Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling
Abstract
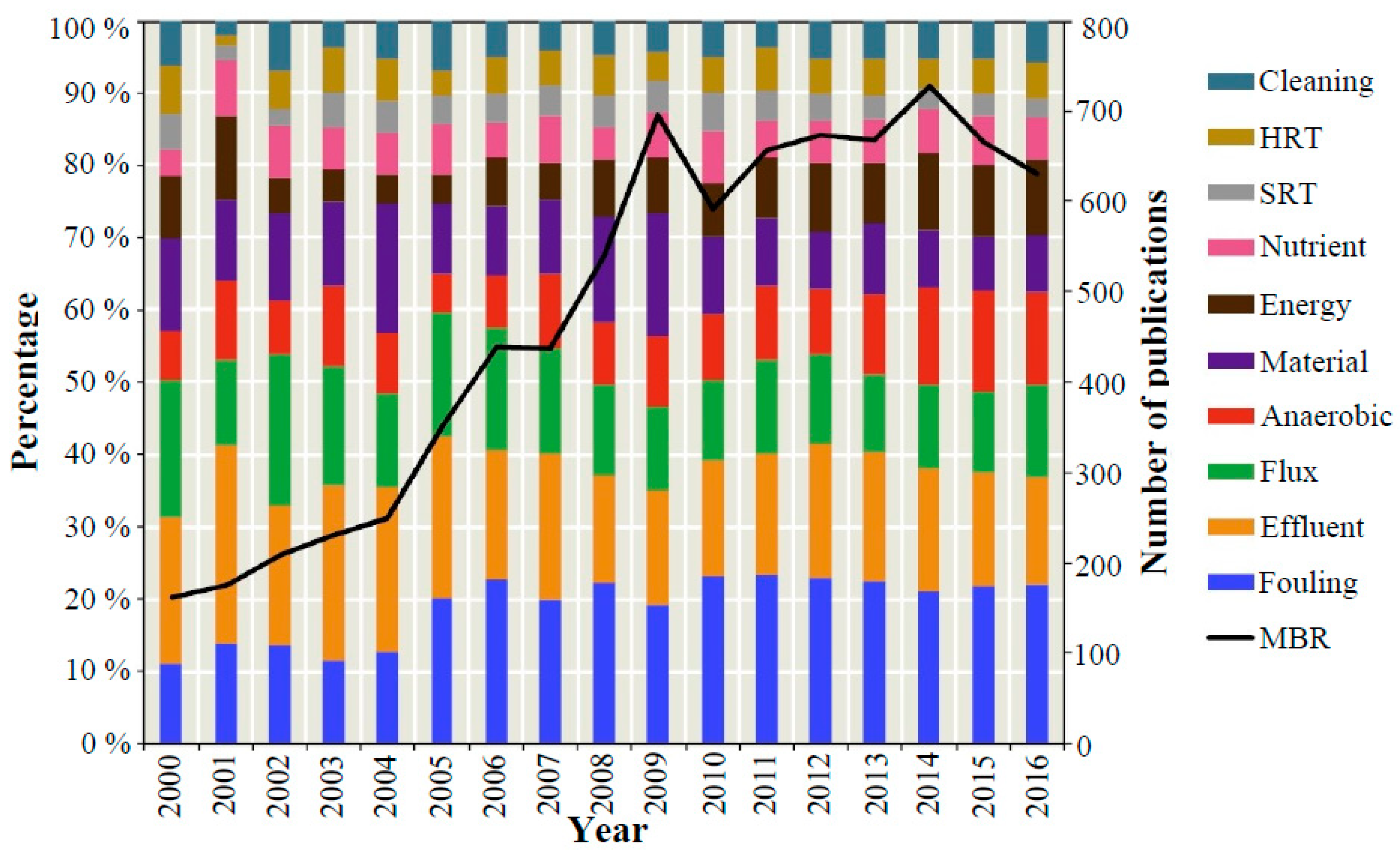
:1. Introduction
2. Major Biomass Characteristics Which Affect Membrane Fouling
2.1. Mixed Liquor Suspended Solids (MLSS)
2.1.1. Misinterpreted Role of MLSS in MBR Systems
2.1.2. Efforts to Correlate MLSS with Fouling-Critical Values
2.1.3. The Weak Correlation between MLSS Concentration and Membrane Fouling
- In a hypothetical side-by-side experiment, the same operating conditions are applied in two reactors, including the feed flow rate, the aeration rate and the flux, except for the MLSS concentration. Under this condition, the reactor with the lower MLSS concentration has a lower SRT and a higher F/M ratio, than its counterpart, resulting in increased fouling. Although low SRT and high F/M ratio are the main causes of higher fouling rate, it may be concluded that membrane fouling is more significant at lower MLSS concentrations.
