Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities
Abstract
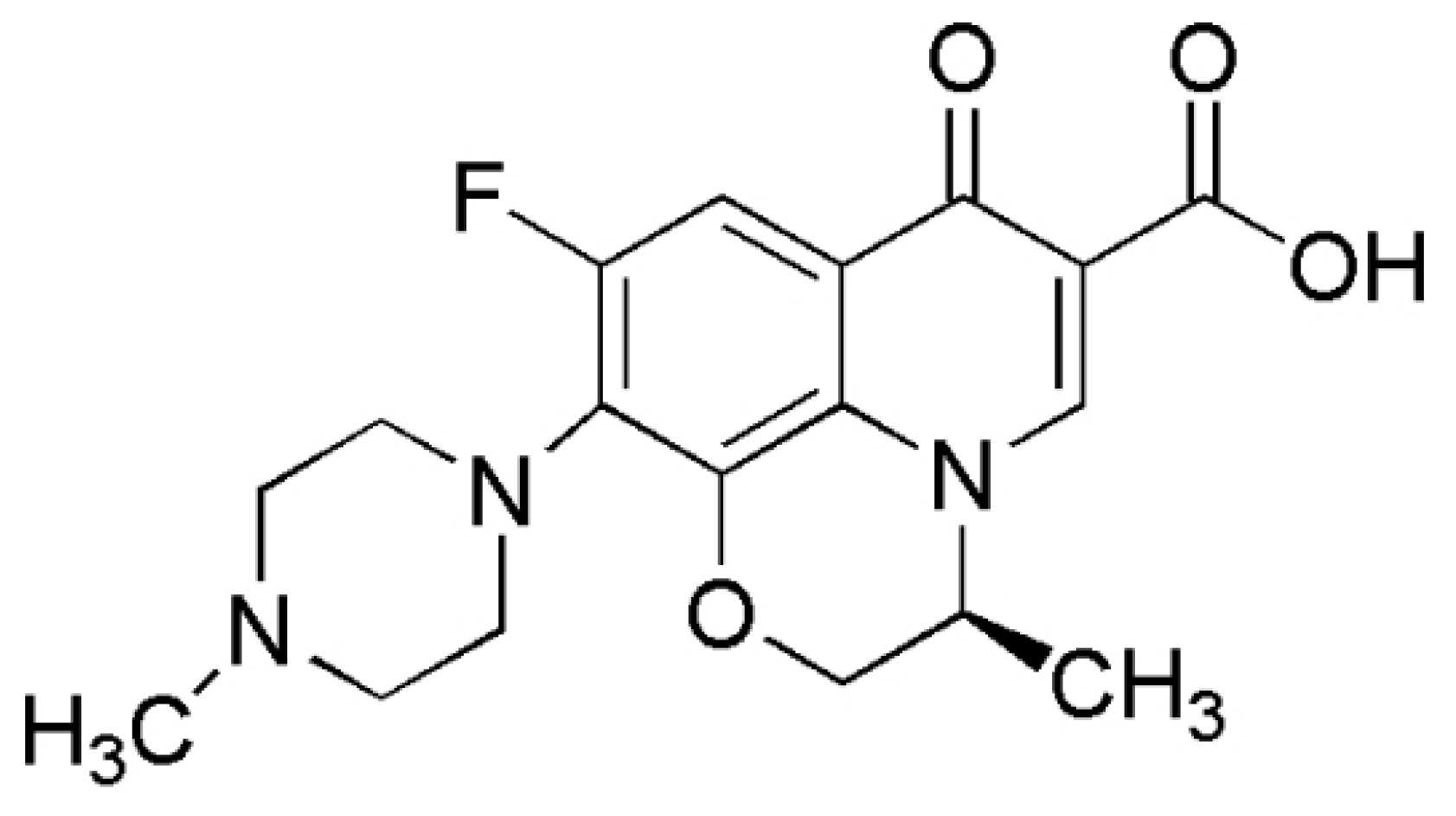
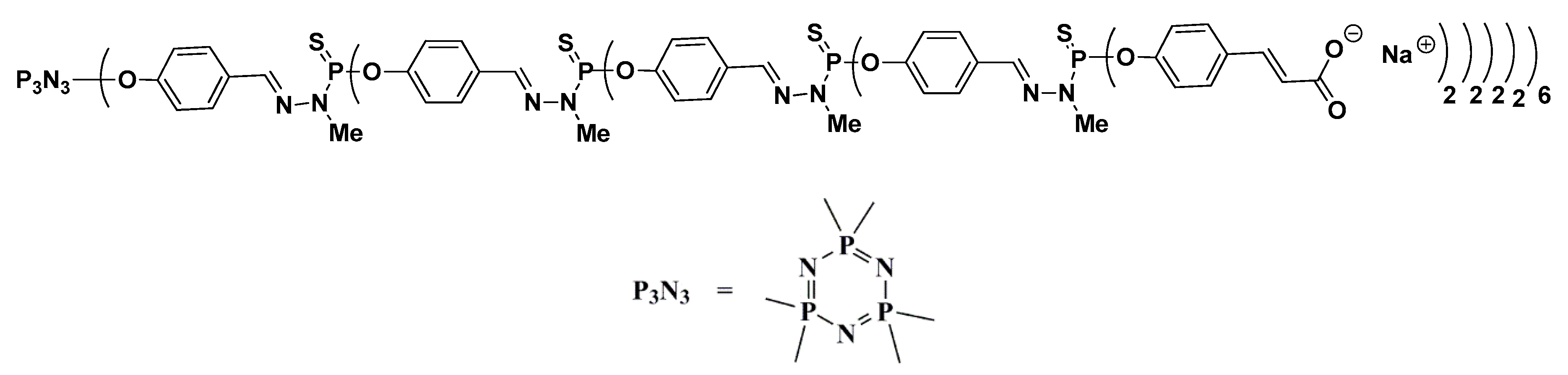
1. Introduction
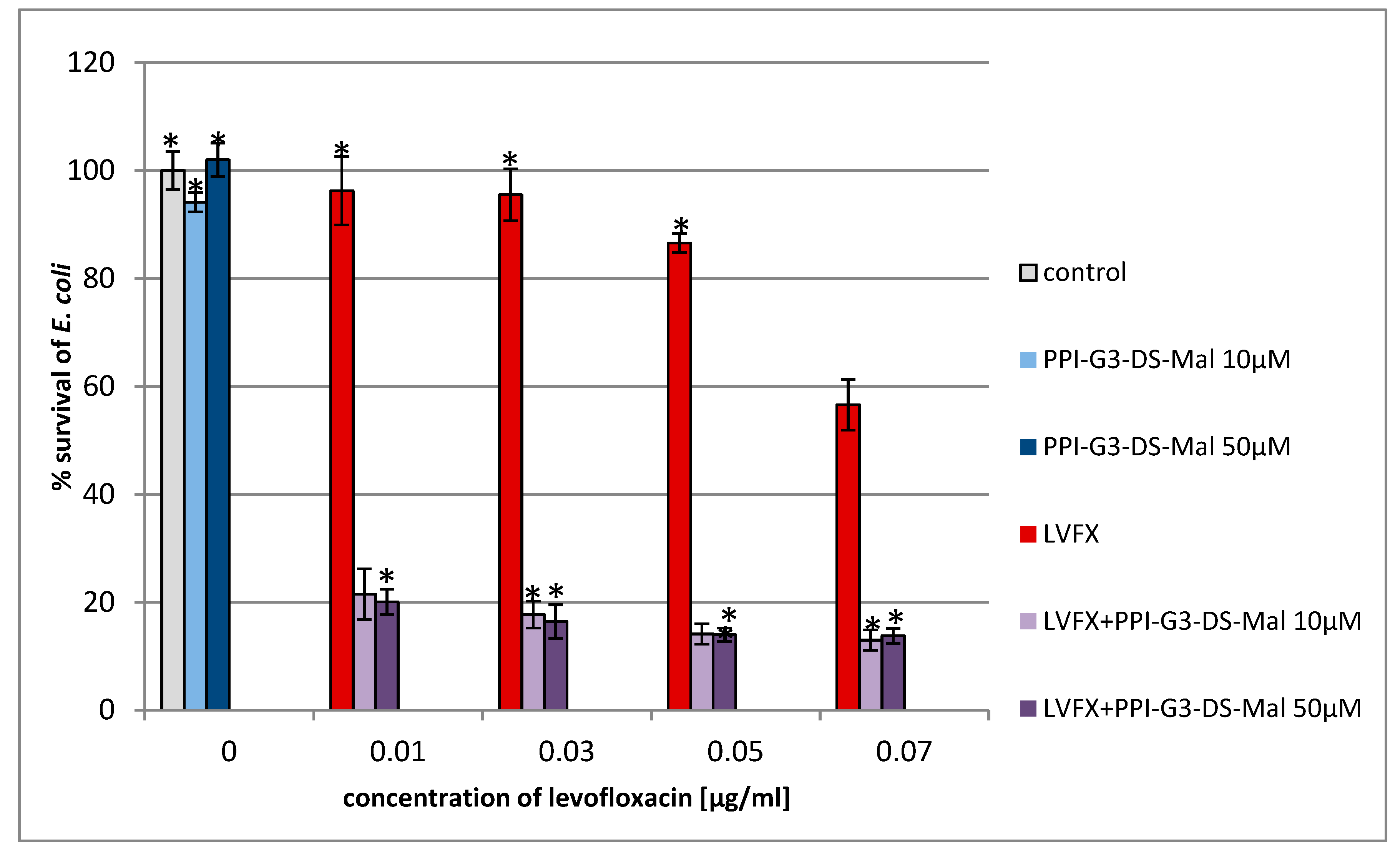
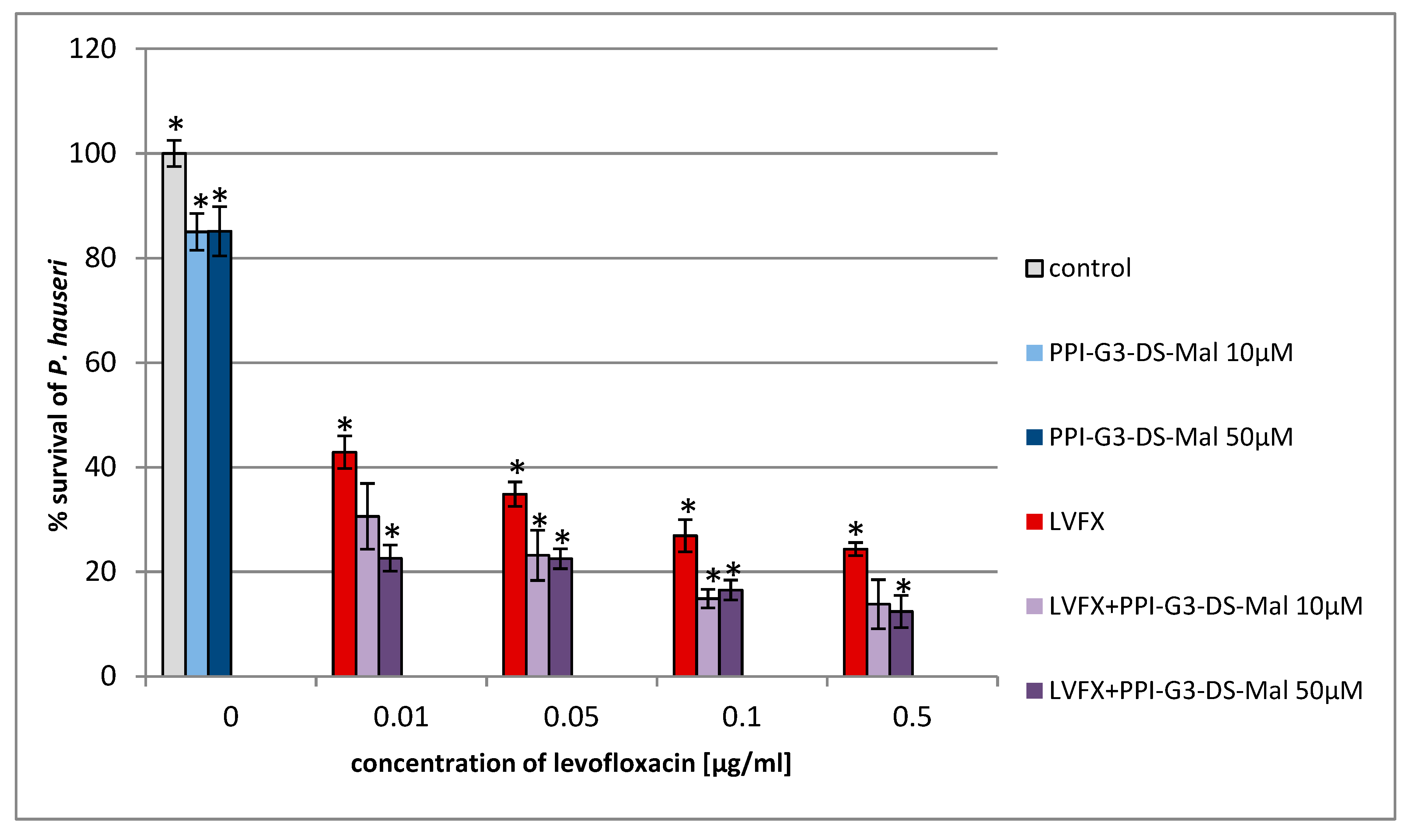
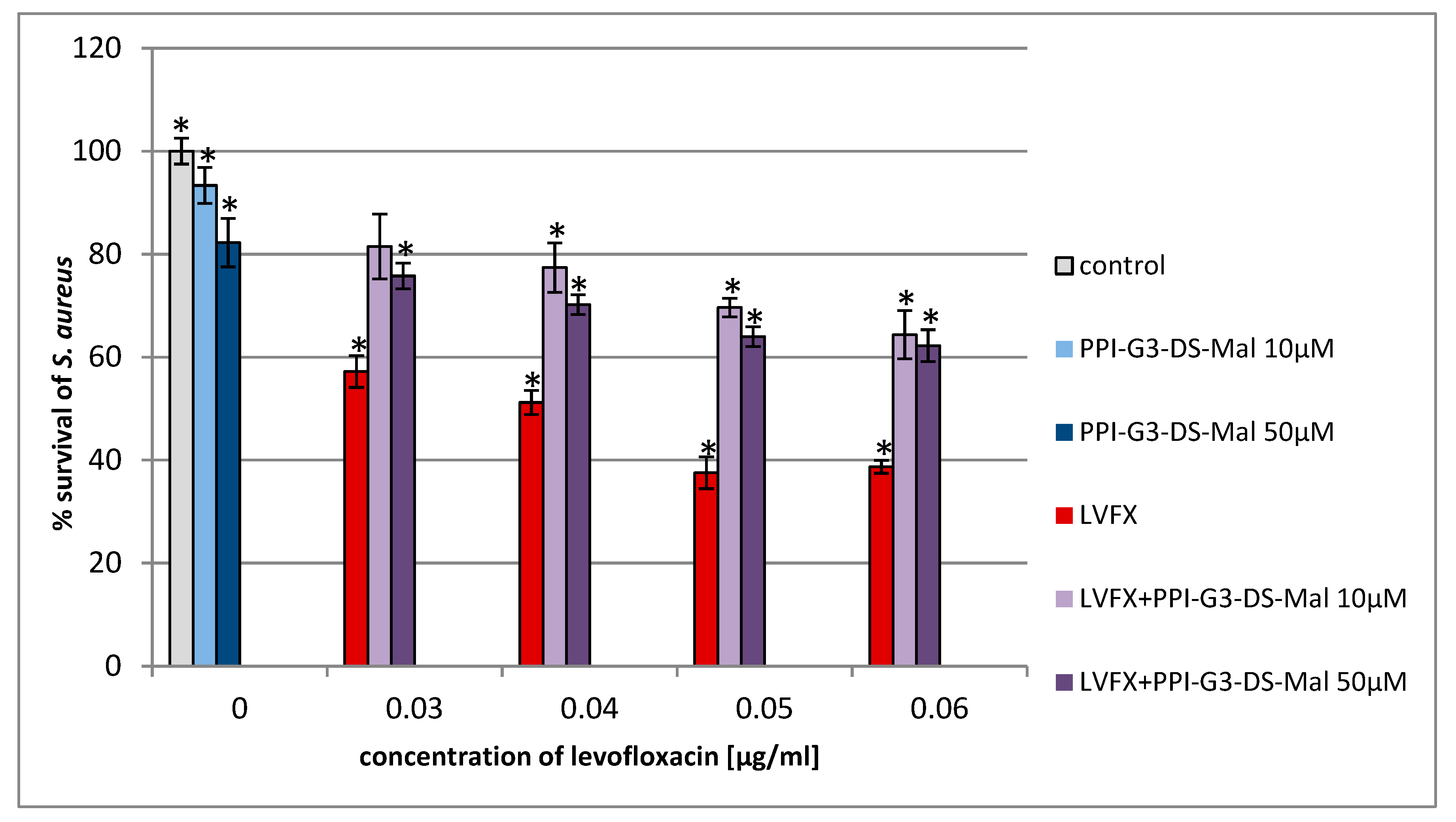
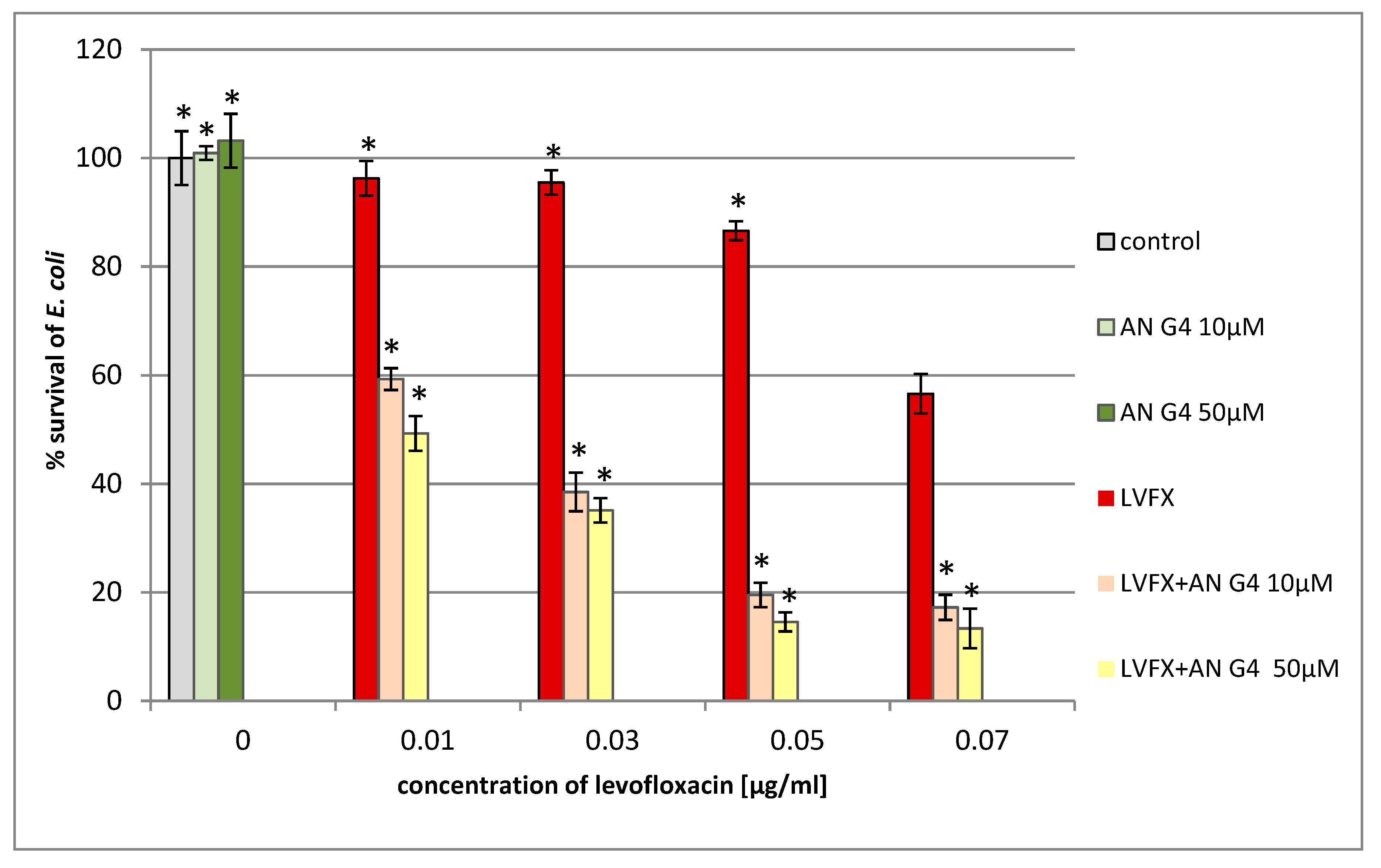
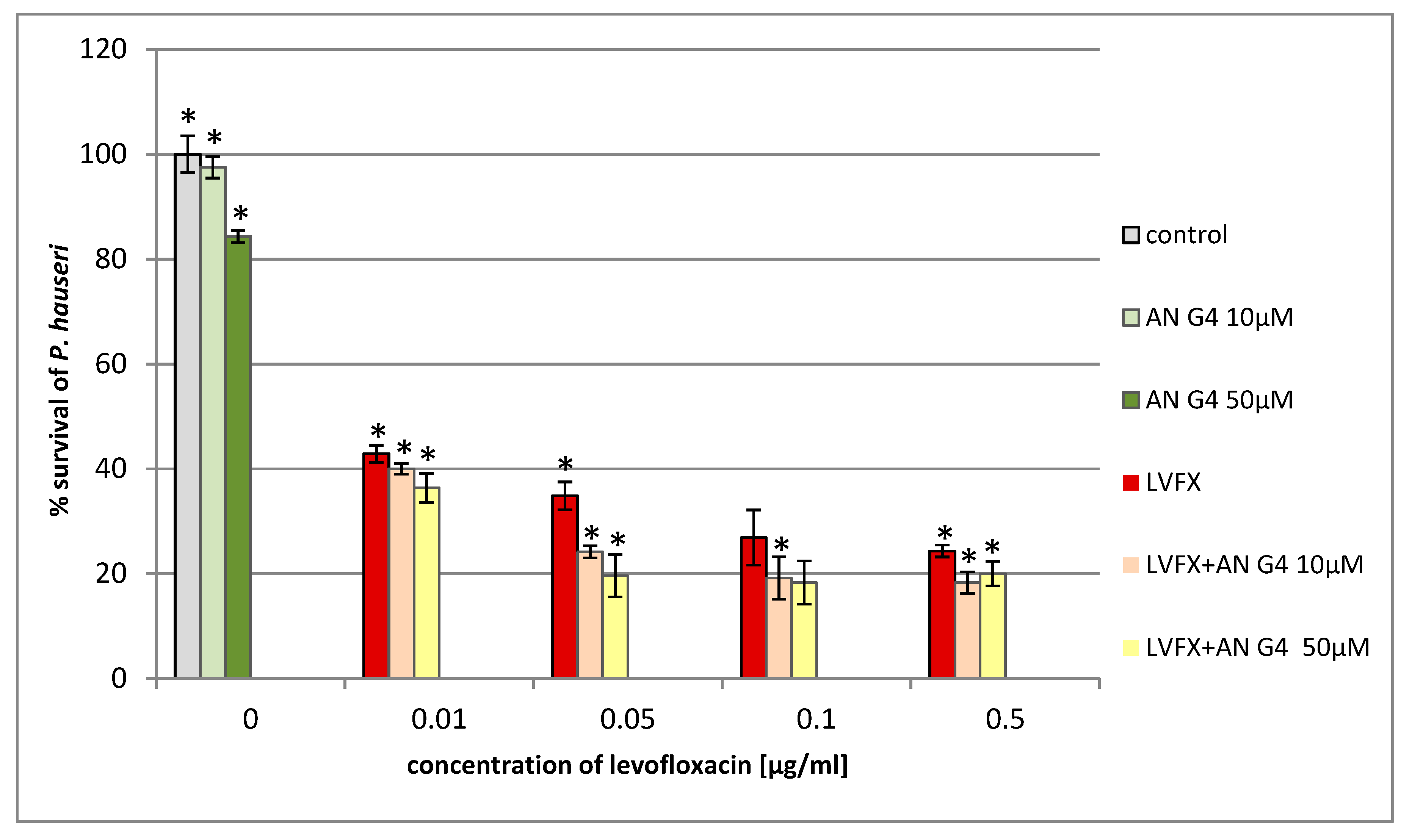
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Determination of Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, L.; Huang, R.; Li, J.; Liu, S.; Huang, S.; Jiang, C. Plasmis pORFhTRail and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials 2011, 32, 1242–1252. [Google Scholar] [CrossRef]
- Beg, S.; Samad, A.; Alam, M.I.; Nazish, I. Dendrimers as novel systems for delivery of neuropharmaceuticals to the brain. CNS Neurol. Discord. Drug Targets 2011, 10, 576–588. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, V.; Palanirajan, V.K.; Tekade, R.K.; Jain, N.K. Dendrimers as therapeutic agents: A systematic review. J. Pharm. Pharmacol. 2009, 61, 989–1003. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, M.K. Dendrimers and their applications as novel drug delivery carriers. J. Appl. Pharm. Sci. 2013, 3, 142–149. [Google Scholar]
- Bodewein, L.; Schmelter, F.; Di Fiore, S.; Hollert, H.; Fischer, R.; Fenske, M. Differences in toxicity and cationic PAMAM and PPI dendrimers in zebrafish embryos and cancer cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleid acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Caminade, A.M.; Laurent, R.; Shi, X.; Majoral, J.P. Recent therapeutic applications of the theranostic principle with dendrimers in oncology. Sci. China Mater. 2018, 61, 1367–1386. [Google Scholar] [CrossRef]
