Synthesis of Ultrastable Gold Nanoparticles as a New Drug Delivery System
Abstract
1. Introduction
2. Results
2.1. Synthesis
2.2. Ultrastability Assays
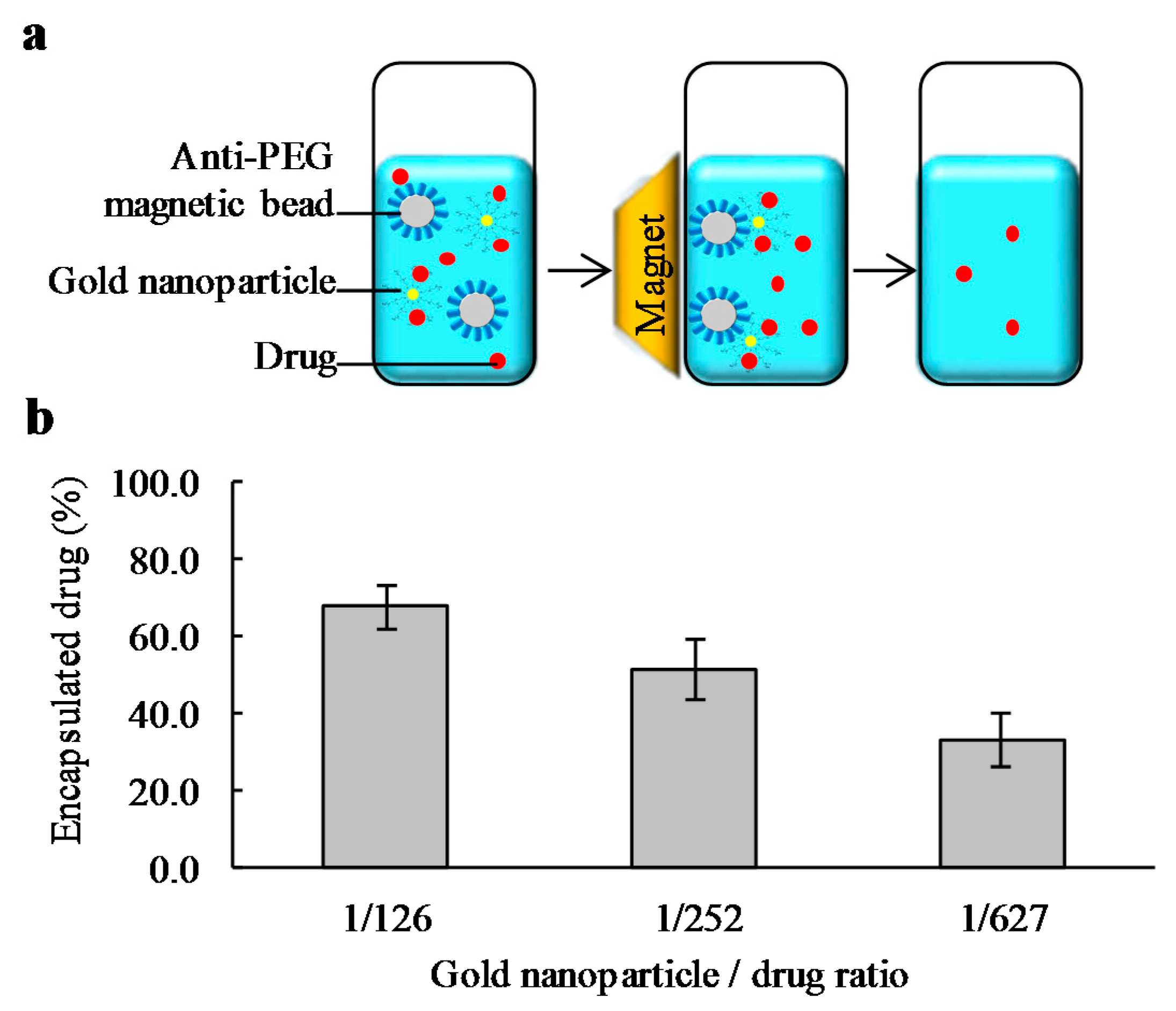
2.3. Drug Encapsulation and Quantification of the Encapsulated Drug
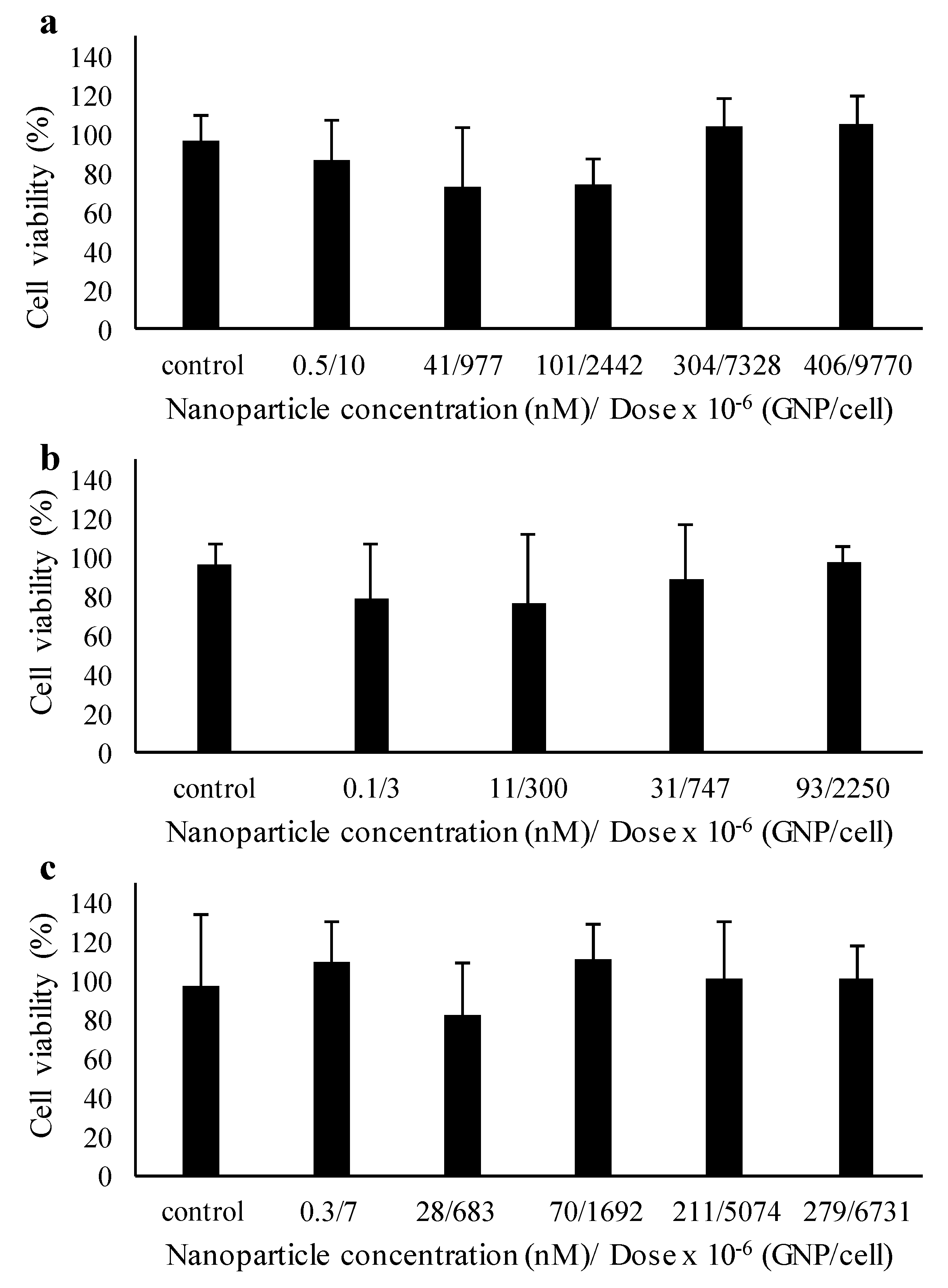
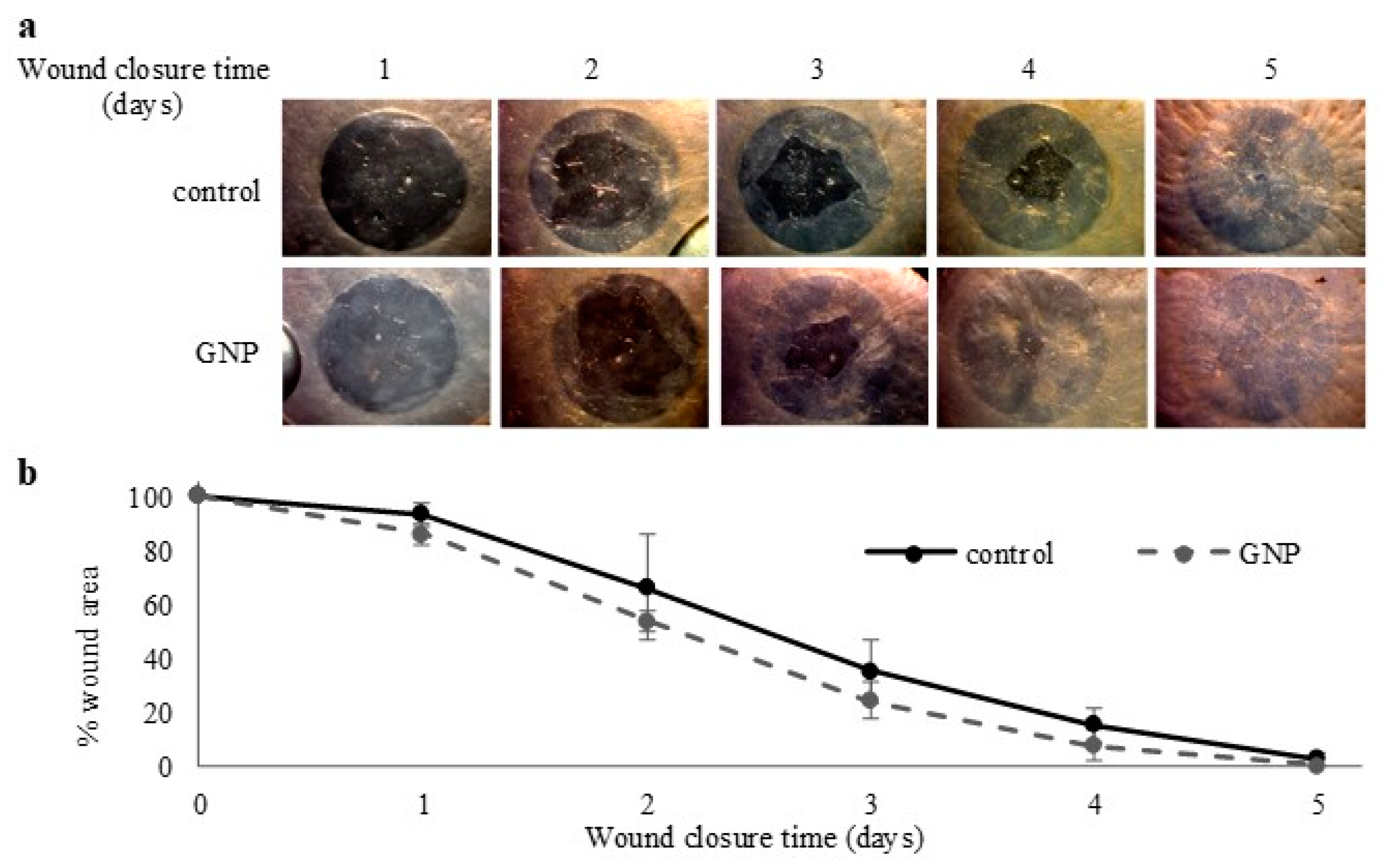
2.4. Toxicity and Wound Healing Assays
3. Materials and Methods
3.1. Chemicals
3.2. Ultrastable Gold Nanoparticles Synthesis
3.3. GNP NUS Synthesis
3.4. GNP CIT Synthesis
3.5. UV-Visible Spectroscopy
3.6. Transmission Electron Microscopy (TEM)
3.7. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
3.8. Elemental Analysis
3.9. Number of Ligand and Molecular Mass Determination
3.10. Stability against Freeze Drying
3.11. Stability against Heat
3.12. Stability against Ultracentrifugation
3.13. Stability against Autoclave Sterilization
3.14. Stability against Phosphate-Buffered Saline (PBS)
3.15. Drug Encapsulation Protocol
Determination of the Encapsulation Time
Quantification of the Encapsulated Drug
Immunoprecipitaton of the Nanoparticles (Adapted from SureBeads Protocol)
3.16. MTS Assay
3.17. Wound Healing Assay
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Mwakwari, S.C.; Oyelere, A.K. Gold nanoparticles: From nanomedicine to nanosensing. Nanotechnol. Sci. Appl. 2008, 1, 45–65. [Google Scholar] [PubMed]