- In another hypothetical example, the MLSS concentration can be gradually increased in order to study its effect on membrane fouling. Excess sludge removal may be paused or reduced to enable the increase of MLSS in this case. For these (dynamic) conditions, the apparent SRT, which is calculated by dividing the total sludge volume in the reactor by the sludge, which is removed every day, is no longer the effective SRT. Meanwhile, the F/M ratio is dynamically decreased, due to the increase of MLSS over time. Since these two crucial, fouling-affecting factors are dynamically changing, any observation made during this condition can be considered as irrelevant to the influence of MLSS. If the DO concentration and the mixing pattern also change as the MLSS concentration increases, then the observations made during the experiment cannot be regarded as valid.
- Conversely, if the MLSS concentration is gradually decreased by increasing the biomass removal, the effect of SRT on the lower MLSS concentration is higher, than the effect of steady-state SRT on MLSS. If the membrane fouling rate is measured under this condition, the apparent conclusion is that fouling increases as the MLSS concentration decreases.
- As the MLSS concentration increases, the OTE tends to decrease, because of the physical hindrance of the solids against the oxygen transfer to liquid, in the gas-liquid interface. As a consequence, the DO concentration can decrease, which in turn causes non-ideal conditions for the microorganisms and promote membrane fouling. If the effect of MLSS on DO is overlooked, then the higher MLSS concentration seems to be the cause of high fouling rate [11].
2.1.4. Bio-Floc Size
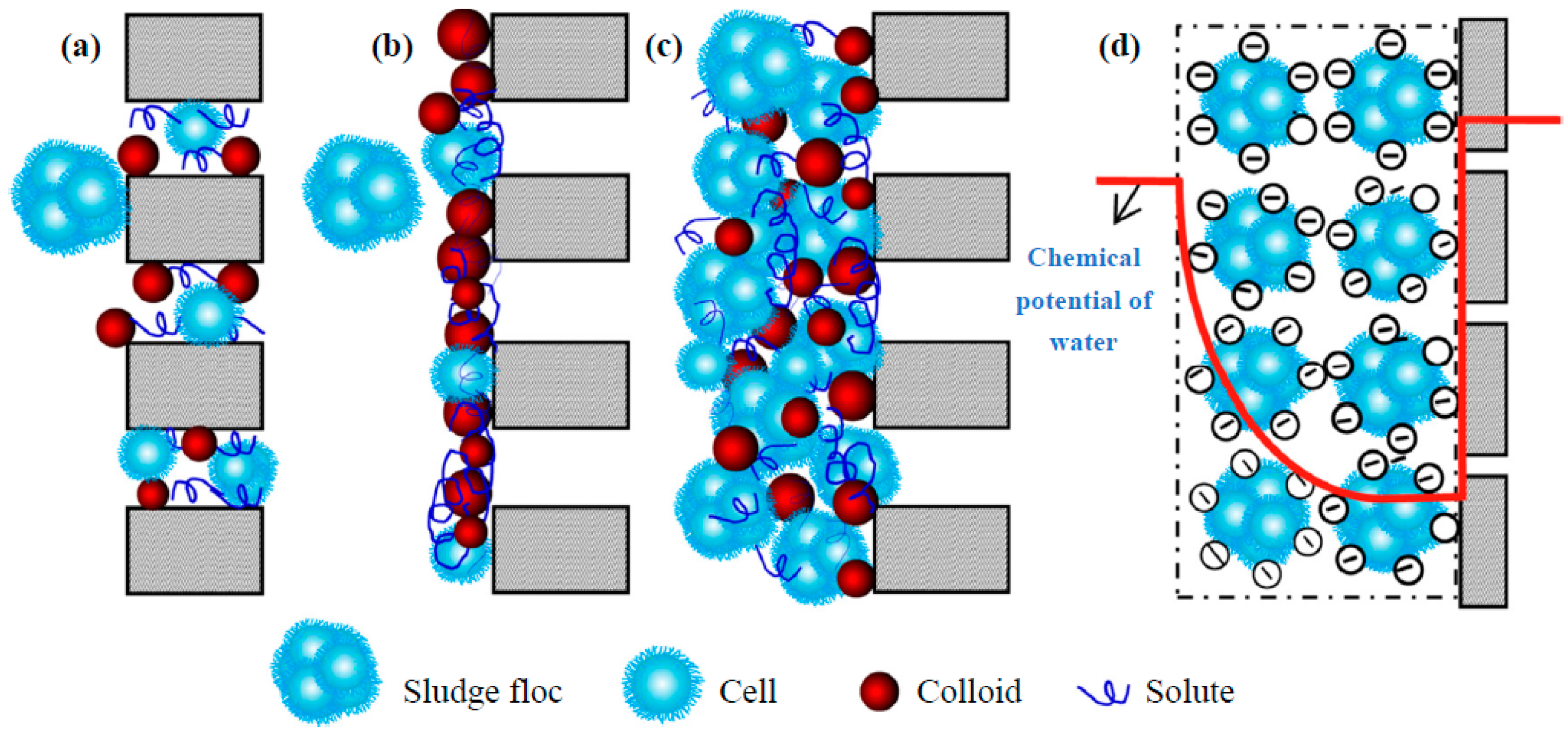
2.2. Colloidal Matter
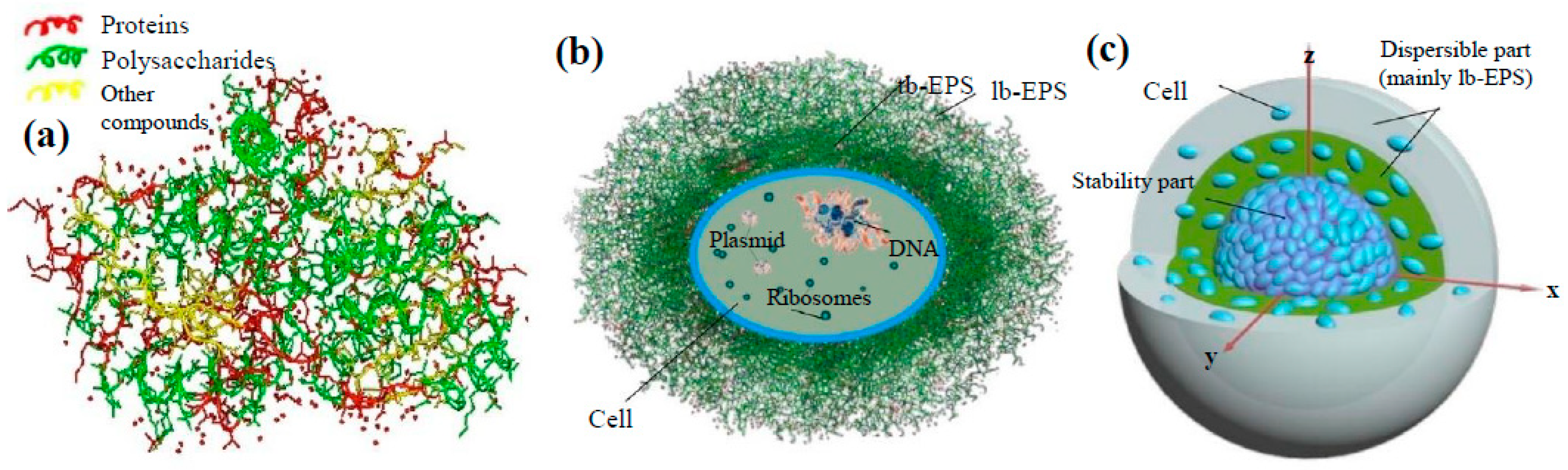
2.3. Extracellular Polymeric Substances (EPS)
2.3.1. Definition and Classification
2.3.2. The Dominant Role of SMP
2.3.3. Carbohydrate and Protein Fraction
2.3.4. Contribution of Humic Substances
2.3.5. Extraction and Quantification of EPS
3. Other Fouling-Related Wastewater and Biomass Characteristics
3.1. Temperature
- The increase of sludge viscosity, which is more significant than the permeate viscosity and can reduce the shear stress generated by the coarse air bubbling, commonly applied for membrane air scouring cleaning purposes.
