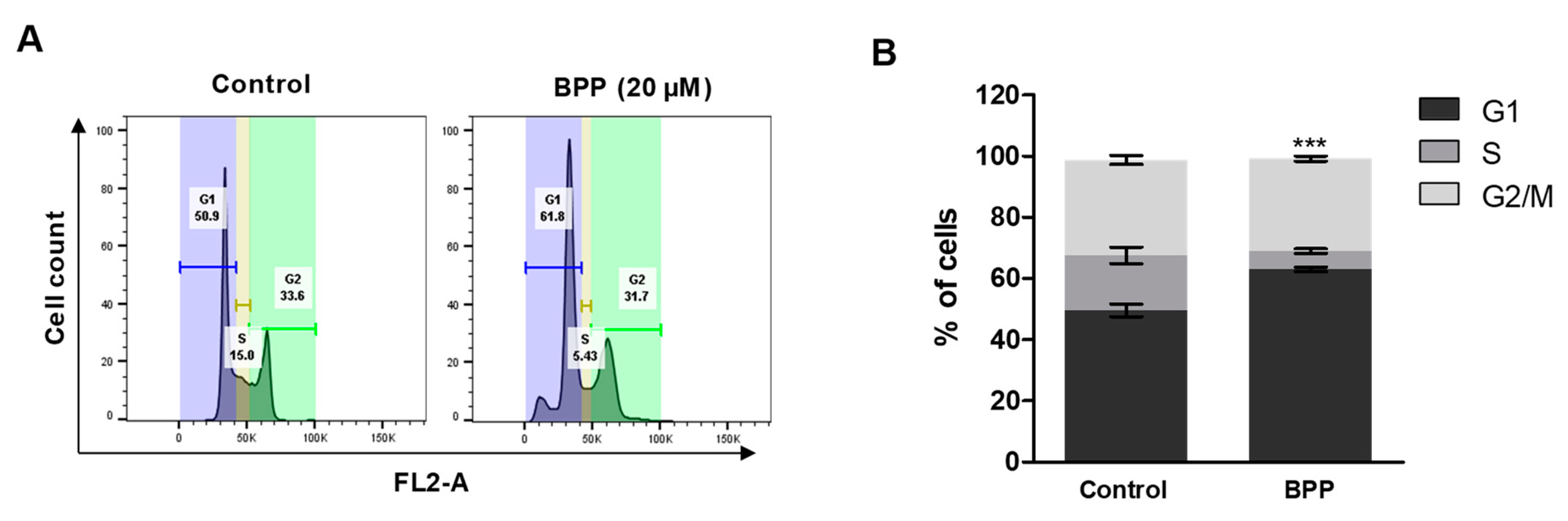
2.3. BPP-Induced Cell Cycle Arrest in RKO Cells
2.4. BPP-Induced Antiproliferative Effects in Leukemic Cells
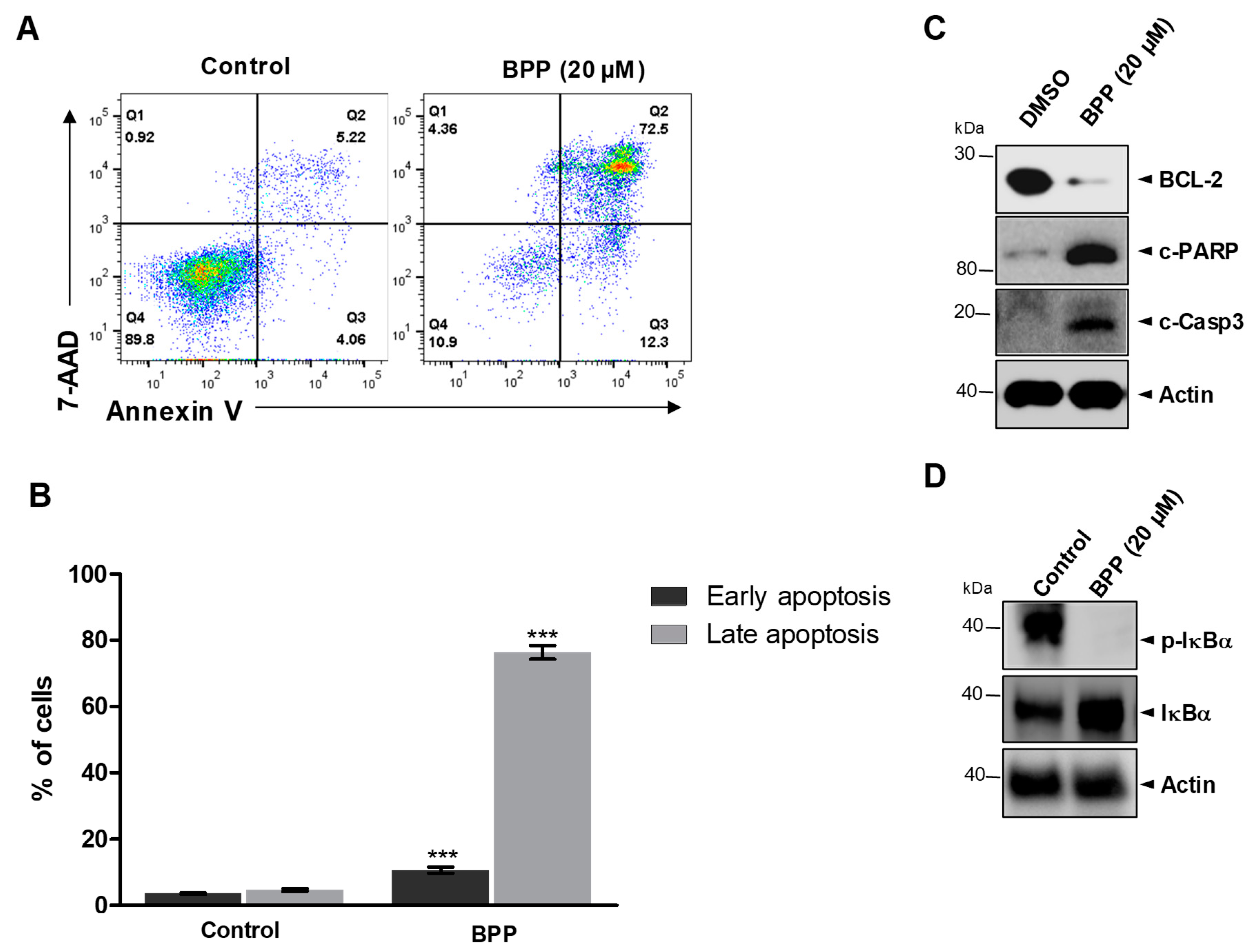
2.5. BPP-Induced Apoptosis in Jurkat Cells
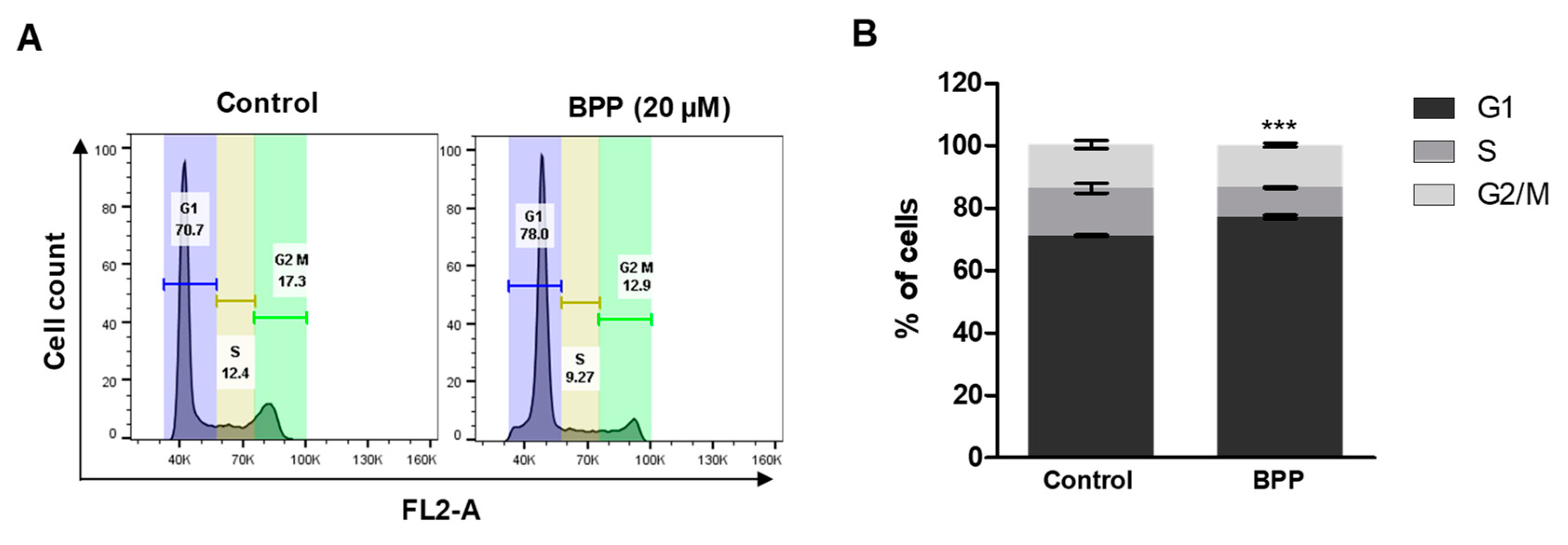
2.6. BPP-Induced Cell Cycle Arrest in Jurkat Cells
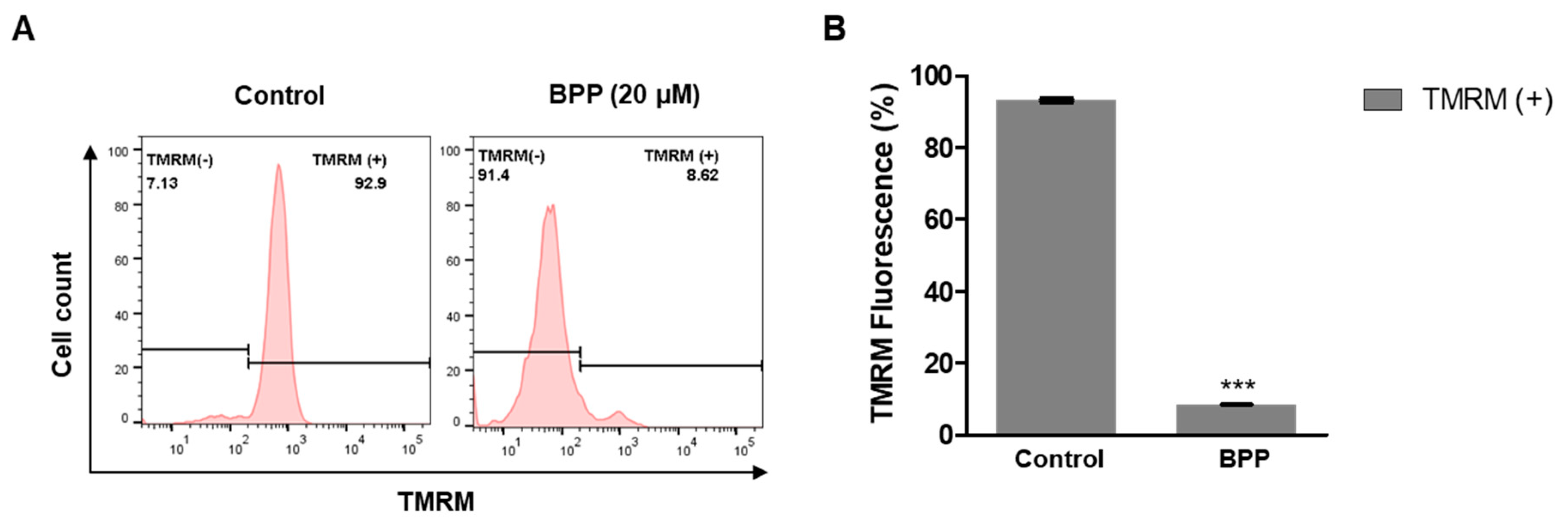
2.7. BPP Effects on Mitochondrial Membrane Potential in Apoptotic Cells
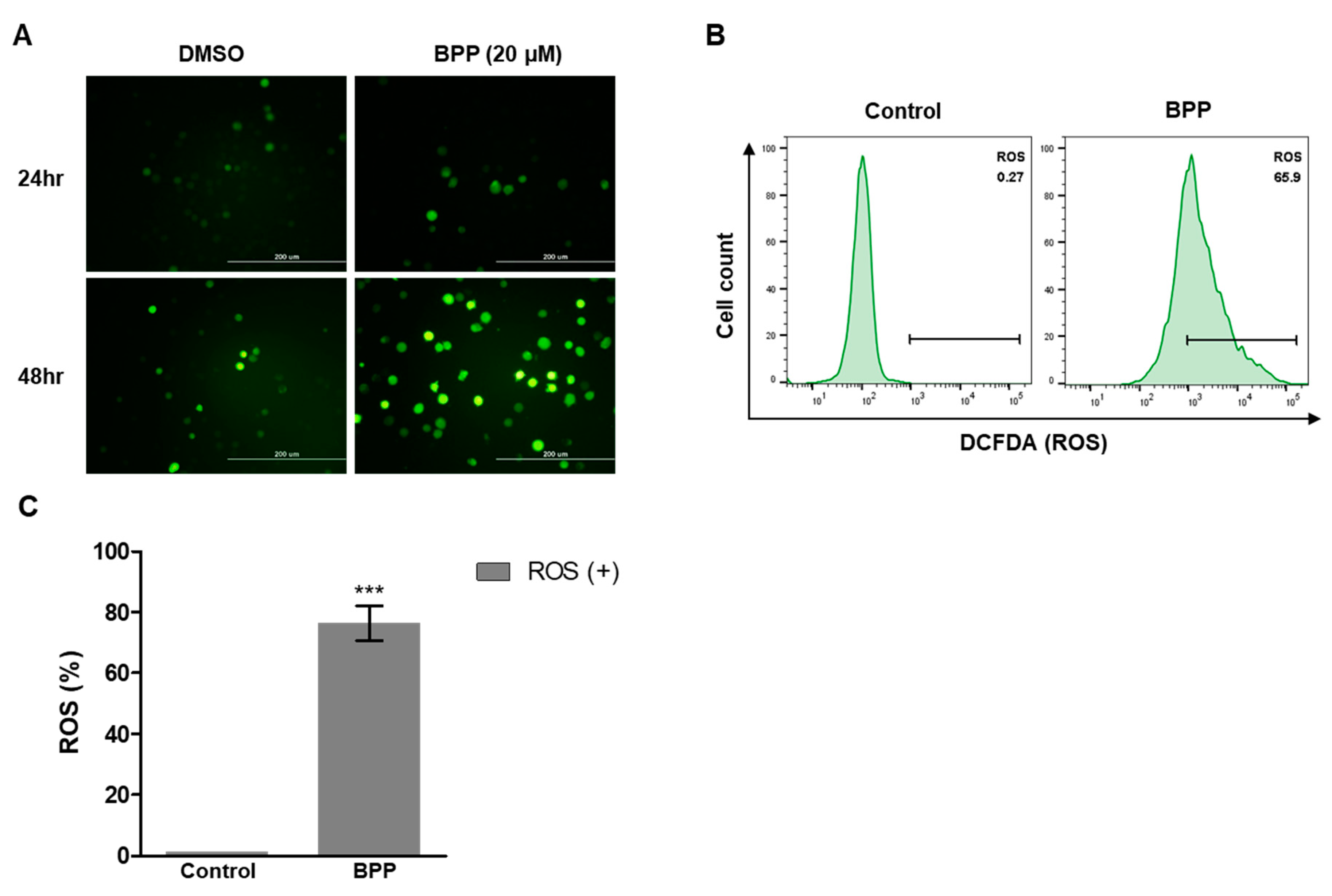
2.8. BPP-Induced ROS in Jurkat Cells
2.9. Live/Dead Assay in Jurkat Cells
3. Materials and Methods
3.1. Plant Material
3.2. Cell Culture
3.3. Evaluating BPP-Induced Antiproliferation of Colon Cancer and Leukemic Cells
3.4. Microscopy
3.5. Cytotoxicity Assay
3.6. Determination of BPP-Induced Apoptosis
3.7. Western Blot
3.8. Tetramethylrhodamine Methyl Ester Perchlorate (TMRM) Assay
3.9. Evaluating BPP-Induced Changes in Cell Cycle Progression
3.10. Measuring Reactive Oxygen Species (ROS)
3.11. Live/Dead Fluorescence Microscopy Assay
3.12. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Van De Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res. Treat. 2018, 50, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Satoh, M.; Kinouchi, M.; Yamamoto, K.; Hasegawa, Y.; Kakugawa, Y.; Kawai, M.; Uchimi, K.; Aizawa, H.; Ohnuma, S.; et al. The use of natural products in colorectal cancer drug discovery. Expert Opin. Drug Discov. 