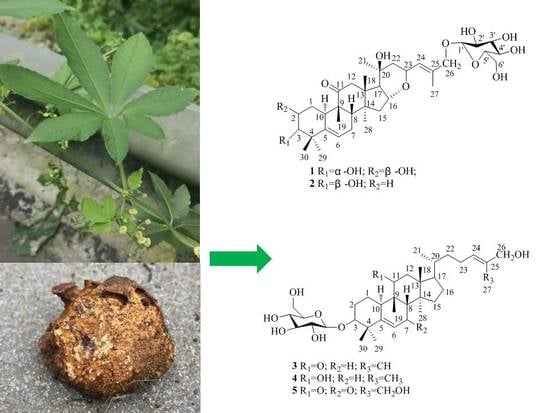
Five New Cucurbitane-Type Triterpenoid Glycosides from the Rhizomes of Hemsleya penxianensis with Cytotoxic Activities
Abstract
:1. Introduction
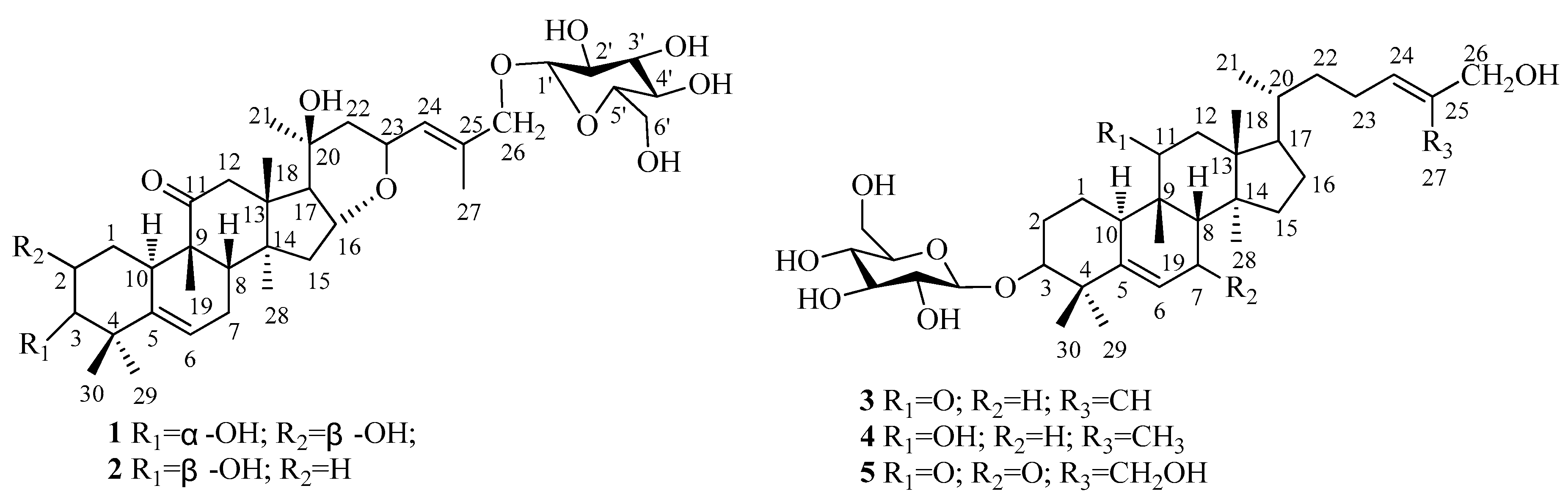
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cytotoxicity Assays
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| HR-ESI-MS | High resolution electrospray ionization mass spectroscopy |
| HMBC | Heteronuclear multiple bond correlation |
| HSQC | Heteronuclear single quantum correlation |
| COSY | Homonuclear chemical shift CorrelationSpectroscopy |
| NOESY | Nuclear overhauser effect spectroscopy |
| ODS | Octadecyl silane |
| HPLC | High performance liquid chromatography |
| MSO | Petroleum ether |
| CH2Cl2 | Dichloromethane |
| EtOAc | Ethyl acetate |
| nBuoH | n-Butanol |
| MeOH | Methanol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMEM | DuIbecco’s modified eagIe’s medium |
References
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1986; pp. 105–128. [Google Scholar]
- The Pharmacopoeia Commission of PRC. The Pharmacopoeia of People’s Republic of China; Part V; Chemical Industrial Publishing Press: Beijing, China, 2005; pp. 539–542. [Google Scholar]
- Yang, Q.F.; Wang, X.Y. The research progress of Traditional herb medicine Hemsleya pengxianensis W.J. Chang and its medicament. China Pharm. 2003, 9, 76–77. [Google Scholar]
- Chen, J.C.; Niu, X.M.; Li, Z.R.; Qiu, M.H. Four New Cucurbitane Glycosides from Hemsleya jinfushanensis. Planta Med. 2005, 71, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.Y.; Yi, Y.H.; Lin, X.W.; Xu, Q.Z.; Lv, T.S. Studies on the active antitumor constituents from Hemsleya sinesis. Acad. J. Sec. Mil. Med. Univ. 1999, 20, 337–339. [Google Scholar]
- Wu, J.; Wu, Y.J.; Yang, B.B. Anticancer activity of Hemsleya amabilis extract. Life Sci. 2002, 71, 2161–2170. [Google Scholar] [CrossRef]
- Boykin, C.; Zhang, G.; Chen, Y.H.; Zhang, R.W.; Fan, X.E.; Yang, W.M.; Lu, Q. Cucurbitacin IIa: A novel class of anti-cancer drug inducing nonreversible actin aggregation and inhibiting surviving independent of JAK2/STAT3 phosphorylation. Br. J. Cancer 2011, 104, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Chen, G.Y.; Liu, J.H.; Lei, M.; Meng, Y.H.; Guo, D.A.; Liu, X.; Hu, L.H. Cytotoxic