- The intense deflocculation at low temperatures, which reduces the bio-floc size and releases EPS.
- The particle back-transport velocity, which decreases linearly with temperature increase, according to Brownian diffusion.
- The biodegradation of COD, which decreases with temperature increase and results in the high concentration of unbiodegraded soluble and particulate COD.
3.2. Viscosity
3.3. Dissolved Oxygen (DO)
3.4. Foaming
3.5. Hydrophobicity and Surface Charge
4. Mixed Liquor/Biomass Characterization
4.1. Direct Test Methods
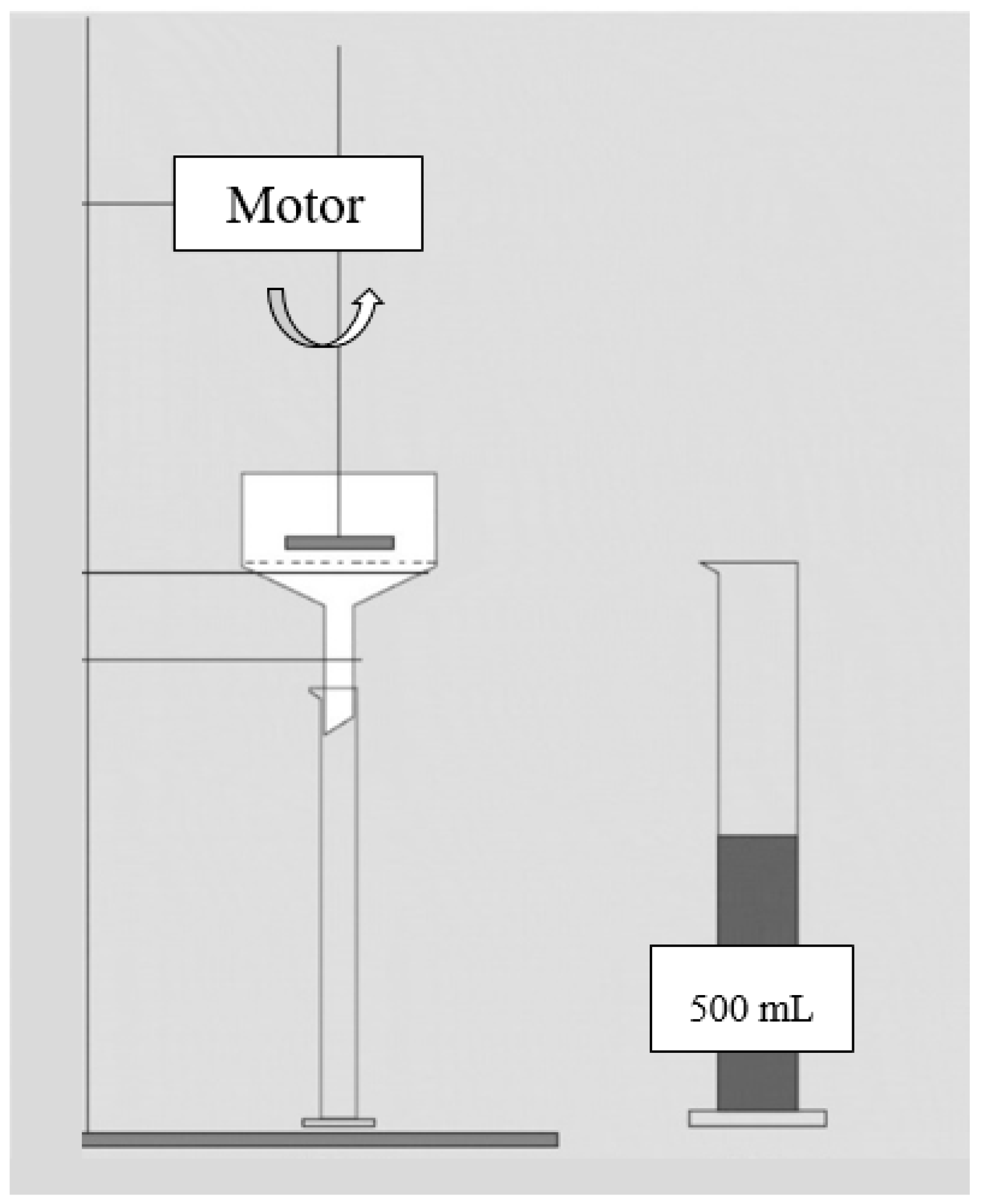
4.1.1. Free Drainage Test (FDT)
- The quality of the results can vary significantly because relatively small amounts of mixed liquor are used.