- Pengo, P.; Sologan, M.; Pasquato, L.; Guida, F.; Pacor, S.; Tossi, A.; Stellacci, F.; Marson, D.; Boccardo, S.; Pricl, S.; et al. Gold nanoparticles with patterned surface monolayers for nanomedicine: Current perspectives. Eur. Biophys. J. 2017, 46, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C. Applications of gold nanoparticles in nanomedicine: Recent advances in vaccines. Molecules 2017, 22, 857. [Google Scholar] [CrossRef] [PubMed]
- Capek, I. Polymer decorated gold nanoparticles in nanomedicine conjugates. Adv. Colloid Interface Sci. 2017, 249, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Zou, T.; Zhang, J.J.; Lin, I.W.; Che, C.M. Controlled-release systems for metal-based nanomedicine: Encapsulated/self-assembled nanoparticles of anticancer gold(iii)/platinum(ii) complexes and antimicrobial silver nanoparticles. Adv. Mat. 2014, 26, 5550–5557. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Masse, F.; Lefebvre-Demers, M.; Maestracci, Q.; Grenier, P.; Millar, R.; Bertrand, N.; Prieto, M.; Boisselier, E. Insights into gold nanoparticles as a mucoadhesive system. Sci. Rep. 2018, 8, 14357. [Google Scholar] [CrossRef] [PubMed]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef]
