Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower
Abstract
1. The Plant of Crocus sativus L.
2. Analytical Methods for Crocus sativus L.
3. Bioactivity of Crocus sativus L. Compounds
3.1. Bioactivity and Bioavailability of the Three Main Compounds of Saffron
3.2. Bioactivity and Bioavailability of Phenolic Compounds of Crocus sativus L. Flower
4. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Moratalla-López, N.; Lorenzo, C.; Alonso, G.L.; Sánchez, A.M. Kaempferol Glycosides in Crocus: Sources, Biosynthesis, and Uses. In Kaempferol: Biosynthesis, Food Sources and Therapeutic Uses; Garde-Cerdán, T., Gonzalo-Diago, A., Eds.; Biochemistry research trends|Includes index; Nova Science Publisher’s, Inc.: Hauppauge, NY, USA, 2016; pp. 151–195. ISBN 9781634858281. [Google Scholar]
- Elmi, A.A. Food Security in the Arab Gulf Cooperation Council States. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 25, pp. 89–114. ISBN 978-3-319-58678-6. [Google Scholar]
- Shufeng, L.; Jingbin, L.; Wang, G.Y.; Hua, L.L. Portable Saffron Harvesting Machine. CN102860176B, 20 January 2016. [Google Scholar]
- González Tornero, D.; Medina Cebrián, J.M. Machine to Collect Saffron Flowers. ES2512165B1, 28 April 2015. [Google Scholar]
- Serrano-Díaz, J.; Sánchez, A.M.; Martínez-Tomé, M.; Winterhalter, P.; Alonso, G.L. A contribution to nutritional studies on Crocus sativus flowers and their value as food. J. Food Compos. Anal. 2013, 31, 101–108. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Alonso, G.L. The Chemical Composition of Saffron: Color, Taste and Aroma; Bomarzo SL: Albacete, Spain, 2006. [Google Scholar]
- Zeka, K.; Randolph, A. Saffron Crocus (Crocus sativus L.) as a source of kaempferol. In Kaempferol: Biosynthesis, Food Sources and Therapeutic Uses; Garde-Cerdán, T., Gonzalo-Diago, A., Eds.; Biochemistry research trends; Nova Science Publisher’s, Inc.: Hauppauge, NY, USA, 2016; pp. 197–215. ISBN 978-1-63485-858-8. [Google Scholar]
- Licón, C.; Carmona, M.; Llorens, S.; Berruga, M.I.; Alonso, G.L. Potential healthy effects of saffron spice (Crocus sativus L. stigmas) consumption. In Functional Plant Science and Biotechnology; Husaini, A.M., Ed.; Global Science Books: Kagawa ken, Japan, 2010; pp. 64–73. [Google Scholar]
- Li, C.Y.; Wu, T.S. Constituents of the pollen of Crocus sativus L. and their tyrosinase inhibitory activity. Chem. Pharm. Bull. 2002, 50, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Harborne, J.B.; Goldblatt, P. Correlations between phenolic patterns and tribal classification in the family iridaceae. Phytochemistry 1986, 25, 2135–2154. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Sánchez, A.M.; Martínez-Tomé, M.; Winterhalter, P.; Alonso, G.L. Flavonoid Determination in the Quality Control of Floral Bioresidues from Crocus sativus L. J. Agric. Food Chem. 2014, 62, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Acero de Mesa, N.; Muñoz-Mingarro, D.; Bielsa Pons, E.M. Biowaste Saffron Extracts as Active Ingredients of Cosmetic Products Antioxidants. ES2646415B1, 28 September 2018. [Google Scholar]
- Serrano-Díaz, J.; Sánchez, A.M.; Maggi, L.; Martínez-Tomé, M.; García-Diz, L.; Murcia, M.A.; Alonso, G.L. Increasing the Applications of Crocus sativus Flowers as Natural Antioxidants. J. Food Sci. 2012, 77, C1162–C1168. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). ISO 3632-1 Saffron (Crocus sativus L.). Part 1 (Specification) and Part 2 (Test Methods); ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin Esters, Picrocrocin and Its Related Compounds Present in Crocus sativus Stigmas and Gardenia jasminoides Fruits. Tentative Identification of Seven New Compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef]
- Aschoff, S. Beiträge sur kenntnis des safrans. Berl. Jb. Pharm. 1818, 19, 142–157. [Google Scholar]
- Moratalla-López, N.; Zalacain, A.; Bagur, M.J.; Salinas, M.R.; Alonso, G.L. Saffron. In FoodIntegrity Handbook. A Guide to Food Authenticity Issues and Analytical Solutions; Morin, J.F., Lees, M., Eds.; Eurofins Analytics France: Nantes, France, 2018. [Google Scholar]
- Sánchez, A.M.; Carmona, M.; Ordoudi, S.A.; Tsimidou, M.Z.; Alonso, G.L. Kinetics of individual crocetin ester degradation in aqueous extracts of saffron (Crocus sativus L.) upon thermal treatment in the dark. J. Agric. Food Chem. 2008, 56, 1627–1637. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; Sánchez, A.M.; Alonso, G.L. Saffron flavor: Compounds involved, biogenesis and human perception. Funct. Plant Sci. Biotechnol. 2010, 4, 45–55. [Google Scholar]
- Chrysanthou, A.; Pouliou, E.; Kyriakoudi, A.; Tsimidou, M.Z. Sensory threshold studies of picrocrocin, the major bitter compound of saffron. J. Food Sci. 2016, 81, S189–S198. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Maggi, L.; Carmona, M.; Alonso, G.L. Authentication of saffron spice (Crocus sativus L.). In Progress in Authentication of Food and Wine; Ebeler, S.E., Takeoka, G.R., Winterhalter, P., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1081, pp. 309–331. ISBN 978-0-8412-2670-8. [Google Scholar]
- Winterstein, E.; Teleczky, J. Constituents of the saffron. I. Picrocrocin. Helv. Chim. Acta 1922, 5, 376–400. [Google Scholar] [CrossRef]
- Kuhn, R.; Winterstein, E. Die dihydrovernindung der isormeren bixine und die elektronen-konfiguration der polyene. Berichte Dtsch. Chem. Ges. 1934, 67, 344–347. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Straubinger, M.; Jezussek, M.; Waibel, R.; Winterhalter, P. Novel glycosidic constituents from saffron. J. Agric. Food Chem. 1997, 45, 1678–1681. [Google Scholar] [CrossRef]