- There is only a slight driving force (i.e., gravity) to filter the mixed liquor, leading to the formation of a much milder, i.e., less compact, cake layer. Therefore, the obtained results are nearly free of the cake layer compaction effect.
- No cross-flow exists in the funnel and thus, all the particles and macromolecules in the sample can contribute to the cake layer formation. In contrast, larger particles are transported back to the bulk and do not contribute to cake layer formation in the actual filtration in MBR systems.
- Unlike the actual filtration in MBRs, where macromolecules (e.g., EPS) are the major components of the cake layer formation, in the FDT solid particles are the major components.
- Filterability is affected by the MLSS concentration, due to the lack of normalization against MLSS.
- Results are affected by the atmospheric pressure.
4.1.2. Modified Free Drainage Test (MFDT), or Sludge Filterability Index (SFI)
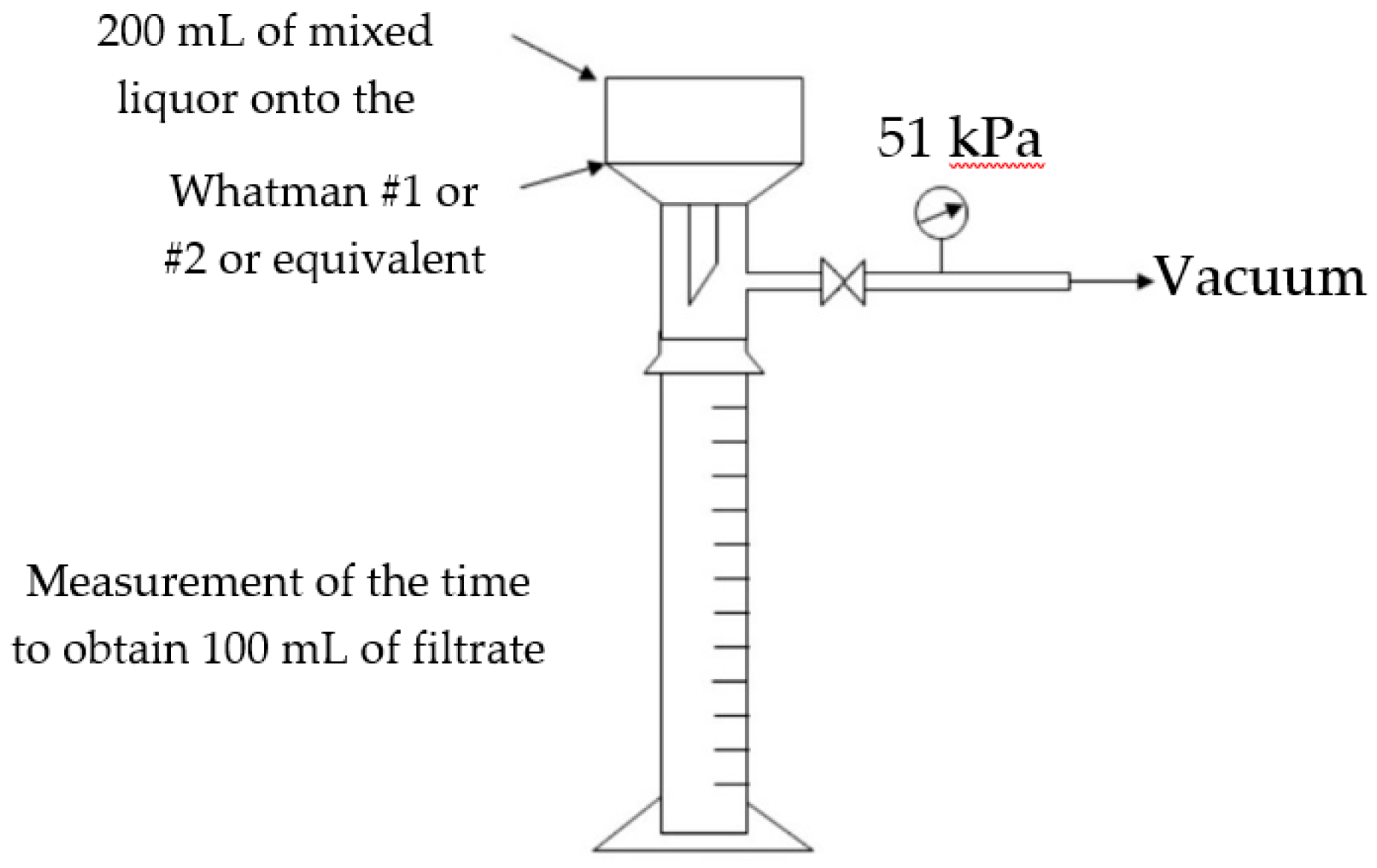
4.1.3. Time to Filter (TTF100)
4.1.4. Modified Fouling Index (MFI)
- 1)
- Filterability, L15 (L/m2∙h/bar), is estimated after a filtrate volume of 15 mL is obtained by dividing the flux by pressure. However, L15 cannot be normalized against a wide range of pressures, because flux is not proportional to the pressure, when the cake layer dominates the filterability decrease. Thus, the best practice to perform this filtration test is at a fixed ΔP (TMP) value Equation (9):
- 1)
- Fouling index, FI30, is calculated by dividing the observed flux after 30 min of filtration, J30, by the initial water flux, Jw,0. Similarly to L15, FI30 is also affected by the TMP and therefore, it should be measured at a fixed TMP value Equation (10):
- The weight of cake layer is not proportional to the filtrate volume in the actual membrane filtration, because of the existence of the particle back-transport phenomenon. Depending on the flux profile during filtration, not only the amount of the deposited particles, but also the structure of cake layer can vary.
- The cake layer compaction plays an important role in cake resistance, but MFI equations do not consider it. Depending on the pressure and flux during the stirred cell test, the extent of cake layer compaction may vary.
- Whichever laboratory test method is used, the hydrodynamics on the membrane surface are not the same as in real membrane filtration. Hydrodynamic conditions determine particle back-transport velocity and eventually the membrane fouling rates.
- The MFI is measured under a membrane fouling condition typically at higher TMP values, than in actual filtration. Cake layer formation and ageing patterns in short-term filtration procedures cannot be the same as in long-term filtration.
4.1.5. Delft Filtration Characterization Method (DFCM)
4.2. Indirect Test Methods
4.2.1. Capillary Suction Time (CST)
4.2.2. Colloidal TOC Measurement
4.2.3. Particle Size Analysis (PSA)
4.2.4. Relative Hydrophobicity (RH) Measurement
5. Conclusions
Funding
Conflicts of Interest
Table of Symbols