- Sun, M.; Liu, F.; Zhu, Y.; Wang, W.; Hu, J.; Liu, J.; Dai, Z.; Wang, K.; Wei, Y.; Bai, J.; et al. Salt-induced aggregation of gold nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Nanoscale 2016, 8, 4452–4457. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef]
- Simon, T.; Shellaiah, M.; Steffi, P.; Sun, K.W.; Ko, F.H. Development of extremely stable dual functionalized gold nanoparticles for effective colorimetric detection of clenbuterol and ractopamine in human urine samples. Anal. Chim. Acta 2018, 1023, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.D.; Adak, A.K.; Yu, C.C.; Hsiao, W.C.; Lin, H.J.; Chen, M.L.; Lin, C.C. Fabrication of highly stable glyco-gold nanoparticles and development of a glyco-gold nanoparticle-based oriented immobilized antibody microarray for lectin (goal) assay. Chemistry 2015, 21, 3956–3967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, F.G.; Liu, P.; Wang, H.Y.; Gu, N.; Chen, Z. Synthesis of ultrastable and multifunctional gold nanoclusters with enhanced fluorescence and potential anticancer drug delivery application. J. Colloid Interface Sci. 2015, 455, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, A.Y.; Lebedev, V.A.; Shirshin, E.A.; Rumyantsev, A.M.; Volikov, A.B.; Zherebker, A.; Garshev, A.V.; Goodilin, E.A.; Perminova, I.V. Non-classical growth of water-redispersible spheroidal gold nanoparticles assisted by leonardite humate. CrystEngComm 2017, 19, 876–886. [Google Scholar] [CrossRef]