- Straubinger, M.; Bau, B.; Eckstein, S.; Fink, M.; Winterhalter, P. Identification of novel glycosidic aroma precursors in saffron (Crocus sativus L.). J. Agric. Food Chem. 1998, 46, 3238–3243. [Google Scholar] [CrossRef]
- del Campo, C.P.; Carmona, M.; Maggi, L.; Kanakis, C.D.; Anastasaki, E.G.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Picrocrocin Content and Quality Categories in Different (345) Worldwide Samples of Saffron (Crocus sativus L.). J. Agric. Food Chem. 2010, 58, 1305–1312. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Carmona, M.; Jarén-Galán, M.; Mínguez Mosquera, M.I.; Alonso, G.L. Picrocrocin Kinetics in Aqueous Saffron Spice Extracts (Crocus sativus L.) upon Thermal Treatment. J. Agric. Food Chem. 2011, 59, 249–255. [Google Scholar] [CrossRef]
- Rödel, W.; Petrizka, M. Analysis of volatile components of saffron. J. High Resolut. Chromatogr. 1991, 14, 771–774. [Google Scholar] [CrossRef]
- Zarghami, N.S.; Heinz, D.E. Monoterpene aldehydes and isophorone-related compounds of saffron. Phytochemistry 1971, 10, 2755–2761. [Google Scholar] [CrossRef]
- Zarghami, N.S.; Heinz, D.E. The volatile constituents of saffron (Crocus sativus L.). Lebensm. Wiss. Technol. 1971, 4, 43–45. [Google Scholar]
- Semiond, D.; Dautraix, S.; Desage, M.; Majdalani, R.; Casabianca, H.; Brazier, J.L. Identification and isotopic analysis of safranal from supercritical fluid extraction and alcoholic extracts of saffron. Anal. Lett. 1996, 29, 1027–1039. [Google Scholar] [CrossRef]
- Alonso, G.L.; Salinas, M.R.; Sánchez-Fernández, M.A.; Garijo, J. Note. Safranal content in Spanish saffron. Food Sci. Technol. Int. 2001, 7, 225–229. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Bioactive volatiles in Sicilian (South Italy) saffron: Safranal and its related compounds. J. Essent. Oil Res. 2017, 29, 221–227. [Google Scholar] [CrossRef]
- Alonso, G.L.; Salinas, M.R. Method to determine the authenticity of aroma of saffron (Crocus sativus L.). J. Food Prot. 1998, 61, 1525–1528. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Salinas, M.R.; Alonso, G.L. A New Approach to Saffron Aroma. Crit. Rev. Food Sci. Nutr. 2007, 47, 145–159. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; del Campo, C.P.; Kanakis, C.D.; Anastasaki, E.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Worldwide market screening of saffron volatile composition. J. Sci. Food Agric. 2009, 89, 1950–1954. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Daferera, D.J.; Tarantilis, P.A.; Polissiou, M.G. Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde (HTCC) in Greek saffron. J. Agric. Food Chem. 2004, 52, 4515–4521. [Google Scholar] [CrossRef]
- Himeno, H.; Sano, K. Synthesis of crocin, picrocrocin and safranal by saffron stigma-like structures proliferated in Vitro. Agric. Biol. Chem. 1987, 51, 2395–2400. [Google Scholar] [CrossRef]
- Iborra, J.L.; Castellar, M.R.; Cánovas, M.; Manjón, A. Analysis of a packed-bed reactor for hydrolysis of picrocrocin by inmobilized β-glucosidase. Enzyme Microb. Technol. 1993, 15, 780–784. [Google Scholar] [CrossRef]
- García-Rodríguez, M.V.; López-Córcoles, H.; Alonso, G.L.; Pappas, C.S.; Polissiou, M.G.; Tarantilis, P.A. Comparative evaluation of an ISO 3632 method and an HPLC-DAD method for safranal quantity determination in saffron. Food Chem. 2017, 221, 838–843. [Google Scholar] [CrossRef]
- O.J.E.C. (Official Journal of the European Communities). Publication of an application for registration pursuant to Article 6(2) of regulation (EEC) No 2081/92 on the protection of geographical indications and designations of origin. PDO Azafrán de La Mancha. 173/4. 22 June 2000. [Google Scholar]
- Alonso, G.L.; Salinas, M.R.; Saez, J.R. Crocin as coloring in the food industry. In Recent Research Development in Agricultural and Food Chemistry; Pandalai, S.G., Ed.; Research Singpost Pandalai: Trivandrum, India, 1998; Volume 2, pp. 141–153. ISBN 81-86481-76-1. [Google Scholar]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods a Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem, 2014; ISBN 978-91-87461-59-0. [Google Scholar]
- García-Rodríguez, M.V.; Serrano-Díaz, J.; Tarantilis, P.A.; López-Córcoles, H.; Carmona, M.; Alonso, G.L. Determination of Saffron Quality by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2014, 62, 8068–8074. [Google Scholar] [CrossRef]
- Vahedi, M.; Kabiri, M.; Salami, S.A.; Rezadoost, H.; Mirzaie, M.; Kanani, M.R. Quantitative HPLC-based metabolomics of some Iranian saffron (Crocus sativus L.) accessions. Ind. Crops Prod. 2018, 118, 26–29. [Google Scholar] [CrossRef]
- Culleré, L.; San-Juan, F.; Cacho, J. Characterisation of aroma active compounds of Spanish saffron by gas chromatography–olfactometry: Quantitative evaluation of the most relevant aromatic compounds. Food Chem. 2011, 127, 1866–1871. [Google Scholar] [CrossRef]
- Aliakbarzadeh, G.; Sereshti, H.; Parastar, H. Pattern recognition analysis of chromatographic fingerprints of Crocus sativus L. secondary metabolites towards source identification and quality control. Anal. Bioanal. Chem. 2016, 408, 3295–3307. [Google Scholar] [CrossRef]
- Rubert, J.; Lacina, O.; Zachariasova, M.; Hajslova, J. Saffron authentication based on liquid chromatography high resolution tandem mass spectrometry and multivariate data analysis. Food Chem. 2016, 204, 201–209. [Google Scholar] [CrossRef]