- Garcia-Gallego, S.; Franci, G.; Falanga, A.; Gomez, R.; Folliero, V.; Galdiero, S.; de la Mata, J.F.; Galdiero, M. Function oriented molecular design: Dendrimers as novel antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.J.; Huang, C.-M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of dendrimers on solubility of hydrophobic drug molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Chauhan, A.S. Dendrimers for enhanced drug solubilization. Nanomedicine 2008, 3, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, B.; Hill, R.A.; Liebenberg, W.; Brits, M.; Villiers, M.M. Comparison of the aqueous solubilization of practically insoluble niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int. J. Pharm. 2005, 304, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Kolhe, P.; Raykova, V.; Glibatec, M.; Kannan, R.M.; Lieh-Lai, M.; Bassett, D. Dynamics of cellular entry and drug delivery by dendritic polymers into human lung epithelial carcinoma cells. J. Biomater. Sci. Polym. Ed. 2004, 15, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly (ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef]
- Lopez, A.I.; Reins, R.Y.; McDermott, A.M.; Trautner, B.W.; Cai, C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol. Biosyst. 2009, 5, 1148–1156. [Google Scholar] [CrossRef]
- Felczak, A.; Wrońska, N.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Appelhans, D.; Voit, B.; Różalska, S.; Lisowska, K. Antimicrobial activity of poly(propylene imine) demdrimers. New J. Chem. 2012, 36, 2215–2222. [Google Scholar] [CrossRef]