2015, 10, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin nanoformulations for colorectal cancer: A Review. Front. Pharmacol. 2019, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Leukaemia “firsts” in cancer research and treatment. Nat. Rev. Cancer 2016, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Kim, M.; Park, H.; Jeong, M.I.; Jung, W.; Kim, B. Natural Products and Acute Myeloid Leukemia: A Review Highlighting Mechanisms of Action. Nutrients 2019, 11, 1010. [Google Scholar] [CrossRef]
- Taylor, W.F.; Moghadam, S.E.; Farimani, M.M.; Ebrahimi, S.N.; Tabefam, M.; Jabbarzadeh, E. A multi-targeting natural compound with growth inhibitory and anti-angiogenic properties re-sensitizes chemotherapy resistant cancer. PloS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Baek, Y.H.; Kim, Y.J.; Lee, J.D.; Choi, D.Y.; Park, D.S. Protective effects of butanol fraction from Betula platyphyla var. japonica on cartilage alterations in a rabbit collagenase-induced osteoarthritis. J. Ethnopharmacol. 2009, 123, 515–521. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Brand, S.; Hölscher, D.; Schierhorn, A.; Svatoš, A.; Schröder, J.; Schneider, B. A type III polyketide synthase from Wachendorfia thyrsiflora and its role in diarylheptanoid and phenylphenalenone biosynthesis. Planta 2006, 224, 413–428. [Google Scholar] [CrossRef]