cucurbitane triterpenoids isolated from the rhizomes of Hemsleya amabilis. Fitoterapia 2014, 94, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.X.; Zheng, Z.F.; Mu, Y.L.; Liu, Y.J.; Wang, H.Y.; Li, L.; Yao, Q.Q. Eight new cucurbitane triterpenoids from “Xue Dan”, the roots of Hemsleya pengxianensis. J. Asian Nat. Prod. Res. 2018, 20, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhou, Y.; Zhou, L.Y.; Kang, L.Y.; Wang, X.; Li, B.L.; Li, Q.; Niu, L.Y. Novel cucurbitane triterpenes from the tubers of Hemsleya amabilis with their cytotoxic acitivity. Molecules 2019, 24, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.L.; Sun, Z.H.; Hu, M.G.; Li, Y.D.; Zhang, D.W.; Wu, H.F.; Tian, Y.; Li, P.F.; Yang, J.S.; Ma, G.X.; et al. Cucurbitane-type triterpenes from the tubers of Hemsleya penxianensis and their bioactive activity. Phytochemistry 2018, 147, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, P.F.; Zhu, N.L.; Hu, M.G.; Wu, H.F.; Tian, Y.; Wu, T.Y.; Zhang, D.W.; Sun, Z.H.; Yang, J.S.; Ma, G.X.; et al. New cucurbitane triterpenoids with cytotoxic activities from Hemsleya penxianensis. Fitoterapia 2017, 120, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Sun, X.B.; Xu, X.D.; Zhou, X.Y.; Zhang, H.Y.; Wang, Y.Q.; Dai, H.N.; Yang, J.S.; Ma, G.X. Chemical constituents from the tubers of Hemsleya penxianensis. Chin. Pharm. J. 2017, 52, 627–630. [Google Scholar]
- Maher, S.; Rasool, S.; Mehmood, R.; Perveen, S.; Tareen, R.B. Trichosides A and B, new withanolide glucosides from Tricholepis eburnean. Nat. Prod. Res. 2018, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.X.; Feng, W.; Sun, Z.H.; Li, P.F.; Zhu, N.L.; Yang, J.S.; Xu, X.D.; Wu, H.F. New stigmastane type of steroidal glycosides from the roots of Vernonia cumingiana. J. Carbohydr. Chem. 2016, 35, 172–179. [Google Scholar] [CrossRef]
- Chen, D.L.; Liu, Y.Y.; Ma, G.X.; Zhu, N.L.; Wu, H.F.; Wang, D.L.; Xu, X.D. Two new rotenoids from the roots of Millettia speciosa. Phytochem. Lett. 2015, 12, 196–199. [Google Scholar] [CrossRef]
- Mua, L.H.; Huang, X.W.; Guo, D.H.; Dong, X.Z.; Liu, P. A new triterpenoid saponin from Ardisia gigantifolia. J. Asian Nat. Prod. Res. 2013, 15, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
 1H-1H COSY;
1H-1H COSY;  HMBC).