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Dourbash, F.A.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly(amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, 395. [Google Scholar] [CrossRef]
- Ortega, P.; Copa-Patino, J.L.; Munoz-Fernandez, M.A.; Soliveri, J.; Gomez, R.; de la Mata, F.J. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org. Biomol. Chem. 2008, 6, 3264–3269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qu, H.; Ma, M.; Xu, Z.; Xu, P.; Fang, Y.; Xu, T.; Wen, L. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Europ. J. Med. Chem. 2007, 42, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Neerman, M.F.; Parrish, A.R.; Simanek, E.E. Cytotoxicity, hemolysis and acute in vitro toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J. Am. Chem. Soc. 2004, 126, 10044–10048. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship between structure and biocompability in vitro and preliminary studies on the biodistribution of 125 I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Janaszewska, A.; Mączyńska, K.; Matuszko, G.; Appelhans, D.; Voit, B.; Klajnet, B.; Bryszewska, M. Cytotoxicity of PAMAM, PPI and maltose modified PPI dendrimers in Chinese hamster ovary CHO) and human ovarian carcinoma (SKOV3) cells. New J. Chem. 2012, 36, 428–437. [Google Scholar] [CrossRef]
- Padilla de Jesus, O.L.; Ihre, H.; Gagne, L.; Frechet, J.M.J.; Szoka, F.C. Polyester dendritic system for drug delivery application: In vitro and in vivo evaluation. Bioconjug. Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O.; Majoral, J.P. Biological properties of phosphorus dendrimers. New J. Chem. 2010, 34, 512–1524. [Google Scholar] [CrossRef]