- Rajaganapathy, B.R.; Thirugnanam, K.; Shanmuganathan, M.V.; Singaravelu, A.; Subadhra, L.B. Molecular basis of the anti-inflammatory potential of a diarylheptanoid in murine macrophage RAW 264.7 cells. Adv. Biol. Chem. 2013, 3, 541–548. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Şoica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.; Gǎluşcan, A.; Munteanu, M.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Cent. J. 2012, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Hong, J.M.; Baek, Y.H.; Lee, J.D.; Choi, D.Y.; Park, D.S. Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. J. Ethnopharmacol. 2011, 135, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ju, E.M.; Lee, S.E.; Hwang, H.J.; Kim, J.H. Antioxidant and anticancer activity of extract from Betula platyphylla var. japonica. Life Sci. 2004, 74, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Tamang, J.P.; Yu, C.Y.; Jung, S.J.; Chung, I.M. Antioxidant, antimicrobial activity and inhibition of α-glucosidase activity by Betula alnoides Buch. bark extract and their relationship with polyphenolic compounds concentration. Immunopharmacol. Immunotoxicol. 2012, 34, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, B.; Satishkumar, M.N.; Gupta, S.; Rawat, M.; Porwal, O.; Murugan, R. Hepatoprotective activity of Betula Utilis bark on D-galactosamine induced hepatic insult. World J. Pharm. Pharma. Sci. 2012, 1, 456–471. [Google Scholar]
- Germanò, M.P.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’Angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula Roth leaves: Gastroprotective effects of an HPLC-fingerprinted methanolic extract. Nat. Prod. Res. 2013, 27, 1569–1575. [Google Scholar] [CrossRef]
- Germanò, M.P.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula leaves: Polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia 2012, 83, 877–882. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, E.J.; Huh, J.; Cho, N.; Kim, T.B.; Jeon, B.J.; Kim, S.H.; Kim, H.P.; Sung, S.H. Cognition-enhancing and neuroprotective activities of the standardized extract of Betula platyphylla bark and its major diarylheptanoids. Phytomedicine 2012, 19, 1315–1320. [Google Scholar] [CrossRef]
- Cho, N.; Kim, H.Y.; Kim, T.B.; Ransom, T.T.; Beutler, J.A.; Sung, S.H. Preparative purification of anti-proliferative diarylheptanoids from Betula platyphylla by high-speed counter-current chromatography. Molecules 2016, 21, 700. [Google Scholar] [CrossRef]