HMBC).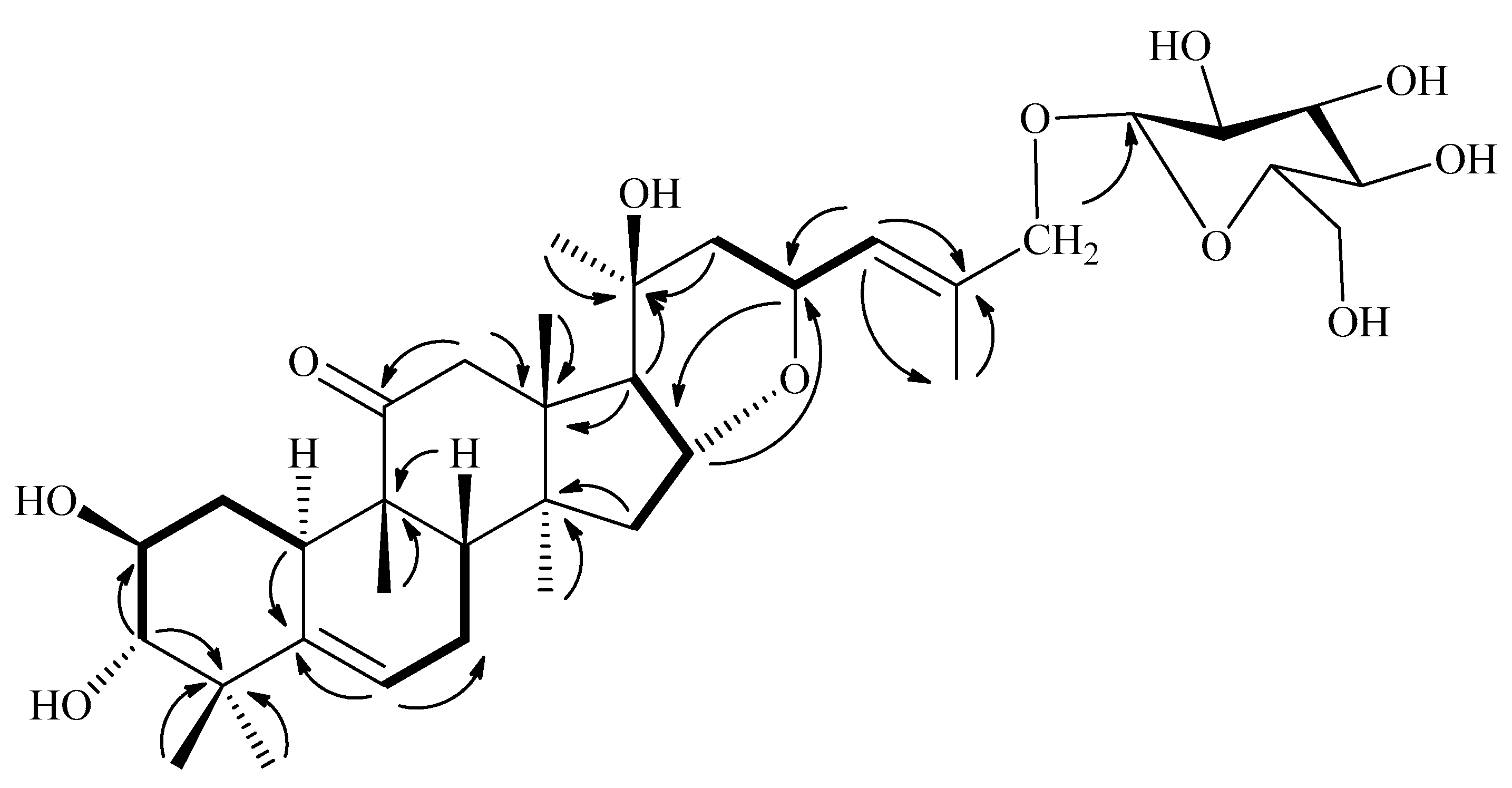
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.52 (1H, m) 2.44 (1H, m) | 2.07 (1H, m) 1.72 (1H, m) | 1.75 (1H, m) 1.67 (1H, m) | 2.92 (1H, m) 2.01 (1H, m) | 2.06 (1H, m) 1.62 (1H, m) |
| 2 | 4.08 (1H, m) | 2.05 (1H, m) 1.89 (1H, m) | 2.37 (1H, m) 1.90 (1H, m) | 2.41 (1H, m) 2.02 (1H, m) | 1.85 (1H, m) 2.38 (1H, m) |
| 3 | 3.40 (1H, d, 12.0) | 3.70 (1H, s) | 3.62 (1H, s) | 3.67 (1H, s) | 3.70 (1H, s) |
| 6 | 5.68 (1H, m) | 5.66 (1H, d, 12.0) | 5.52 (1H, d, 6.0) | 5.49 (1H, d, 6.0) | 6.31 (1H, s) |
| 7 | 1.85 (1H, m) 2.28 (1H, m) | 2.24 (1H, m) 1.78 (1H, m) | 1.81 (1H, m) 1.94 (1H, m) | 2.29 (1H, m) 1.70 (1H, m) | |
| 8 | 1.93 (1H, m) | 1.84 (1H, m) | 1.80 (1H, m) | 1.62 (1H, m) | 2.62 (1H, s) |
| 10 | 2.66 (1H, m) | 2.54 (1H, d, 14.4) | 2.47 (1H, m) | 2.79 (1H, d, 10.8) | 2.98 (1H, m) |
| 11 | 4.18 (1H, m) | ||||
| 12 | 2.64 (1H, m) 3.17 (1H, d, 12.0) | 3.21 (1H, d, 14.4) 2.68 (1H, d, 14.4) | 2.49 (1H, m) 2.94 (1H, d, 12.0) | 2.12 (1H, m) 2.07 (1H, m) | 2.94 (1H, d, 18.0) 2.52 (1H, d, 12.0) |
| 15 | 1.61 (1H, m) 1.92 (1H, m) | 1.90 (1H, m) 1.62 (1H, m) | 1.30 (1H, m) 1.38 (1H, m) | 1.24 (1H, m) 1.07 (1H, m) | 1.40 (1H, m) 1.80 (1H, m) |
| 16 | 5.06 (1H, m) | 5.21 (1H, t, 6.0) | 1.27 (1H, m) 2.13 (1H, m) | 1.87 (1H, m) 1.18 (1H, m) | 1.26 (1H, m) 1.88 (1H, m) |
| 17 | 2.14 (1H, d, 12.0) | 2.16 (1H, d, 9.0) | 1.68 (1H, m) | 1.61 (1H, m) | 1.64 (1H, m) |
| 18 | 1.26 (3H, s) | 1.27 (3H, s) | 0.70 (3H, s) | 0.89 (3H, s) | 0.68 (3H, s) |
| 19 | 1.21 (3H, s) | 1.24 (3H, s) | 1.14 (3H, s) | 1.31 (3H, s) | 1.12 (3H, s) |
| 20 | 1.45 (1H, m) | 1.58 (1H, m) | 1.38 (1H, m) | ||
| 21 | 1.44 (3H, s) | 1.45 (3H, s) | 0.89 (3H, s) | 0.94 (3H, s) | 0.80 (3H, s) |