- El Brahmi, N.; Mignani, S.; Caron, J.; El Kazzouli, S.; Bousmina, M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. Investigations of dendrimer space reveal solid and liquid tumor growth-inhibition by original phosphorus-based dendrimers and the corresponding monomers and dendrons with ethacrynic acid motifs. Nanoscale 2015, 7, 3915–3922. [Google Scholar] [CrossRef]
- Mignani, S.; El Brahmi, N.; El Kazzouli, S.; Cresteil, T.; Majoral, J.P. First-in-Class Combination Therapy of a Copper (II) Metallo-Phosphorus Dendrimer with Cytotoxic Agents. Oncology 2018, 94, 324–328. [Google Scholar] [CrossRef]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.P. Dendrimer space concept for innovative nanomedicine: A futuristic vision for medicinal chemistry. Adv. Drug Deliv. Rev. 2013, 64, 1316–1330. [Google Scholar] [CrossRef]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Tourin, C.O.; Caminade, A.M.; Fournie, J.J.; et al. Design of phosphorylated dendritic architecture to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Tsapis, N.; Andreana, I.; Chamarat, A.; Foged, C.; Delomenie, C.; Noiray, M.; El Brahmi, N.; Majoral, J.P.; Mignani, S.; et al. Anti-Inflammatory Effect of Anti-TNF-alpha SiRNA Cationic Phosphorus Dendrimer Nanocomplexes Administered Intranasally in a Murine Acute Lung Injury Model. Biomacromolecules 2017, 18, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Posadasa, I.; Romero-Castillo, L.; El Brahmi, N.; Manzanaresa, D.; Mignani, S.; Majoral, J.P.; Ceña, V. Neutral high-generation phosphorus dendrimers inhibit macrophage-mediated inflammatory response in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2018, 114, E7660–E7669. [Google Scholar] [CrossRef] [PubMed]