- Maggi, L.; Sánchez, A.M.; Carmona, M.; Kanakis, C.D.; Anastasaki, E.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Rapid determination of safranal in the quality control of saffron spice (Crocus sativus L.). Food Chem. 2011, 127, 369–373. [Google Scholar] [CrossRef]
- Liu, J.; Chen, N.; Yang, J.; Yang, B.; Ouyang, Z.; Wu, C.; Yuan, Y.; Wang, W.; Chen, M. An integrated approach combining HPLC, GC/MS, NIRS, and chemometrics for the geographical discrimination and commercial categorization of saffron. Food Chem. 2018, 253, 284–292. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. The potentiality of NMR-based metabolomics in food science and food authentication assessment. Magn. Reson. Chem. 2018. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. Chapter 4—Nuclear Magnetic Resonance and Chemometrics to Assess Geographical Origin and Quality of Traditional Food Products. In Advances in Food and Nutrition Research; Taylor, S.L., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 59, pp. 87–165. ISBN 1043-4526. [Google Scholar]
- Yilmaz, A.; Nyberg, N.T.; Mølgaard, P.; Asili, J.; Jaroszewski, J.W. 1H NMR metabolic fingerprinting of saffron extracts. Metabolomics 2010, 6, 511–517. [Google Scholar] [CrossRef]
- Consonni, R.; Ordoudi, A.S.; Cagliani, R.L.; Tsiangali, M.; Tsimidou, Z.M. On the Traceability of Commercial Saffron Samples Using 1H-NMR and FT-IR Metabolomics. Molecules 2016, 21, 286. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Cagliani, L.R.; Lalou, S.; Naziri, E.; Tsimidou, M.Z.; Consonni, R. 1H NMR-based metabolomics of saffron reveals markers for its quality deterioration. Food Res. Int. 2015, 70, 1–6. [Google Scholar] [CrossRef]
- Carmona, M.; Sánchez, A.M.; Ferreres, F.; Zalacain, A.; Tomás-Barberán, F.; Alonso, G.L. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Rocchi, R.; Mascini, M.; Sergi, M.; Compagnone, D.; Mastrocola, D.; Pittia, P. Crocins pattern in saffron detected by UHPLC-MS/MS as marker of quality, process and traceability. Food Chem. 2018, 264, 241–249. [Google Scholar] [CrossRef]
- Carmona, M.; Martínez, J.; Zalacain, A.; Rodríguez-Méndez, M.L.; de Saja, J.A.; Alonso, G.L. Analysis of saffron volatile fraction by TD–GC–MS and e-nose. Eur. Food Res. Technol. 2006, 223, 96–101. [Google Scholar] [CrossRef]
- Nenadis, N.; Heenan, S.; Tsimidou, M.Z.; Van Ruth, S. Applicability of PTR-MS in the quality control of saffron. Food Chem. 2016, 196, 961–967. [Google Scholar] [CrossRef]
- Anastasaki, E.G.; Kanakis, C.D.; Pappas, C.; Maggi, L.; Zalacain, A.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Quantification of Crocetin Esters in Saffron (Crocus sativus L.) Using Raman Spectroscopy and Chemometrics. J. Agric. Food Chem. 2010, 58, 6011–6017. [Google Scholar] [CrossRef]
- Zalacain, A.; Ordoudi, S.A.; Díaz-Plaza, E.M.; Carmona, M.; Blázquez, I.; Tsimidou, M.Z.; Alonso, G.L. Near-Infrared Spectroscopy in Saffron Quality Control: Determination of Chemical Composition and Geographical Origin. J. Agric. Food Chem. 2005, 53, 9337–9341. [Google Scholar] [CrossRef]
- del Campo, C.P.; Garde-Cerdán, T.; Sánchez, A.M.; Maggi, L.; Carmona, M.; Alonso, G.L. Determination of free amino acids and ammonium ion in saffron (Crocus sativus L.) from different geographical origins. Food Chem. 2009, 114, 1542–1548. [Google Scholar] [CrossRef]
- Anastasaki, E.; Kanakis, C.; Pappas, C.; Maggi, L.; del Campo, C.P.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Differentiation of saffron from four countries by mid-infrared spectroscopy and multivariate analysis. Eur. Food Res. Technol. 2010, 230, 571–577. [Google Scholar] [CrossRef]
- Alonso, G.L.; Sanchez-Fernandez, M.A.; Saez, J.R.; Zalacain, A.; Salinas, M.R. Evaluation of the color of Spanish saffron using tristimulus colorimetry. Ital. J. Food Sci. 2003, 15, 249–258. [Google Scholar]
- Bohm, B.A. Introduction to Flavonoids; Harwood Academic Publishers: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Calderon-Montano, J.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- Straubinger, M.; Jezussek, M.; Waibel, R.; Winterhalter, P. Two kaempferol sophorosides from Crocus sativus. Nat. Prod. Lett. 1997, 10, 213–216. [Google Scholar] [CrossRef]
- Zeka, K.; Ruparelia, K.C.; Continenza, M.A.; Stagos, D.; Vegliò, F.; Arroo, R.R.J. Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia 2015, 107, 128–134. [Google Scholar] [CrossRef]
- Goupy, P.; Vian, M.A.; Chemat, F.; Caris-Veyrat, C. Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of Crocus sativus by ultra performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections. Ind. Crops Prod. 2013, 44, 496–510. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Montoro, P.; Fenu, M.A.; Pizza, C. Antioxidant activity, cytotoxic activity and metabolic profiling of juices obtained from saffron (Crocus sativus L.) floral by-products. Food Chem. 2016, 199, 18–27. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Jerz, G.; Serrano-Díaz, J.; Alonso, G.L.; Winterhalter, P. Flavonol composition and isolation of kaempferol 3-sophoroside from saffron (Crocus sativus L.) floral bio-residues. In Proceedings of the 40. Deutscher Lebensmittelchemikertag 231, Halle, Germany, 12–14 September 2011. [Google Scholar]
- Moratalla-López, N.; Lorenzo, C.; Chaouqi, S.; Sánchez, A.M.; Alonso, G.L. Kinetics of polyphenol content of dry flowers and floral bio-residues of saffron at different temperatures and relative humidity conditions. Food Chem. 2019, 290, 87–94. [Google Scholar] [CrossRef]