- Rahme, K.; Nolan, M.T.; Doody, T.; McGlacken, G.P.; Morris, M.A.; O’Driscoll, C.; Holmes, J.D. Highly stable pegylated gold nanoparticles in water: Applications in biology and catalysis. RSC Adv. 2013, 3, 21016–21024. [Google Scholar] [CrossRef]
- Deka, J.; Mech, R.; Ianeselli, L.; Amenitsch, H.; Cacho-Nerin, F.; Parisse, P.; Casalis, L. Surface passivation improves the synthesis of highly stable and specific DNA-functionalized gold nanoparticles with variable DNA density. ACS Appl Mater Interfaces 2015, 7, 7033–7040. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, B.; Rozynek, Z.; Xie, Z.; Fossum, J.O.; Yu, X.; Raaen, S. Minute synthesis of extremely stable gold nanoparticles. Nanotechnology 2009, 20, 505606. [Google Scholar] [CrossRef] [PubMed]
- Hinman, S.S.; McKeating, K.S.; Cheng, Q. DNA linkers and diluents for ultrastable gold nanoparticle bioconjugates in multiplexed assay development. Anal. Chem. 2017, 89, 4272–4279. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yuan, H.; Xu, A.; Wang, J.; Wu, L. Rapid synthesis of stable and functional conjugates of DNA/gold nanoparticles mediated by tween 80. Langmuir 2011, 27, 13629–13634. [Google Scholar] [CrossRef]
- Zhong, Z.; Sim, D.; Teo, J.; Luo, J.; Zhang, H.; Gedanken, A. D-glucose-derived polymer intermediates as templates for the synthesis of ultrastable and redispersible gold colloids. Langmuir 2008, 24, 4655–4660. [Google Scholar] [CrossRef]