| ASP | Activated Sludge Process |
| bEPS | Bound Extracellular Polymeric Substances |
| BAP | Biomass Associated Products |
| BPC | Bio-Polymeric Clusters |
| BSA | Bovine Serum Albumin |
| COD | Chemical Oxygen Demand |
| DO | Dissolved Oxygen |
| EPS | Extracellular Polymeric Substances |
| F/M | Food to Microorganisms |
| FTIR | Fourier Transform Infra-Red |
| HF | Hollow Fibre |
| HPSEC | High-Performance Size Exclusion Chromatography |
| HRT | Hydraulic Residence Time |
| lb-EPS | Loosely Bound Extracellular Polymeric Substances |
| LC-OCND | Liquid Chromatography-Organic Carbon and Nitrogen Detector |
| MATH | Microbial Adhesion To Hydrocarbons |
| MBR | Membrane Bio-Reactor |
| MLSS | Mixed Liquor Suspended Solids |
| MW | Molecular Weight |
| NMR | Nuclear Magnetic Resonance |
| NOM | Natural Organic Matter |
| OTE | Oxygen Transfer Efficiency |
| PSA | Particle Size Analysis |
| PSD | Particle Size Distribution |
| RO | Reverse Osmosis |
| SEM | Scanning Electron Microscopy |
| sEPS | Soluble Extracellular Polymeric Substances |
| SMP | Soluble Microbial Products |
| SS | Suspended Solids |
| SRT | Solids Retention Time |
| tb-EPS | Tightly bound Extracellular Polymeric Substances |
| TEP | Transparent Exopolymer Particles |
| TMP | Trans-Membrane Pressure |
| TOC | Total Organic Carbon |
| UAP | Utilization Associated Products |
References
- Gkotsis, P.K.; Banti, D.C.; Peleka, E.N.; Zouboulis, A.I.; Samaras, P.E. Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies. Processes 2014, 2, 795–866. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Memb. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, M.; Leung, K.T.; Chen, J.; Hong, H.; Lin, H.; Liao, B.Q. A unified thermodynamic mechanism underlying fouling behaviors of soluble microbial products (SMPs) in a membrane bioreactor. Water Res. 2019, 149, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Bin, C.; Xiaochang, W.; Enrang, W. Effects of TMP, MLSS concentration and intermittent membrane permeation on a hybrid submerged MBR fouling. In Proceedings of the water-environment technology conference, Seoul, Korea, 7–10 June 2004. [Google Scholar]
- Chang, I.S.; Kim, S.N. Wastewater treatment using membrane filtration—Effect of biosolids concentration on cake resistance. Process Biochem. 2005, 40, 1307–1314. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, W.Y.; Lee, C.H. Comparison of the filtration characteristics between attached and suspended growth microorganisms in submerged membrane bioreactor. Water Res. 2001, 35, 2435–2445. [Google Scholar] [CrossRef]
- Rosenberger, S.; Evenblij, H.; te Poele, S.; Wintgens, T.; Laabs, C. The importance of liquid phase analyses to understand fouling in membrane assisted activated sludge processes—Six case studies of different European research groups. J. Memb. Sci. 2005, 263, 113–126. [Google Scholar] [CrossRef]