| 22 | 1.79 (1H, m) 2.07 (1H, q, 6.0) | 2.09 (1H, m) 1.81 (1H, m) | 1.52 (1H, m) 1.18 (1H, m) | 1.45 (1H, m) 1.09 (1H, m) | 1.17 (1H, m) 1.58 (1H, m) |
| 23 | 5.19 (1H, t, 6.0) | 5.11 (1H, m) | 2.18 (2H, m) | 2.11 (1H, m) 1.98 (1H, m) | 2.16 (1H, m) 2.30 (1H, m) |
| 24 | 6.89 (1H, d, 6.0) | 6.92 (1H, d, 9) | 5.72 (1H, t, 6.0) | 5.65 (1H, t, 7.2) | 5.88 (1H, t, 6.0) |
| 26 | 4.85 (1H, d, 6.0) 4.44 (1H, d, 6.0) | 4.86 (1H, d, 12.0) 4.45 (1H, d, 12.0) | 4.31 (2H, s) | 4.32 (2H, s) | 4.73 (2H, s) |
| 27 | 1.91 (3H, s) | 1.91 (3H, s) | 1.83 (3H, s) | 1.80 (3H, s) | 4.70 (2H, s) |
| 28 | 1.34 (3H, s) | 1.37 (3H, s) | 0.98 (3H, s) | 0.91 (3H, s) | 1.05 (3H, s) |
| 29 | 1.28 (3H, s) | 1.13 (3H, s) | 1.10 (3H, s) | 1.15 (3H, s) | 1.18 (3H, s) |
| 30 | 1.45 (3H, s) | 1.41 (3H, s) | 1.54 (3H, s) | 1.56 (3H, s) | 1.58 (3H, s) |
| Glc | |||||
| 1′ | 4.80 (1H, d, 6.0) | 4.81 (1H, d, 7.8) | 4.83 (1H, d, 6.0) | 4.91 (1H, d, 7.8) | 4.86 (1H, d, 6.0) |
| 2′ | 4.02 (1H, m) | 4.05 (1H, m) | 3.95 (1H, m) | 3.98 (1H, m) | 3.97 (1H, m) |
| 3′ | 4.17 (1H, m) | 4.22 (1H, m) | 4.18 (1H, m) | 4.21 (1H, m) | 4.21 (1H, m) |
| 4′ | 4.18 (1H, m) | 4.21 (1H, m) | 4.16 (1H, m) | 4.21 (1H, m) | 4.20 (1H, m) |
| 5′ | 3.89 (1H, m) | 3.95 (1H, m) | 3.92 (1H, m) | 3.93 (1H, m) | 3.95 (1H, m) |
| 6′ | 4.55 (1H, d, 6.0) 4.35 (1H, m) | 4.58 (1H, d, 12.0) 4.39 (1H, m) | 4.50 (1H, d, 12.0) 4.35 (1H, m) | 4.57 (1H, d, 12.0) 4.41 (1H, m) | 4.55 (1H, d, 12.0) 4.40 (1H, m) |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 35.9 | 21.6 | 22.6 | 27.2 | 22.5 |
| 2 | 72.2 | 30.3 | 28.8 | 30.0 | 28.4 |
| 3 | 82.7 | 76.0 | 87.7 | 88.3 | 87.2 |
| 4 | 44.1 | 42.4 | 42.5 | 42.8 | 43.9 |