- Dabrzalska, M.; Zabłocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. Phosphorus dendrimers and photodynamic therapy. Spectroscopic studies on two dendrimer-photosensitizer complexes: Cationic phosphorus dendrimer with rose bengal and anionic phosphorus dendrimer with methylene blue. Int. J. Pharm. 2015, 492, 266–274. [Google Scholar] [CrossRef]
- Machuca, J.; Briales, A.; Diaz-de-Alba, P.; Martinez-Martinez, L.; Pascal, A.; Rodriquez-Martinez, J.M. Effect of the efflux pump QepA2 combined with chromosomally mediated mechanisms on quinolone resistance and bacterial fitness in Escherichia coli. J. Antimicrob. Chemother. 2015, 70, 2524–2527. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, C.A.; Papanastasiou, F.A.; Juba, M.; Bishop, B. Covalent modification of ten-residue cationic antimicrobial peptide with levofloxacin. Front. Chem. 2014, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Andreu, V. Fluoroquinolones in soil-risks and challenges. Anal. Bioanal. Chem. 2007, 387, 1287–1299. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Michalak, K.; Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Sobolewska, J.; Woźniak, P.; Sobolewski, B. Treatment of the fluoroquinolone-associated disability: The pathobiochemical implications. Oxid. Med. Cell. Longev. 2017, 2017, 8023935. [Google Scholar] [CrossRef]
- Sarro, A.D.; Sarro, G.D. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects. Curr. Med. Chem. 2001, 8, 371–384. [Google Scholar] [CrossRef]