- Ulbricht, C.; Conquer, J.; Costa, D.; Hollands, W.; Iannuzzi, C.; Isaac, R.; Jordan, J.K.; Ledesma, N.; Ostroff, C.; Serrano, J.M.G.; et al. An evidence-based systematic review of saffron (Crocus sativus) by the natural standard research collaboration. J. Diet. Suppl. 2011, 8, 58–114. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Avicenna’s (Ibn Sina) the canon of medicine and saffron (Crocus sativus): A review. Phytother. Res. 2013, 27, 475–483. [Google Scholar] [CrossRef]
- Bolhassani, A.; Khavari, A.; Bathaie, S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta BBA Rev. Cancer 2014, 1845, 20–30. [Google Scholar] [CrossRef]
- Bagur, M.J.; Alonso Salinas, G.; Jiménez-Monreal, A.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2018, 23, 30. [Google Scholar] [CrossRef]
- Razak, S.I.A.; Anwar Hamzah, M.S.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A review on medicinal properties of saffron toward major diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iran. J. Basic Med. Sci. 2018, 21, 1091–1099. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Ansari, M.A.; Ahmad, M.; Saleem, S.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005, 81, 805–813. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Z.; Jin, T. Crocin protects against cerebral-ischemia-induced damage in aged rats through maintaining the integrity of blood-brain barrier. Restor. Neurol. Neurosci. 2017, 35, 65–75. [Google Scholar] [CrossRef]
- Ginwala, R.; McTish, E.; Raman, C.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.; Sagar, D.; Jain, P.; Khan, Z.K. Apigenin, a natural flavonoid, attenuates EAE severity through the modulation of dendritic cell and other immune cell functions. J. Neuroimmune Pharmacol. 2016, 11, 36–47. [Google Scholar] [CrossRef]
- Moure, A.; Franco, D.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Lema, J.M. Evaluation of extracts from Gevuina avellana Hulls as antioxidants. J. Agric. Food Chem. 2000, 48, 3890–3897. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Sinakos, Z.; Papageorgiou, V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother. Res. 2005, 19, 997–1000. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Tajmir-Riahi, H.A.; Polissiou, M.G. Crocetin, dimethylcrocetin, and safranal bind human serum albumin: Stability and antioxidative properties. J. Agric. Food Chem. 2007, 55, 970–977. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Pappas, C.; Bariyanga, J.; Tajmir-Riahi, H.A.; Polissiou, M.G. An overview of structural features of DNA and RNA complexes with saffron compounds: Models and antioxidant activity. J. Photochem. Photobiol. B 2009, 95, 204–212. [Google Scholar] [CrossRef]
- Mashmoul, M.; Azlan, A.; Khaza’ai, H.; Yusof, B.N.M.; Noor, S.M. Saffron: A natural potent antioxidant as a promising anti-obesity drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef]
- Farahmand, S.K.; Samini, F.; Samini, M.; Samarghandian, S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology 2013, 14, 63–71. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Food Biotechnol. Plant Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef]
- Erdman, J.L., Jr.; Bierer, T.T.; Gugger, E. Absorption and transport of carotenoids. Ann. N. Y. Acad. Sci. 2006, 691, 75–85. [Google Scholar] [CrossRef]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [CrossRef]
- Chryssanthi, D.G.; Lamari, F.N.; Georgakopoulos, C.D.; Cordopatis, P. A new validated SPE-HPLC method for monitoring crocetin in human plasma—Application after saffron tea consumption. J. Pharm. Biomed. Anal. 2011, 55, 563–568. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Musumeci, T.; Cardile, V.; Graziano, A.; Salerno, L.; Raciti, G.; Crascì, L.; Panico, A.; Puglisi, G. Nanotechnological approach to increase the antioxidant and cytotoxic Efficacy of crocin and crocetin. Planta Med. J. 2018, 85, 258–265. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Xu, G.; Zheng, S.; Sun, S.; Wen, N.; Sheng, L.; Shi, Y.; Zhang, Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007, 18, 64–72. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z.; O’Callaghan, Y.C.; Galvin, K.; O’Brien, N.M. Changes in total and individual crocetin esters upon in vitro gastrointestinal digestion of saffron aqueous extracts. J. Agric. Food Chem. 2013, 61, 5318–5327. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; O’Callaghan, Y.C.; Galvin, K.; Tsimidou, M.Z.; O’Brien, N.M. Cellular transport and bioactivity of a major saffron apocarotenoid, picrocrocin (4-(β-d-Glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde). J. Agric. Food Chem. 2015, 63, 8662–8668. [Google Scholar] [CrossRef]
- Lautenschläger, M.; Sendker, J.; Hüwel, S.; Galla, H.J.; Brandt, S.; Düfer, M.; Riehemann, K.; Hensel, A. Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine 2015, 22, 36–44. [Google Scholar] [CrossRef]
- Linardaki, Z.I.; Orkoula, M.G.; Kokkosis, A.G.; Lamari, F.N.; Margarity, M. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem. Toxicol. 2013, 52, 163–170. [Google Scholar] [CrossRef]
- Yoshino, F.; Yoshida, A.; Umigai, N.; Kubo, K.; Lee, M.C.I. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J. Clin. Biochem. Nutr. 2011, 49, 182–187. [Google Scholar] [CrossRef]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J. Agric. Food Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef]