- Yoo, M.; Kim, S.; Lim, J.; Kramer, E.J.; Hawker, C.J.; Kim, B.J.; Bang, J. Facile synthesis of thermally stable core−shell gold nanoparticles via photo-cross-linkable polymeric ligands. Macromolecules 2010, 43, 3570–3575. [Google Scholar] [CrossRef]
- Huang, W.; Chen, S.; Liu, Y.; Fu, H.; Wu, G. The controlled synthesis of stable gold nanoparticles in quaternary ammonium ionic liquids by simple heating. Nanotechnology 2011, 22, 025602. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.; Ramesh, K.; Rajeshkumar, S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Kumara, C.; Jupally, V.R.; Dass, A. Gold thiolate nanomolecules: Synthesis, mass spectrometry, and characterization. In Gold Clusters, colloids and Nanoparticles I; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 155–187. [Google Scholar]
- Lees, E.E.; Nguyen, T.L.; Clayton, A.H.; Muir, B.W.; Mulvaney, P. The preparation of colloidally stable, water-soluble, biocompatible, semiconductor nanocrystals with a small hydrodynamic diameter. ACS Nano 2009, 3, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, W. Progress in the synthesis and characterization of gold nanoclusters. In Gold Clusters, Colloids and Nanoparticles I; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 117–153. [Google Scholar]