| 5 | 143.7 | 141.9 | 141.7 | 144.7 | 168.3 |
| 6 | 120.0 | 119.4 | 118.9 | 118.9 | 125.4 |
| 7 | 25.5 | 24.7 | 24.6 | 25.0 | 199.6 |
| 8 | 44.1 | 43.5 | 44.4 | 43.9 | 60.0 |
| 9 | 50.50 | 50.2 | 49.5 | 40.5 | 49.5 |
| 10 | 35.5 | 36.1 | 36.4 | 37.3 | 38.0 |
| 11 | 214.4 | 213.8 | 214.2 | 78.6 | 211.7 |
| 12 | 50.1 | 49.4 | 49.2 | 41.5 | 49.1 |
| 13 | 50.0 | 49.3 | 49.4 | 47.8 | 48.9 |
| 14 | 49.9 | 49.2 | 50.0 | 50.1 | 49.7 |
| 15 | 42.9 | 42.2 | 35.0 | 34.9 | 35.2 |
| 16 | 72.1 | 71.2 | 28.5 | 28.7 | 28.2 |
| 17 | 57.3 | 56.5 | 50.1 | 51.0 | 49.6 |
| 18 | 21.4 | 20.5 | 17.4 | 19.2 | 17.4 |
| 19 | 21.8 | 20.8 | 20.8 | 26.7 | 21.3 |
| 20 | 73.8 | 73.0 | 36.4 | 36.5 | 36.3 |
| 21 | 31.4 | 30.6 | 18.7 | 19.7 | 18.8 |
| 22 | 47.7 | 47.0 | 36.8 | 37.5 | 37.0 |
| 23 | 71.8 | 71.0 | 25.1 | 25.2 | 24.8 |
| 24 | 133.5 | 132.7 | 125.4 | 127.7 | 127.7 |
| 25 | 135.7 | 134.8 | 136.7 | 136.7 | 141.3 |
| 26 | 68.0 | 67.1 | 68.5 | 68.5 | 65.8 |
| 27 | 23.2 | 22.4 | 14.5 | 17.4 | 58.9 |
| 28 | 22.4 | 21.6 | 19.0 | 28.1 | 18.9 |
| 29 | 23.6 | 28.2 | 28.9 | 26.8 | 28.6 |
| 30 | 26.7 | 26.7 | 26.3 | 22.3 | 25.6 |
| Glc | |||||
| 1′ | 103.9 | 103.0 | 107.8 | 107.8 | 107.6 |
| 2′ | 76.3 | 75.6 | 75.9 | 75.9 | 75.9 |
| 3′ | 79.9 | 79.1 | 79.1 | 79.1 | 79.1 |
| 4′ | 73.0 | 72.2 | 72.1 | 72.2 | 72.1 |
| 5′ | 79.8 | 79.0 | 78.6 | 78.2 | 78.8 |
| 6′ | 64.1 | 63.3 | 63.4 | 63.4 | 63.4 |
| Compounds | Hela | MCF-7 | A-549 | L-02 | ||||
|---|---|---|---|---|---|---|---|---|
| 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | |
| 1 | 34.38±2.05 | 50.56 ± 4.28 | 45.09 ± 3.52 | 57.85 ± 5.16 | 49.44 ± 2.67 | 68.82 ± 4.33 | >100 | >100 |