- Hong, S.P.; Bae, T.H.; Tak, T.M.; Hong, S.; Randall, A. Fouling control in activated sludge submerged hollow fiber membrane bioreactors. Desalination 2002, 143, 219–228. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Yoon, S.H. Membrane Bioreactor Processes: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cho, J.; Song, K.G.; Ahn, K.H. The activated sludge and microbial substances influences on membrane fouling in submerged membrane bioreactor: Unstirred batch cell test. Desalination 2005, 183, 425–429. [Google Scholar] [CrossRef]
- Lubbecke, S.; Vogelpohl, A.; Dewjanin, W. Wastewater treatment in a biological high performance system with high biomass concentration. Water Res. 1995, 29, 793–802. [Google Scholar] [CrossRef]
- Deng, L.; Guo, W.; Ngo, H.H.; Zhang, H.; Wang, J.; Li, J.; Xia, S.; Wu, Y. Biofouling and control approaches in membrane bioreactors. Bioresour. Technol. 2016, 221, 565–665. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, X. Effect of mixed liquor properties on fouling propensity in membrane bioreactors. J. Memb. Sci. 2009, 342, 88–96. [Google Scholar] [CrossRef]
- Lousada-Ferreira, M.; Geilvoet, S.; Moreau, A.; Atasoy, E.; Krzeminski, P.; van Nieuwenhuijzen, A.; van der Graaf, J. MLSS concentration: Still a poorly understood parameter in MBR filterability. Desalination 2010, 250, 618–622. [Google Scholar] [CrossRef]
- Ren, N.; Chen, Z.; Wang, A.; Hu, D. Removal of organic pollutants and analysis of MLSS-COD removal relationship at different HRTs in a submerged membrane bioreactor. Int. Biodeterior. Biodegradation 2005, 55, 279–284. [Google Scholar] [CrossRef]
- Flemming, H.C.; Schaule, G.; Griebe, G.; Schmitt, T.; Tamachkiarowa, A. Biofouling—the Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Ma, D.; Meng, Y.; Xia, C.; Gao, B.; Wang, Y. Fractionation, characterization and C-, N-disinfection byproduct formation of soluble microbial products in MBR processes. Bioresour. Technol. 2015, 198, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.J.; Bligh, M.W.; Kinsela, A.S.; Waite, T.D. Effect of iron on membrane fouling by alginate in the absence and presence of calcium. J. Memb. Sci. 2016, 497, 289–299. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors-characterisation, contradictions, cause and cures. J. Memb. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbourgh, N.J.; Farr, A.R.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Lee, W.; Jeon, J.H.; Cho, Y.; Chung, K.Y.; Min, B.R. Behavior of TMP according to membrane pore size. In Proceedings of the International congress on membranes and membrane processes (ICOM), Seoul, Korea, 21–26 August 2005. [Google Scholar]
- Kang, I.J.; Lee, C.H.; Kim, K.J. Characteristics of filtration membranes in a membrane coupled sequencing batch reactor system. Water Res. 2003, 37, 1192–1197. [Google Scholar] [CrossRef]
- Faust, L.; Temmink, H.; Zwijnenburg, A.; Kemperman, A.J.B.; Rijnaarts, H.H.M. Effect of dissolved oxygen concentration on the bioflocculation process in high loaded MBRs. Water Res. 2014, 66, 199–207. [Google Scholar] [CrossRef]