- He, K.; Si, P.; Wang, H.; Tahir, U.; Chen, K.; Xiao, J.; Duan, X.; Huang, R.; Xiang, G. Crocetin induces apoptosis of BGC-823 human gastric cancer cells. Mol. Med. Rep. 2014, 9, 521–526. [Google Scholar] [CrossRef]
- Zheng, S.; Qian, Z.; Tang, F.; Sheng, L. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem. Pharmacol. 2005, 70, 1192–1199. [Google Scholar] [CrossRef]
- Umigai, N.; Murakami, K.; Ulit, M.V.; Antonio, L.S.; Shirotori, M.; Morikawa, H.; Nakano, T. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine 2011, 18, 575–578. [Google Scholar] [CrossRef]
- Christodoulou, E.; Grafakou, M.; Skaltsa, E.; Kadoglou, N.; Kostomitsopoulos, N.; Valsami, G. Preparation, chemical characterization and determination of crocetin’s pharmacokinetics after oral and intravenous administration of saffron (Crocus sativus L.) aqueous extract to C57/ BL 6J mice. J. Pharm. Pharmacol. 2019, 71, 753–764. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Rahimi, A. Effect of safranal from Crocus sativus on extracellular hippocampal levels of glutamate and aspartate during kainic acid seizures in anesthetized rats. Planta Med. 2008, 73, 1441–1445. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghnia, H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of GABAergic and opioids systems. Phytomedicine 2007, 14, 256–262. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Talebzadeh, F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia 2005, 76, 722–724. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar]
- Gout, B.; Bourges, C.; Paineau-Dubreuil, S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr. Res. 2010, 30, 305–313. [Google Scholar] [CrossRef]
- Wani, B.A.; Hamza, A.K.R.; Mohidin, F.A. Saffron: A repository of medicinal properties. J. Med. Plant Res. 2011, 5, 2131–2135. [Google Scholar]
- Agha-Hosseini, M.; Kashani, L.; Aleyaseen, A.; Ghoreishi, A.; Rahmanpour, H.; Zarrinara, A.; Akhondzadeh, S. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: A double-blind, randomised and placebo-controlled trial. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 515–519. [Google Scholar] [CrossRef]
- Sugiura, M.; Shoyama, Y.; Saito, H.; Abe, K. The effects of ethanol and crocin on the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Jpn. J. Pharmacol. 1995, 67, 395–397. [Google Scholar] [CrossRef]
- Abe, K.; Sugiura, M.; Yamaguchi, S.; Shoyama, Y.; Saito, H. Saffron extract prevents acetaldehyde-induced inhibition of long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1999, 851, 287–289. [Google Scholar] [CrossRef]
- Ghazavi, A.; Mosayebi, G.; Salehi, H.; Abtahi, H. Effect of ethanol extract of saffron (Crocus sativus L.) on the inhibition of experimental autoimmune encephalomyelitis in C57bl/6 mice. Pak. J. Biol. Sci. 2009, 12, 690–695. [Google Scholar]
- Ghaffari, S.; Hatami, H.; Dehghan, G. Saffron ethanolic extract attenuates oxidative stress, spatial learning, and memory impairments induced by local injection of ethidium bromide. Res. Pharm. Sci. 2015, 10, 222–232. [Google Scholar]
- Zheng, Y.Q.; Liu, J.X.; Wang, J.N.; Xu, L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007, 1138, 86–94. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Liu, C.; Fang, C. Protective effects of crocetin pretreatment on myocardial injury in an ischemia/reperfusion rat model. Eur. J. Pharmacol. 2014, 741, 290–296. [Google Scholar] [CrossRef]
- Gainer, J.V.; Nugent, R. Effect of increasing the plasma oxygen diffusivity on experimental cryogenic edema. J. Neurosurg. 1976, 45, 535–538. [Google Scholar] [CrossRef]
- Seyde, W.C.; McKernan, D.J.; Laudeman, T.; Gainer, J.L.; Longnecker, D.E. Carotenoid compound crocetin improves cerebral oxygenation in hemorrhaged rats. J. Cereb. Blood Flow Metab. 1986, 6, 703–707. [Google Scholar] [CrossRef]
- Holloway, G.M.; Gainer, J.L. The carotenoid crocetin enhances pulmonary oxygenation. J. Appl. Physiol. 1988, 65, 683–686. [Google Scholar] [CrossRef]
- Gainer, J.L.; Stennett, A.K.; Murray, R.J. The effect of trans sodium crocetinate (TSC) in a rat oleic acid model of acute lung injury. Pulm. Pharmacol. Ther. 2005, 18, 213–216. [Google Scholar] [CrossRef]
- Gainer, J.L. Trans-sodium crocetinate for treating hypoxia/ischemia. Expert Opin. Investig. Drugs 2008, 17, 917–924. [Google Scholar] [CrossRef]
- Yang, R.; Vernon, K.; Thomas, A.; Morrison, D.; Qureshi, N.; Van Way, C.W., 3rd. Crocetin reduces activation of hepatic apoptotic pathways and improves survival in experimental hemorrhagic shock. JPEN J. Parenter. Enteral Nutr. 2011, 35, 107–113. [Google Scholar] [CrossRef]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid. Based Complement. Altern. Med. ECAM 2012, 2012, 429124. [Google Scholar] [CrossRef]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Vis. Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef]
- Baziar, S.; Aqamolaei, A.; Khadem, E.; Mortazavi, S.H.; Naderi, S.; Sahebolzamani, E.; Mortezaei, A.; Jalilevand, S.; Mohammadi, M.R.; Shahmirzadi, M.; et al. Crocus sativus L. versus methylphenidate in treatment of children with attention-deficit/hyperactivity disorder: A randomized, double-blind pilot study. J. Child Adolesc. Psychopharmacol. 2019, 29, 205–212. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Sabet, M.S.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Shafiee Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology 2010, 207, 637–643. [Google Scholar] [CrossRef]