- Rubinson, K.A.; Krueger, S. Poly(ethylene glycol)s 2000–8000 in water may be planar: A small-angle neutron scattering (sans) structure study. Polymer 2009, 50, 4852–4858. [Google Scholar] [CrossRef]
- Pai, S.S.; Hammouda, B.; Hong, K.; Pozzo, D.C.; Przybycien, T.M.; Tilton, R.D. The conformation of the poly(ethylene glycol) chain in mono-pegylated lysozyme and mono-pegylated human growth hormone. Bioconjug. Chem. 2011, 22, 2317–2323. [Google Scholar] [CrossRef]
- Boisselier, E.; Diallo, A.K.; Salmon, L.; Ornelas, C.; Ruiz, J.; Astruc, D. Encapsulation and stabilization of gold nanoparticles with “click” polyethyleneglycol dendrimers. J. Am. Chem. Soc. 2010, 132, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Gao, K.; Ou, Q.; Fu, X.; Man, S.Q.; Guo, J.; Liu, Y. Calculation extinction cross sections and molar attenuation coefficient of small gold nanoparticles and experimental observation of their uv-vis spectral properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 191, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ding, X.; Xu, Q.; Wang, J.; Wang, L.; Lou, X. Zeta-potential data reliability of gold nanoparticle biomolecular conjugates and its application in sensitive quantification of surface absorbed protein. Colloids Surf. B Biointerfaces 2016, 148, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Iglesias, N.; Bettmer, J. Complementary