- Gao, D.W.; Fu, Y.; Tao, Y.; Li, X.X.; Xing, M.; Gao, X.H.; Ren, N.Q. Linking microbial community structure to membrane biofouling associated with varying dissolved oxygen concentrations. Bioresour. Technol. 2011, 102, 5626–5633. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, J. Influence of aeration on microbial polymers and membrane fouling in submerged membrane bioreactors. J. Memb. Sci. 2006, 276, 168–177. [Google Scholar] [CrossRef]
- Zhang, K.; Choi, H.; Dionysiou, D.D.; Sorial, G.A.; Oerther, D.B. Identifying pioneer bacterial species responsible for biofouling membrane bioreactors. Environ. Microbiol. 2006, 8, 433–440. [Google Scholar] [CrossRef]
- Banti, D.C.; Karayannakidis, P.D.; Samaras, P.; Mitrakas, M.G. An innovative bioreactor set-up that reduces membrane fouling by adjusting the filamentous bacterial population. J. Memb. Sci. 2017, 542, 430–438. [Google Scholar] [CrossRef]
- Thiemig, C. The importance of measuring the sludge filterability at an MBR: Introduction of a new method. Water Sci.Technol. 2012, 66, 9–14. [Google Scholar] [CrossRef]
- APHA. Standard Method for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Gkotsis, P.K.; Mitrakas, M.M.; Tolkou, A.K.; Zouboulis, A.I. Batch and continuous dosing of conventional and composite coagulation agents for fouling control in a pilot-scale MBR. Chem. Eng. J. 2017, 311, 255–264. [Google Scholar] [CrossRef]
- Côté, P. Future of membrane technology in worldwide sanitation. In Proceedings of the IWS Young Water Professionals Conference, Berlin, Germany, 4–6 June 2007. [Google Scholar]
- Tarnacki, K.; Lyko, S.; Wintgens, T.; Melin, T.; Natau, F. Impact of extra-cellular polymeric substances on the filterability of activated sludge in membrane bioreactors for landfill leachate treatment. Desalination 2005, 179, 181–190. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, H.; Husain, H. Identification of wastewater sludge characteristics to predict critical flux for membrane bioreactor processes. Water Res. 2006, 40, 205–212. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.H.; Chang, I.S. Effect of pump shear on the performance of a crossflow membrane bioreactor. Water Res. 2001, 35, 2137–2144. [Google Scholar] [CrossRef]
- Saini, G. Bacterial hydrophobicity: Assessment techniques, applications and extension to colloids. PhD. Thesis, Oregon State University, Corvallis, OR, USA, June 2010. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkotsis, P.K.; Zouboulis, A.I. Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling. Molecules 2019, 24, 2867. https://doi.org/10.3390/molecules24162867
Gkotsis PK, Zouboulis AI. Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling. Molecules. 2019; 24(16):2867. https://doi.org/10.3390/molecules24162867
Chicago/Turabian StyleGkotsis, Petros K., and Anastasios I. Zouboulis. 2019. "Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling" Molecules 24, no. 16: 2867. https://doi.org/10.3390/molecules24162867
APA StyleGkotsis, P. K., & Zouboulis, A. I. (2019). Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling. Molecules, 24(16), 2867. https://doi.org/10.3390/molecules24162867