- Farokhnia, M.; Shafiee Sabet, M.; Iranpour, N.; Gougol, A.; Yekehtaz, H.; Alimardani, R.; Farsad, F.; Kamalipour, M.; Akhondzadeh, S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 351–359. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Fallah-Pour, H.; Afkham, K.; Jamshidi, A.H.; Khalighi-Cigaroudi, F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement. Altern. Med. 2004, 4, 12. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Tahmacebi-Pour, N.; Noorbala, A.A.; Amini, H.; Fallah-Pour, H.; Jamshidi, A.H.; Khani, M. Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother. Res. 2005, 19, 148–151. [Google Scholar] [CrossRef]
- Noorbala, A.A.; Akhondzadeh, S.; Tahmacebi-Pour, N.; Jamshidi, A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef]
- Shahmansouri, N.; Farokhnia, M.; Abbasi, S.H.; Kassaian, S.E.; Noorbala Tafti, A.A.; Gougol, A.; Yekehtaz, H.; Forghani, S.; Mahmoodian, M.; Saroukhani, S.; et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014, 155, 216–222. [Google Scholar] [CrossRef]
- Talaei, A.; Hassanpour Moghadam, M.; Sajadi Tabassi, S.A.; Mohajeri, S.A. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: A randomized, double-blind, placebo-controlled, pilot clinical trial. J. Affect. Disord. 2015, 174, 51–56. [Google Scholar] [CrossRef]
- Ghajar, A.; Neishabouri, S.; Velayati, N.; Jahangard, L.; Matinnia, N.; Haghighi, M.; Ghaleiha, A.; Afarideh, M.; Salimi, S.; Meysamie, A.; et al. Crocus sativus L. versus citalopram in the treatment of major depressive disorder with anxious distress: A double-blind, controlled clinical trial. Pharmacopsychiatry 2017, 50, 152–160. [Google Scholar] [CrossRef]
- De Monte, C.; Carradori, S.; Chimenti, P.; Secci, D.; Mannina, L.; Alcaro, F.; Petzer, A.; N’Da, C.I.; Gidaro, M.C.; Costa, G.; et al. New insights into the biological properties of Crocus sativus L.: Chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014, 82, 164–171. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Hoshyar, R.; Miri, H.; Sadeghizadeh, M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem. Cell Biol. 2013, 91, 397–403. [Google Scholar] [CrossRef]
- Samarghandian, S.; Shoshtari, M.E.; Sargolzaei, J.; Hossinimoghadam, H.; Farahzad, J.A. Anti-tumor activity of safranal against neuroblastoma cells. Pharmacogn. Mag. 2014, 10, S419–S424. [Google Scholar]
- Bakshi, H.; Sam, S.; Rozati, R.; Sultan, P.; Rathore, B.; Lone, Z.; Sharma, M.; Triphati, J.; Saxena, R.C. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by crocin from Kashmiri saffron in a human pancreatic cancer cell line. Asian Pac. J. Cancer Prev. 2010, 11, 675–679. [Google Scholar]
- Sun, Y.; Xu, H.J.; Zhao, Y.X.; Wang, L.Z.; Sun, L.R.; Wang, Z.; Sun, X.F. Crocin exhibits antitumor effects on human leukemia HL-60 cells in vitro and in vivo. Evid. Based Complement. Altern. Med. ECAM 2013, 2013, 690164. [Google Scholar] [CrossRef]
- Li, C.Y.; Huang, W.F.; Wang, Q.L.; Wang, F.; Cai, E.; Hu, B.; Du, J.C.; Wang, J.; Chen, R.; Cai, X.J.; et al. Crocetin induces cytotoxicity in colon cancer cells via p53-independent mechanisms. Asian Pac. J. Cancer Prev. 2012, 13, 3757–3761. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 2014, 6, 99–107. [Google Scholar] [CrossRef]
- Samarghandian, S.; Tavakkol Afshari, J.; Davoodi, S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl. Biochem. Biotechnol. 2011, 164, 238–247. [Google Scholar] [CrossRef]
- Escribano, J.; Alonso, G.L.; Coca-Prados, M.; Fernández, J.A. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996, 100, 23–30. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A.; Farahmand, S.K.; Afshari, R.; Davoodi, S. Crocus sativus L. (saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. BioMed Res. Int. 2013, 2013, 417928. [Google Scholar] [CrossRef]
- D’Alessandro, A.M.; Mancini, A.; Lizzi, A.R.; De Simone, A.; Marroccella, C.E.; Gravina, G.L.; Tatone, C.; Festuccia, C. Crocus Sativus stigma extract and its major constituent crocin possess significant antiproliferative properties against human prostate cancer. Nutr. Cancer 2013, 65, 930–942. [Google Scholar] [CrossRef]
- Gutheil, W.G.; Reed, G.; Ray, A.; Anant, S.; Dhar, A. Crocetin: An agent derived from saffron for prevention and therapy for cancer. Curr. Pharm. Biotechnol. 2012, 13, 173–179. [Google Scholar] [CrossRef]
- Gainer, J.L.; Chisolm, G.M. Oxygen diffusion and atherosclerosis. Atherosclerosis 1974, 19, 135–138. [Google Scholar] [CrossRef]
- Gainer, J.L.; Jones, J.R. The use of crocetin in experimental atherosclerosis. Experientia 1975, 31, 548–549. [Google Scholar] [CrossRef]
- Miller, T.L.; Willett, S.L.; Moss, M.E.; Miller, J.; Belinka, B.A. Binding of crocetin to plasma albumin. J. Pharm. Sci. 1982, 71, 173–177. [Google Scholar] [CrossRef]
- DiLuccio, R.C.; Gainer, J.L. Increasing alveolar oxygen transport. Aviat. Space Environ. Med. 1980, 51, 18–20. [Google Scholar]
- Gainer, J.L.; Rudolph, D.B.; Caraway, D.L. The effect of crocetin on hemorrhagic shock in rats. Circ. Shock 1993, 41, 1–7. [Google Scholar]
- Lari, P.; Abnous, K.; Imenshahidi, M.; Rashedinia, M.; Razavi, M.; Hosseinzadeh, H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol. Ind. Health 2015, 31, 367–376. [Google Scholar] [CrossRef]
- Termentzi, A.; Kokkalou, E. LC-DAD-MS (ESI+) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta Med. 2008, 74, 573–581. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z.E. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Estevan, C.; Sogorb, M.Á.; Carmona, M.; Alonso, G.L.; Vilanova, E. Cytotoxic effect against 3T3 fibroblasts cells of saffron floral bio-residues extracts. Food Chem. 2014, 147, 55–59. [Google Scholar] [CrossRef]