- Anderson, V.E.; Osheroff, N. Type II topoisomerases as targets for quinolone antibacterials: Turning Dr. Jekyll into Mr. Hyde. Curr. Pharm. Des. 2001, 7, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, N.; Alipanahi, Z.; Alipour, E.; Emami, S.; Faramarzi, M.A.; Samadi, N.; Khoshnevis, N.; Shafiee, A.; Foroumadi, A. Synthesis and antibacterial activity of novel levofloxacin derivatives containing a substitued thienylethyl moiety. DARU J. Pharm. Sci. 2012, 20, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Segatore, B.; Aetacci, D.; Perilli, M.; Franceschini, N.; Marchetti, F.; Amicosante, G. Bactericidal activity of levofloxacin and ciprofloxacin on clinical isolates of different phenotypes of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2000, 13, 223–226. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Jumbe, N.; Louie, A.; Leary, R.; Liu, W.; Deziel, M.R.; Tam, V.H.; Bachhawat, R.; Freeman, C.; Kahn, J.B.; Bush, K.; et al. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J. Clin. Investig. 2003, 112, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.R.; Talan, D.A.; Nichols, R.L.; Lucasti, C.; Corrado, M.; Morgan, N.; Fowler, C.L. Once-daily, high-dose levofloxacin versus ticarcillin-clavulanate alone or followed by amoxicillin-clavulanate for compilated skin and skin-structure infections: A randomized, open-label trial. Clin. Infect. Dis. 2002, 35, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.-H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Frade, V.M.F.; Dias, M.; Teixeira, A.C.S.C.; Palma, M.S.A. Environmental contamination by fluoroquinolones. Braz. J. Pharm. Sci. 2014, 50, 41–54. [Google Scholar] [CrossRef]