- Moos, P.J.; Edes, K.; Mullally, J.E.; Fitzpatrick, F.A. Curcumin impairs tumor suppressor p53 function in colon cancer cells. Carcinogenesis 2004, 25, 1611–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uto, T.; Tung, N.H.; Appiah-Opong, R.; Aning, A.; Morinaga, O.; Edoh, D.; Nyarko, A.K.; Shoyama, Y. Antiproliferative and pro-apoptotic activity of diarylheptanoids isolated from the bark of Alnus japonica in human leukemia cell lines. Am. J. Chin. Med. 2015, 43, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Novaković, M.; Pešić, M.; Trifunović, S.; Vučković, I.; Todorović, N.; Podolski-Renić, A.; Dinić, J.; Stojković, S.; Tešević, V.; Vajs, V.; et al. Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochemistry 2014, 97, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. Inflammation: A driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.G.; Lee, S.Y.; Huang, Z.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.K.; Paul, M.; Paul, S. Curcumin induces caspase mediated apoptosis in JURKAT cells by disrupting the redox balance. Asian Pac. J. Cancer Prev. 2014, 15, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R.R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Kummrow, A.; Frankowski, M.; Bock, N.; Werner, C.; Dziekan, T.; Neukammer, J. Quantitative assessment of cell viability based on flow cytometry and microscopy. Cytometry A 2013, 83, 197–204. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Thuy, N.T.T.; Lee, J.; Cho, N.; Yoo, H.M. Platyphylloside Isolated from Betula platyphylla is Antiproliferative and Induces Apoptosis in Colon Cancer and Leukemic Cells. Molecules 2019, 24, 2960. https://doi.org/10.3390/molecules24162960
Lee J-E, Thuy NTT, Lee J, Cho N, Yoo HM. Platyphylloside Isolated from Betula platyphylla is Antiproliferative and Induces Apoptosis in Colon Cancer and Leukemic Cells. Molecules. 2019; 24(16):2960. https://doi.org/10.3390/molecules24162960
Chicago/Turabian StyleLee, Joo-Eun, Nguyen Thi Thanh Thuy, Jina Lee, Namki Cho, and Hee Min Yoo. 2019. "Platyphylloside Isolated from Betula platyphylla is Antiproliferative and Induces Apoptosis in Colon Cancer and Leukemic Cells" Molecules 24, no. 16: 2960. https://doi.org/10.3390/molecules24162960
APA StyleLee, J.-E., Thuy, N. T. T., Lee, J., Cho, N., & Yoo, H. M. (2019). Platyphylloside Isolated from Betula platyphylla is Antiproliferative and Induces Apoptosis in Colon Cancer and Leukemic Cells. Molecules, 24(16), 2960. https://doi.org/10.3390/molecules24162960