mass spectrometric techniques for the quantification of the protein corona: A case study on gold nanoparticles and human serum proteins. Nanoscale 2015, 7, 14324–14331. [Google Scholar] [CrossRef] [PubMed]
- Francois, A.; Laroche, A.; Pinaud, N.; Salmon, L.; Ruiz, J.; Robert, J.; Astruc, D. Encapsulation of docetaxel into pegylated gold nanoparticles for vectorization to cancer cells. ChemMedChem 2011, 6, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [PubMed]
- Couture, C.; Desjardins, P.; Zaniolo, K.; Germain, L.; Guerin, S.L. Enhanced wound healing of tissue-engineered human corneas through altered phosphorylation of the creb and akt signal transduction pathways. Acta Biomater. 2018, 73, 312–325. [Google Scholar] [CrossRef]
- Boisselier, E.; Salmon, L.; Ruiz, J.; Astruc, D. How to very efficiently functionalize gold nanoparticles by “click” chemistry. Chem. Commun. 2008, 5788–5790. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

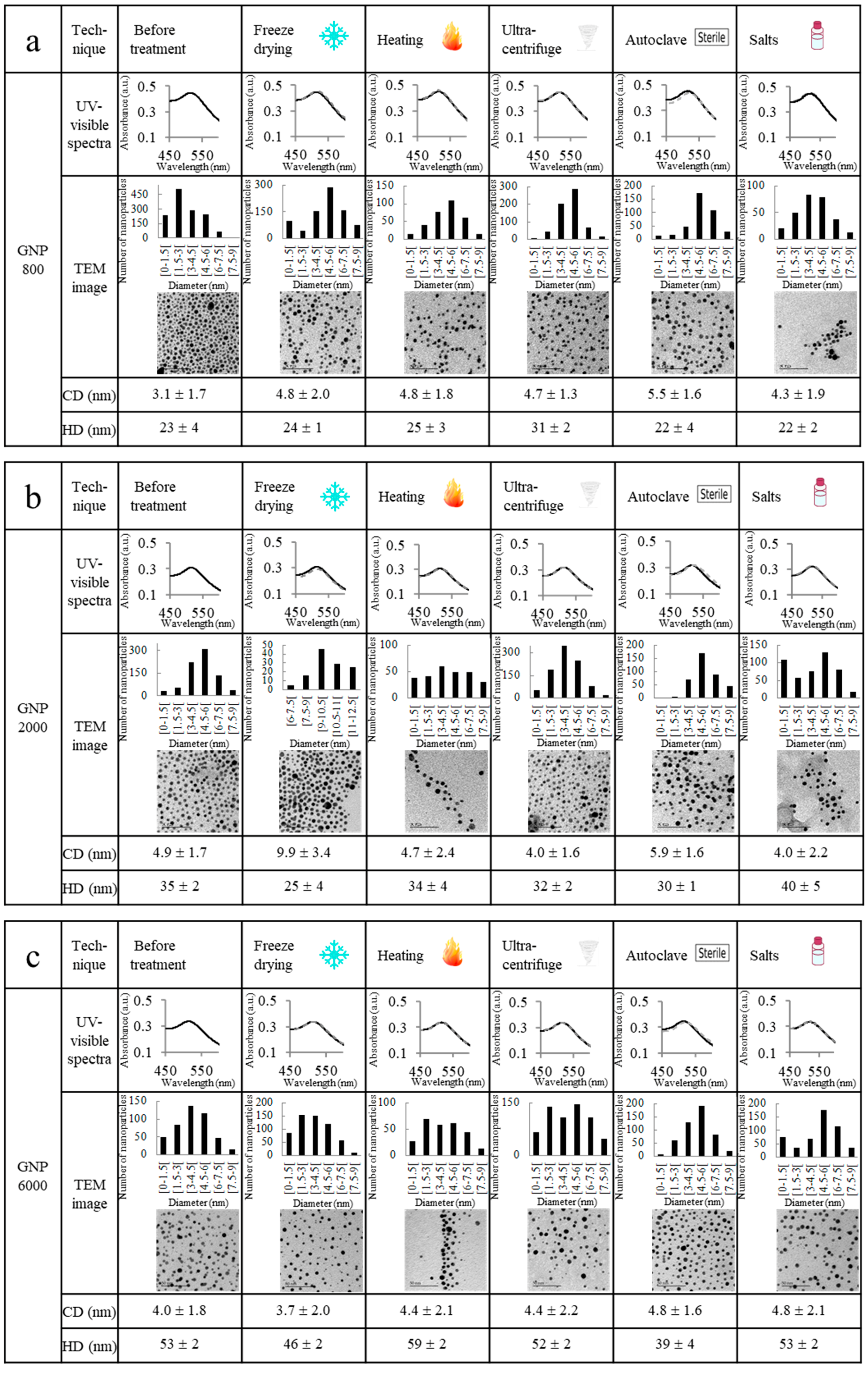
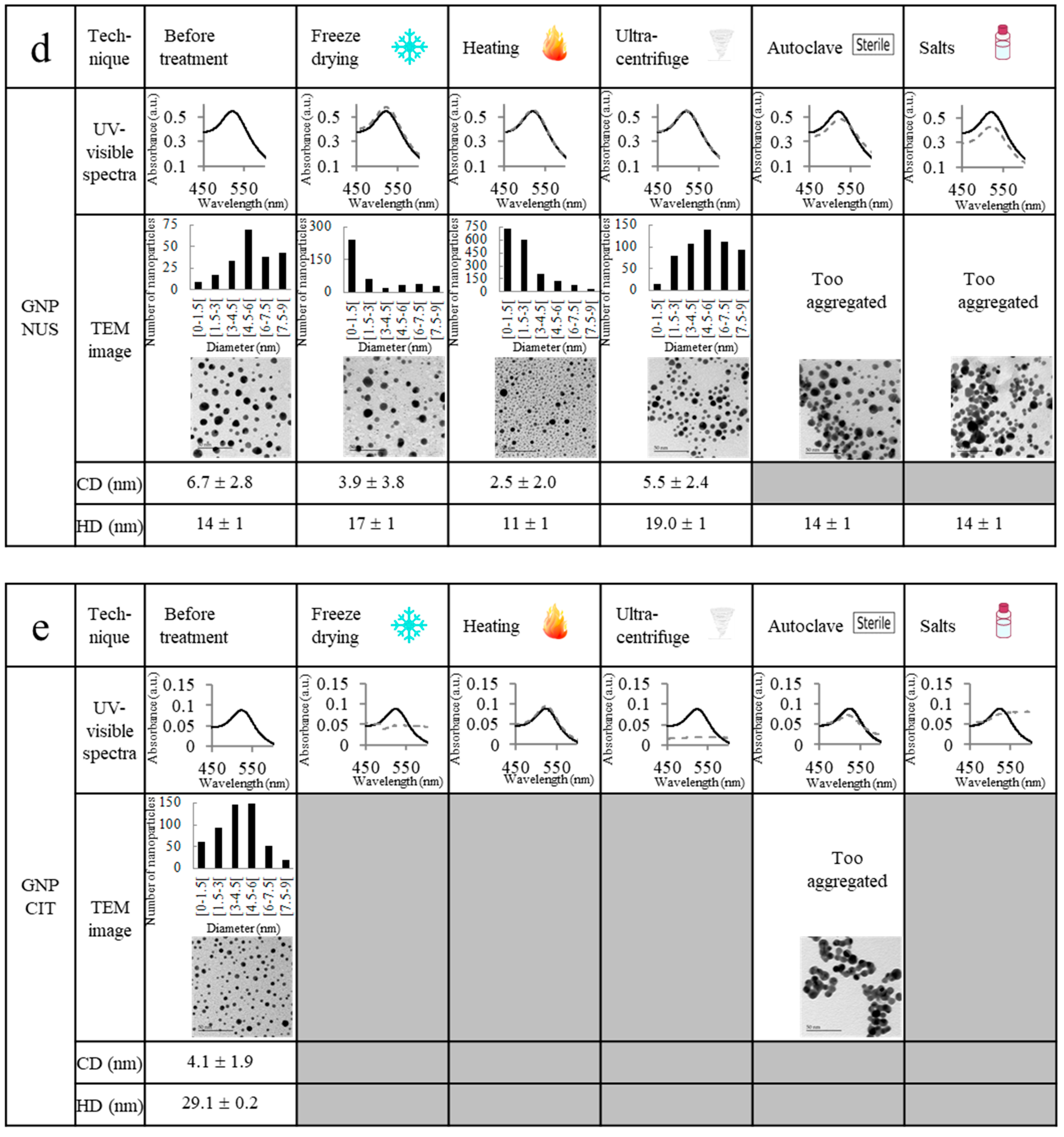
| Characterization | GNP 800 | GNP 2000 | GNP 6000 |
|---|---|---|---|
| % Au atoms 1 | 73.0 | 54.1 | 49.6 |
| % S atoms 1 | 1.5 | 0.5 | 1.4 |
| Core diameter (nm) 2 | 3.1 ± 1.7 | 4.9 ± 1.7 | 4.0 ± 1.8 |
| Hydrodynamic diameter (nm) 3 | 23 ± 4 | 35 ± 2 | 53 ± 2 |