- Yamashita, N.; Yasojima, M.; Nakada, N.; Miyajima, K.; Komori, K.; Suzuki, Y.; Tanaka, H. Effects of antibacterial agents, levofloxacin and clarithromycin, on aquatic organisms. Water Sci. Technol. 2006, 53, 65–72. [Google Scholar] [CrossRef]
- Różalski, A.; Torzewska, A.; Moryl, M.; Kwil, I.; Maszewska, A.; Ostrowska, K.; Drzewiecka, D.; Zabłotni, A.; Palusiak, A.; Siwińska, M.; et al. Proteus sp.—An opportunistic bacterial pathogen-classification, swarming growth, clinical significance and virulence factors. Folia Biol. Oecol. 2012, 8, 1–17. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fovler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Appelhans, D.; Komber, H.; Morgner, R.; Schwarz, S.; Richter, S.; Brutschy, B.; Ionov, M.; Tonkikh, A.K.; Bryszewska, M.; et al. The influence of densely organized maltose shells on the biological properties of poly (propylene imine) dendrimers: New effects dependent on hydrogen bonding. Chem. Eur. J. 2008, 14, 7030–7041. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Appelhans, D.; Schwarz, S.; Klajnert, B.; Bryszewska, M.; Voit, B.; Rogers, M. Influence of surface functionality of poly (propylene imine) dendrimers on protease resistance and propagation of the scrapie prion protein. Biomacromolecules 2010, 11, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Wronska, N.; Felczak, A.; Zawadzka, K.; Poszepczyńska, M.; Różalska, S.; Bryszewska, M.; Appelhans, D.; Lisowska, K. Poly(propylene imine) dendrimers and amoxicillin as dual-action antibacterial agents. Molecules 2015, 20, 19330–19342. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Multidrug resistance in Gram-negative bacteria. Curr. Opin. Microbiol. 2001, 4, 500–508. [Google Scholar] [CrossRef]