| 2 | 31.75 ± 1.45 | 40.32 ± 2.56 | 45.88 ± 0.92 | 60.74 ± 4.73 | 47.58 ± 0.84 | 80.65 ± 5.16 | >100 | >100 |
| 3 | 7.55 ± 1.75 | 13.15 ± 1.88 | 10.88 ± 2.77 | 26.12 ± 1.22 | 8.55 ± 1.78 | 20.12 ± 1.08 | 68.25 ± 3.78 | >100 |
| 4 | 14.77 ± 2.15 | 25.38 ± 3.72 | 12.54 ± 1.32 | 25.44 ± 3.15 | 18.72 ± 2.35 | 40.18 ± 3.02 | 89.55 ± 4.60 | >100 |
| 5 | 2.25 ± 0.42 | 4.88 ± 1.05 | 4.72 ± 0.54 | 12.65 ± 2.36 | 5.33 ± 0.68 | 12.45 ± 1.28 | 50.52 ± 2.15 | >100 |
| doxorubicin | 1.32 ± 0.03 | 2.15 ± 0.06 | 2.45 ± 0.05 | 3.02 ± 0.04 | 3.85 ± 0.05 | 6.10 ± 0.26 | 15.42 ± 0.28 | 26.56 ± 1.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.-L.; Xu, X.-D.; Li, R.-T.; Wang, B.-W.; Yu, M.; Liu, Y.-Y.; Ma, G.-X. Five New Cucurbitane-Type Triterpenoid Glycosides from the Rhizomes of Hemsleya penxianensis with Cytotoxic Activities. Molecules 2019, 24, 2937. https://doi.org/10.3390/molecules24162937
Chen D-L, Xu X-D, Li R-T, Wang B-W, Yu M, Liu Y-Y, Ma G-X. Five New Cucurbitane-Type Triterpenoid Glycosides from the Rhizomes of Hemsleya penxianensis with Cytotoxic Activities. Molecules. 2019; 24(16):2937. https://doi.org/10.3390/molecules24162937
Chicago/Turabian StyleChen, De-Li, Xu-Dong Xu, Rong-Tao Li, Bo-Wen Wang, Meng Yu, Yang-Yang Liu, and Guo-Xu Ma. 2019. "Five New Cucurbitane-Type Triterpenoid Glycosides from the Rhizomes of Hemsleya penxianensis with Cytotoxic Activities" Molecules 24, no. 16: 2937. https://doi.org/10.3390/molecules24162937
APA StyleChen, D.-L., Xu, X.-D., Li, R.-T., Wang, B.-W., Yu, M., Liu, Y.-Y., & Ma, G.-X. (2019). Five New Cucurbitane-Type Triterpenoid Glycosides from the Rhizomes of Hemsleya penxianensis with Cytotoxic Activities. Molecules, 24(16), 2937. https://doi.org/10.3390/molecules24162937