| Charge (mV) 4 | −0.1 ± 1.0 | −0.2 ± 0.5 | −1.8 ± 4.3 |
| Number of ligands 5 | 93 | 165 | 275 |
| Molecular weight (g.mol−1) 6 | 146,922 | 616,059 | 1,805,630 |
| Molar extinction coefficient (L.mol−1.cm−1) 7 | 618,204 | 545,219 | 3,711,567 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masse, F.; Desjardins, P.; Ouellette, M.; Couture, C.; Omar, M.M.; Pernet, V.; Guérin, S.; Boisselier, E. Synthesis of Ultrastable Gold Nanoparticles as a New Drug Delivery System. Molecules 2019, 24, 2929. https://doi.org/10.3390/molecules24162929
Masse F, Desjardins P, Ouellette M, Couture C, Omar MM, Pernet V, Guérin S, Boisselier E. Synthesis of Ultrastable Gold Nanoparticles as a New Drug Delivery System. Molecules. 2019; 24(16):2929. https://doi.org/10.3390/molecules24162929
Chicago/Turabian StyleMasse, Florence, Pascale Desjardins, Mathieu Ouellette, Camille Couture, Mahmoud Mohamed Omar, Vincent Pernet, Sylvain Guérin, and Elodie Boisselier. 2019. "Synthesis of Ultrastable Gold Nanoparticles as a New Drug Delivery System" Molecules 24, no. 16: 2929. https://doi.org/10.3390/molecules24162929
APA StyleMasse, F., Desjardins, P., Ouellette, M., Couture, C., Omar, M. M., Pernet, V., Guérin, S., & Boisselier, E. (2019). Synthesis of Ultrastable Gold Nanoparticles as a New Drug Delivery System. Molecules, 24(16), 2929. https://doi.org/10.3390/molecules24162929