- Nørbæk, R.; Brandt, K.; Nielsen, J.K.; Ørgaard, M.; Jacobsen, N. Flower pigment composition of Crocus species and cultivars used for a chemotaxonomic investigation. Biochem. Syst. Ecol. 2002, 30, 763–791. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Rodríguez-Conde, M.F.; Reina-Ureña, J.V.; Escolano-Tercero, M.A.; Herraiz-Peñalver, D.; Santana-Méridas, O. In vitro antioxidant and metal chelating properties of corm, tepal and leaf from saffron (Crocus sativus L.). Ind. Crops Prod. 2012, 39, 149–153. [Google Scholar] [CrossRef]
- Zheng, C.J.; Li, L.; Ma, W.H.; Han, T.; Qin, L.P. Chemical constituents and bioactivities of the liposoluble fraction from different medicinal parts of Crocus sativus. Pharm. Biol. 2011, 49, 756–763. [Google Scholar] [CrossRef]
- Omidi, A.; Riahinia, N.; Torbati, M.B.M. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J. Phytomed. 2014, 4, 7. [Google Scholar]
- Omidi, A.; Totrabi, Z. The protective role of saffron petal extracts on gentamicininduced nephrotoxicity in rats. Vet. Sci. Dev. 2016, 6. [Google Scholar] [CrossRef]
- Babaei, A.; Arshami, J.; Haghparast, A.; Mesgaran, M.D. Effects of saffron (Crocus sativus) petal ethanolic extract on hematology, antibody response, and spleen histology in rats. Avis. J. Phytomed. 2014, 4, 103–109. [Google Scholar]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Colombo, M.; Melchiades, G.; Roberta Michels, L.; Figueiró, F.; Bassani, V.; Ferreira Teixeira, H.; Koester, L. Solid dispersion of kaempferol: Formulation development, characterization, and oral bioavailability assessment. AAPS Pharm. Sci. Tech. 2019, 20, 106. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef]
- Mohebbati, R.; Khazdair, M.R.; Hedayati, M. Neuroprotective effects of medicinal plants and their constituents on different induced neurotoxicity methods: A review. J. Rep. Pharm. Sci. 2017, 6, 18. [Google Scholar]
- Qian, J.; Chen, X.; Chen, X.; Sun, C.; Jiang, Y.; Qian, Y.; Zhang, Y.; Khan, Z.; Zhou, J.; Liang, G.; et al. Kaempferol reduces K63-linked polyubiquitination to inhibit nuclear factor-κB and inflammatory responses in acute lung injury in mice. Toxicol. Lett. 2019, 306, 53–60. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, P.; Zhong, J.; Hu, Y.; Fan, Y.; Zhuang, S.; Liu, Z. Kaempferol inhibits multiple pathways involved in the secretion of inflammatory mediators from LPS-induced rat intestinal microvascular endothelial cells. Mol. Med. Rep. 2019, 19, 1958–1964. [Google Scholar] [CrossRef]
- Yeon, M.J.; Lee, M.H.; Kim, D.H.; Yang, J.Y.; Woo, H.J.; Kwon, H.J.; Moon, C.; Kim, S.H.; Kim, J.B. Anti-inflammatory effects of kaempferol on Helicobacter pylori-induced inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 166–173. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Peng, Y.; Deng, Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab. Brain Dis. 2019, 34, 485–494. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Kim, T.H.; Ku, S.K.; Bae, J.S. Inhibitory effects of kaempferol-3-O-sophoroside on HMGB1-mediated proinflammatory responses. Food Chem. Toxicol. 2012, 50, 1118–1123. [Google Scholar] [CrossRef]
- Palanichamy, S.; Nagarajan, S. Analgesic activity of Cassia alata leaf extract and kaempferol 3-O-sophoroside. J. Ethnopharmacol. 1990, 29, 73–78. [Google Scholar] [CrossRef]
- Vidya Priyadarsini, R.; Senthil Murugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Chen, S.F.; Nien, S.; Wu, C.H.; Liu, C.L.; Chang, Y.C.; Lin, Y.S. Reappraisal of the anticancer efficacy of quercetin in oral cancer cells. J. Chin. Med. Assoc. 2013, 76, 146–152. [Google Scholar] [CrossRef]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef]
- Viegas, O.; Faria, M.A.; Sousa, J.B.; Vojtek, M.; Gonçalves-Monteiro, S.; Suliburska, J.; Diniz, C.; Ferreira, I.M. Delphinidin-3-O-glucoside inhibits angiogenesis via VEGFR2 downregulation and migration through actin disruption. J. Funct. Foods 2019, 54, 393–402. [Google Scholar] [CrossRef]
- Manach, C.; Donovan, J. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004, 38, 771–785. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pérez, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Bioavailability of dietary phenolic compounds: Review. Span. J. Hum. Nutr. Diet. 2016, 20, 140–147. [Google Scholar] [CrossRef]
- de Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 638, 60–65. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Morten, K.; Justesenb, U.; Schoua, A.; Dragsted, L.O. Human absorption and excretion of flavonoids after broccoli consumption. Cancer Lett. 1997, 114, 173–174. [Google Scholar] [CrossRef]
- DuPont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Chen, F.; Hua, A.; Wang, S.; Wu, M. Pharmacokinetics and bioavailability of kaempferol in rat plasma by UPLC-MS/MS. Lat. Am. J. Pharm. 2019, 39, 80–84. [Google Scholar]

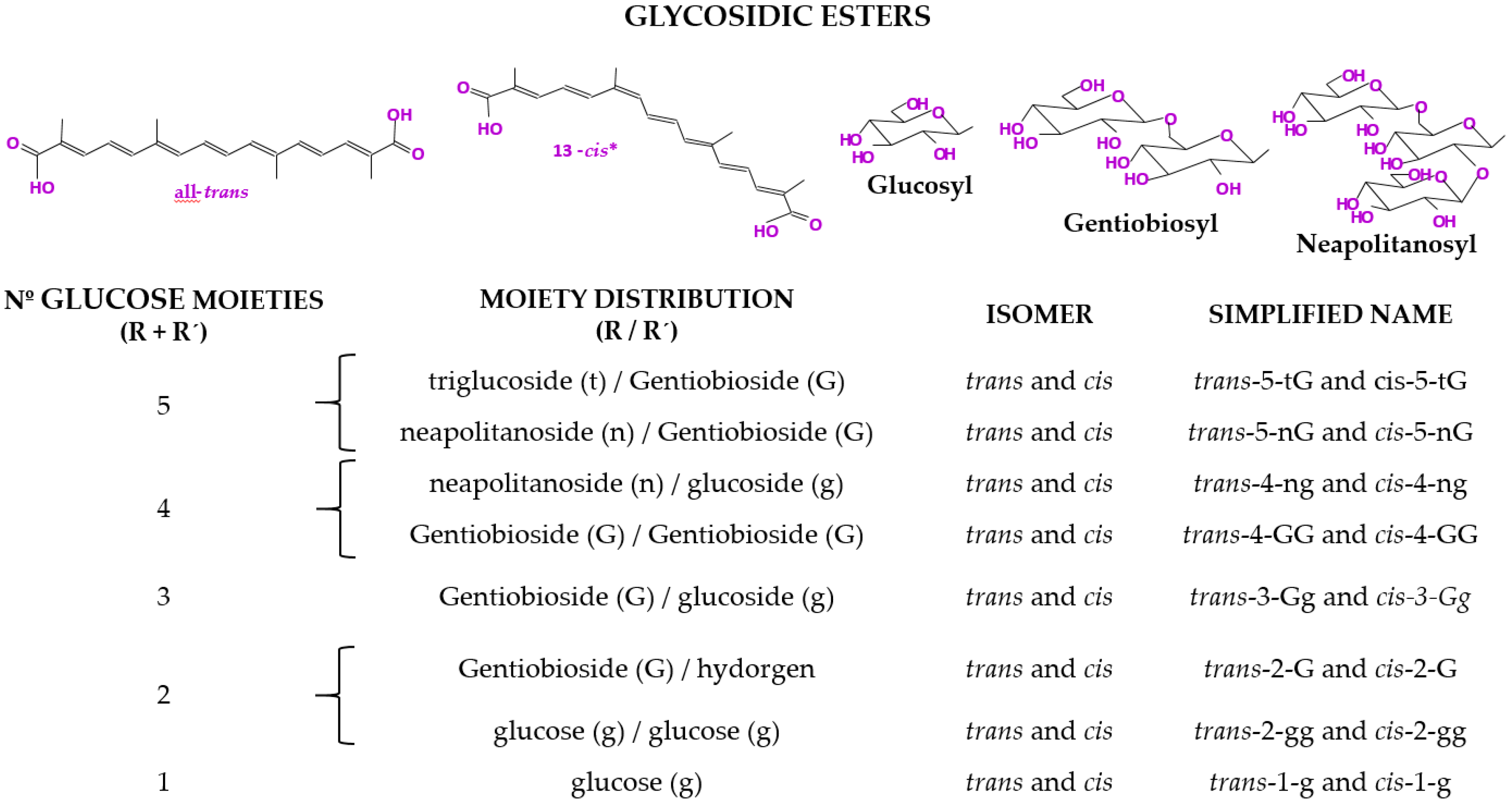
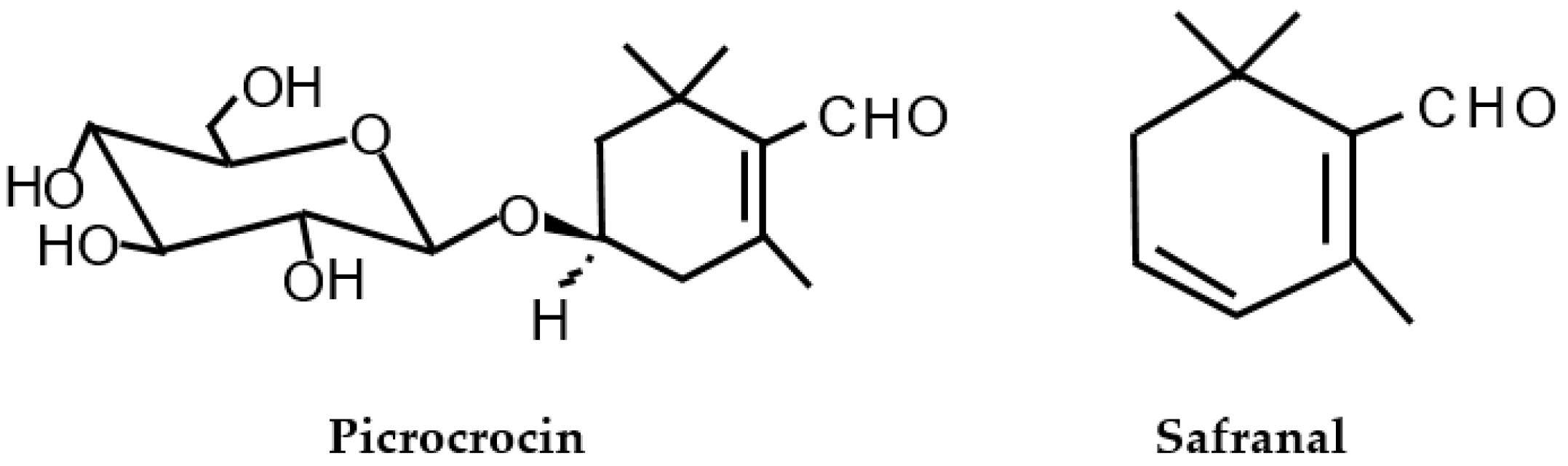

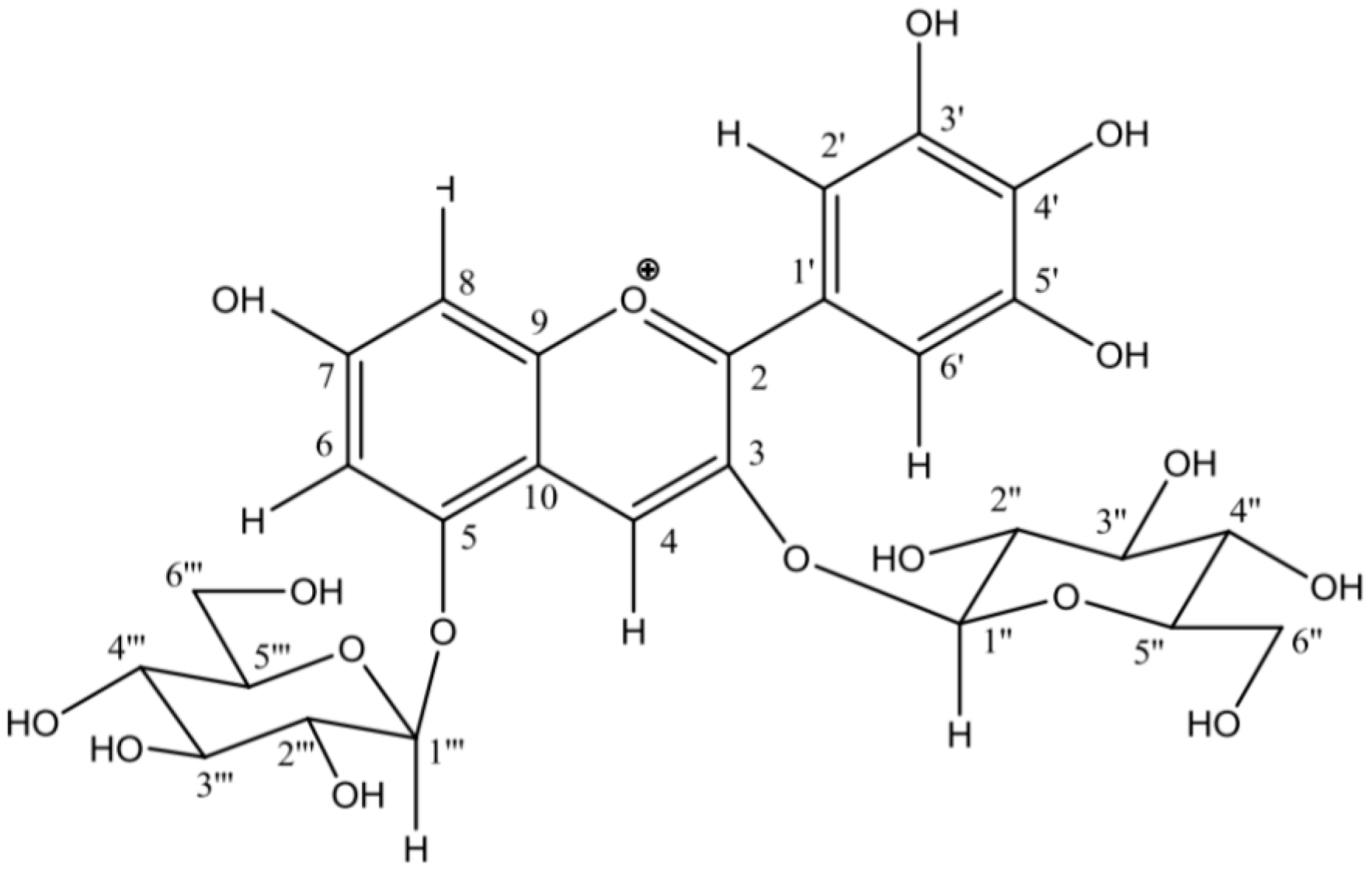
| Analytical Technique [Reference] | Indicative Data or Analyte | Information |
|---|---|---|
| Spectrophotometry UV-vis [15] | Coloring strength, 257 nm and 330 nm | ISO 3632:2011 |
| HPLC–DAD [46,47] | Crocins, picrocrocin, and safranal | Saffron quality |
| LC/DAD/MS/MS [58] | Crocins, picrocrocin, and flavonoids | Identification of saffron metabolites |