- Heiden, T.C.; Dengler, E.; Kao, W.J.; Heideman, W.; Peterson, R.E. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol. Appl. Pharmacol. 2007, 225, 70–79. [Google Scholar] [CrossRef]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic amphiphilic dendrimers as antibacterial agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Rookmaker, M. Poly(propyleneimine) Dendrimers. In Polymer Data Handbook, 2nd ed.; Mark, J.E., Ed.; Oxford University Press: New York, NY, USA, 2009; pp. 979–982. [Google Scholar]
- Katir, N.; El Brahmi, N.; Marcotte, N.; Majoral, J.P.; Bousmina, M.; El Kadib, A. Orthogonal synthesis of covalent polydendrimer frameworks by fussing classical and onion-pell phosphorus-based dendritic units. Macromolecules 2016, 49, 5796–5805. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrońska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities. Molecules 2019, 24, 2894. https://doi.org/10.3390/molecules24162894
Wrońska N, Majoral JP, Appelhans D, Bryszewska M, Lisowska K. Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities. Molecules. 2019; 24(16):2894. https://doi.org/10.3390/molecules24162894
Chicago/Turabian StyleWrońska, Natalia, Jean Pierre Majoral, Dietmar Appelhans, Maria Bryszewska, and Katarzyna Lisowska. 2019. "Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities" Molecules 24, no. 16: 2894. https://doi.org/10.3390/molecules24162894
APA StyleWrońska, N., Majoral, J. P., Appelhans, D., Bryszewska, M., & Lisowska, K. (2019). Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities. Molecules, 24(16), 2894. https://doi.org/10.3390/molecules24162894