| UHPLC–MS/MS [59] | Crocins | Differentiation of the obtaining process of saffron |
| NMR [55] | Saffron compounds | Metabolic fingerprinting |
| DHS–GC–MS [38] | Safranal and other volatile compounds | Quality of aroma |
| e-Nose [60] | Volatiles of saffron as a whole | Determination geographical origin |
| PTR–TOFMS [61] | Volatile compounds | Quality of aroma |
| Raman spectroscopy [62] | Sum of crocins and coloring strength | Saffron quality |
| NIR spectroscopy [63] | Control of saffron quality | Saffron Quality/Geographical origin |
| Derivatization–HPLC–DAD [64] | Free amino acids and ammonium | Determination of geographical origin |
| MIR spectroscopy [65] | FT-IR spectra saffron filaments | Determination of geographical origin |
| Tristimulus colorimetry [66] | Color | Saffron quality |
| Bioactive Compound | Bioactivity [Reference] | Model | Dose |
|---|---|---|---|
| trans-crocetin | Cross the blood–brain barrier and reach the central nervous system [102,103] | Rats | Oral administration (100 mg/kg) |
| Crocetin | Neuroprotection [83] | Hemi-Parkinson rats | Peripheral administration (25, 50 and 75 µg/kg body weight) |
| Improved post-shock survival and reduced apoptosis [127] | Rats | Bolus injection (2 mg/kg body weight) | |
| Cardioprotective effects (after myocardial ischemia reperfusion injury) [121] | Adult male Wistar rats | Intragastric administration (50 mg/kg/day) | |
| Crocins | Hepatoprotective effects [158] | Rats | Intraperitoneally (25 mg/ kg body weight/day for 4 weeks) |
| Safranal | Antidepressant [110] | Rats | Peripheral administration (15.5 mg/kg body weight.) |
| Anticonvulsant [111] | Mice | Injected (0.15 and 0.35 mg/kg) | |
| Picrocrocin | Antitumor effects [100] | Human colon adenocarcinoma (Caco-2-cell model) | 8–24 µM |
| Saffron extracts | Satiating [113] | Human (randomized, double-blind, placebo-controlled, parallel-group) | Oral administration (capsule: 176.5 mg extract/day for 8 weeks) |
| Reduce cognitive deterioration (Alzheimer’s disease) [134] | Patients (randomized double-blind parallel-group) | Oral administration (capsule: 30 mg/day for 12 months) | |
| Saffron | Premenstrual syndrome [115] | Women (double-blind, randomized and placebo-controlled trial) | Oral administration (capsule: 30 mg/day for 6 months) |
| Neuroprotection (macular degeneration) [130] | Albino rats with light-induced photoreceptors degenerations | Oral administration (1 mg/kg/day for 6 weeks) | |
| Improve the symptoms of children with deficit hyperactivity disorder [131] | Children | Oral administration (20–30 mg/ day for 6 weeks) | |
| Effective treatment in depression and anxiety [140] | Patients (double-blind controlled clinical trial) | Oral administration (30 mg/day per 6 weeks) |
| Bioactive Compound | Bioactivity [Reference] | Model | Dose |
|---|---|---|---|
| Tepal (ethanol; 80%) extracts of C.s. flower | Hepatoprotective effects [165] | Rats | Administration by oral gavage (20 mg/kg body weight for 6 days) |
| Ameliorative effects on kidney failures [166] | Rats | Intraperitoneal injection (40 mg/kg body weight for 7/13 days) | |
| Increase antibody response [167] | Rats | Intraperitoneal injection (75 mg/kg body weight for 14 days) | |
| Kaempferol aglycone | Antitumor effects [170] | Colon cancer cells | 75 µM |
| Anti-inflammatory effects in acute lung injury [171] | Cell | 100 µM | |
| Mice | Intraperitoneal injection (50 mg/kg body weight) | ||
| Anti-inflammatory effects [173] | Cellular model of intestinal inflammation in rats | 12.5, 25 and 50 µM | |
| Anti-inflammatory effects on Helicobacter pylory-induced inflammation [174] | Gastric adenocarcinoma cell | 6.25, 12.5, and 25 µM | |
| Antidepressant effects [175] | Chronic social defeat stress mouse model | Intraperitoneal injection (20 mg/kg body weight) | |
| Wound healing effects [176] | Incisional and excisional wound models on diabetic and nondiabetic rats | Topically applied (1% weight/weight for 14 days) | |
| Kaempferol 3-O-β-sophoroside | Anti-inflammatory effects [177] | Human endothelial cells | >0.05 µM |
| Analgesic effects [178] | Mice | Intraperitoneal injection (50 mg/kg body weight) | |
| Quercetin | Chemopreventive effects. Inhibit cell growth and invasion/migration of the cells [180] | Cultured oral squamous cell carcinoma cells | 2 mg/mL |
| Chemopreventive effects [181] | Male Sprague Dawley rats | Oral administration (200 mg/kg body weigh/ trice a week for 16 weeks) | |
| Delphinidin 3-O-β-glucoside | Prevent tumor progress by inhibiting angiogenesis and cell migration [183] | Breast cancer cells | 200 µM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Martínez-Navarro, M.E.; Salinas, M.R.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. https://doi.org/10.3390/molecules24152827
Moratalla-López N, Bagur MJ, Lorenzo C, Martínez-Navarro ME, Salinas MR, Alonso GL. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules. 2019; 24(15):2827. https://doi.org/10.3390/molecules24152827
Chicago/Turabian StyleMoratalla-López, Natalia, María José Bagur, Cándida Lorenzo, M.E. Martínez-Navarro, M. Rosario Salinas, and Gonzalo L. Alonso. 2019. "Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower" Molecules 24, no. 15: 2827. https://doi.org/10.3390/molecules24152827
APA StyleMoratalla-López, N., Bagur, M. J., Lorenzo, C., Martínez-Navarro, M. E., Salinas, M. R., & Alonso, G. L. (2019). Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules, 24(15), 2827. https://doi.org/10.3390/molecules24152827





