A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates
Abstract
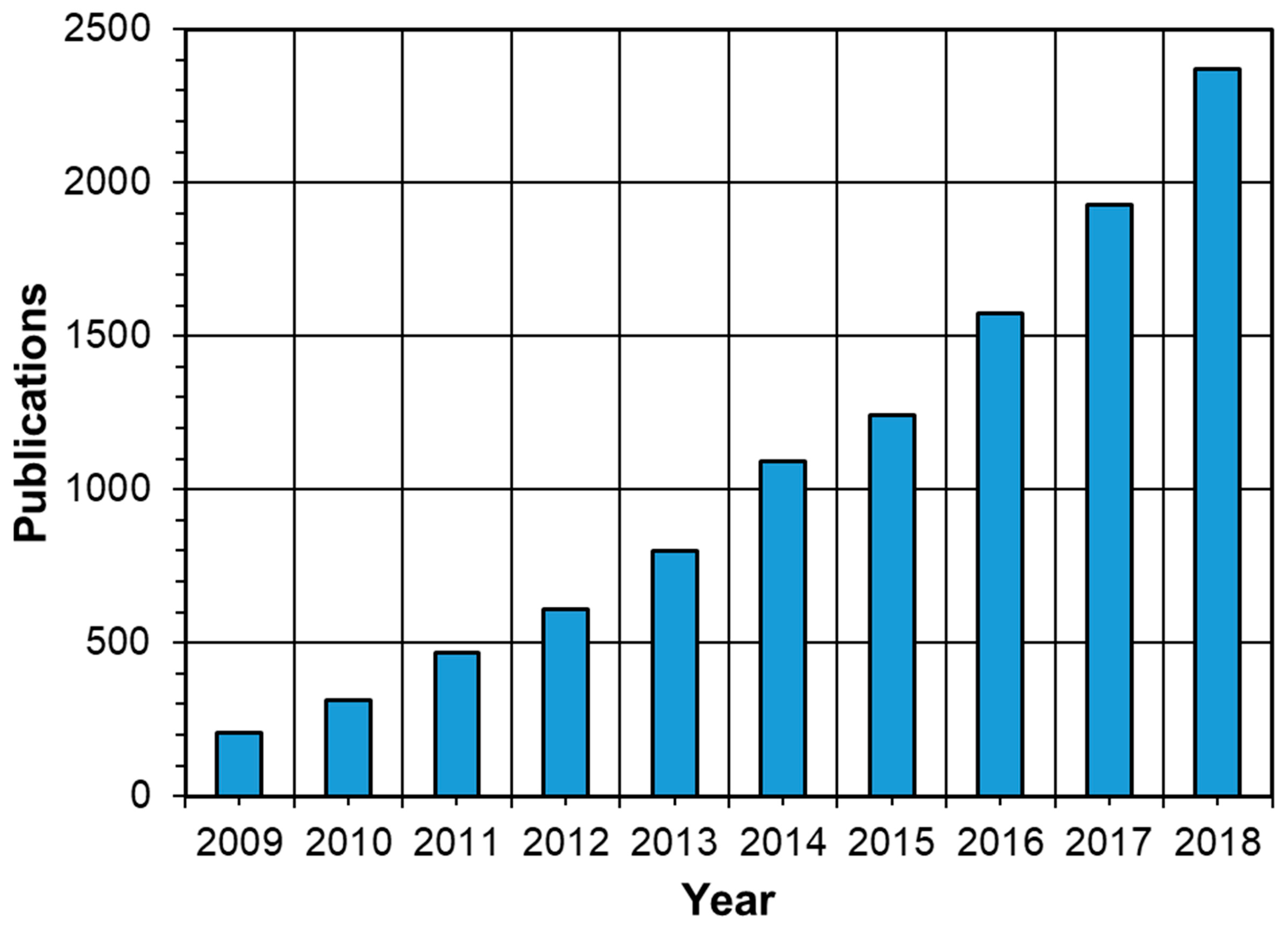
1. Introduction
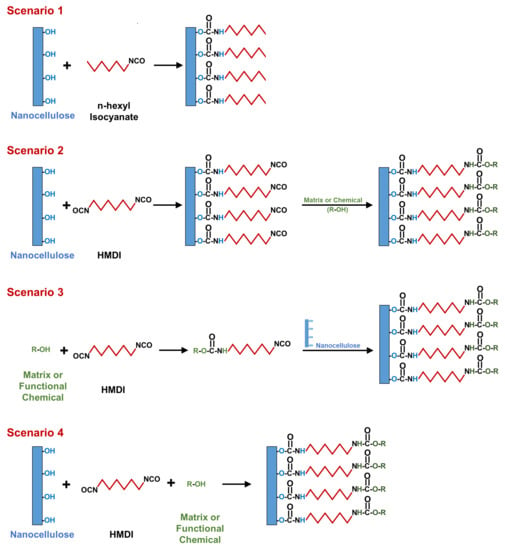
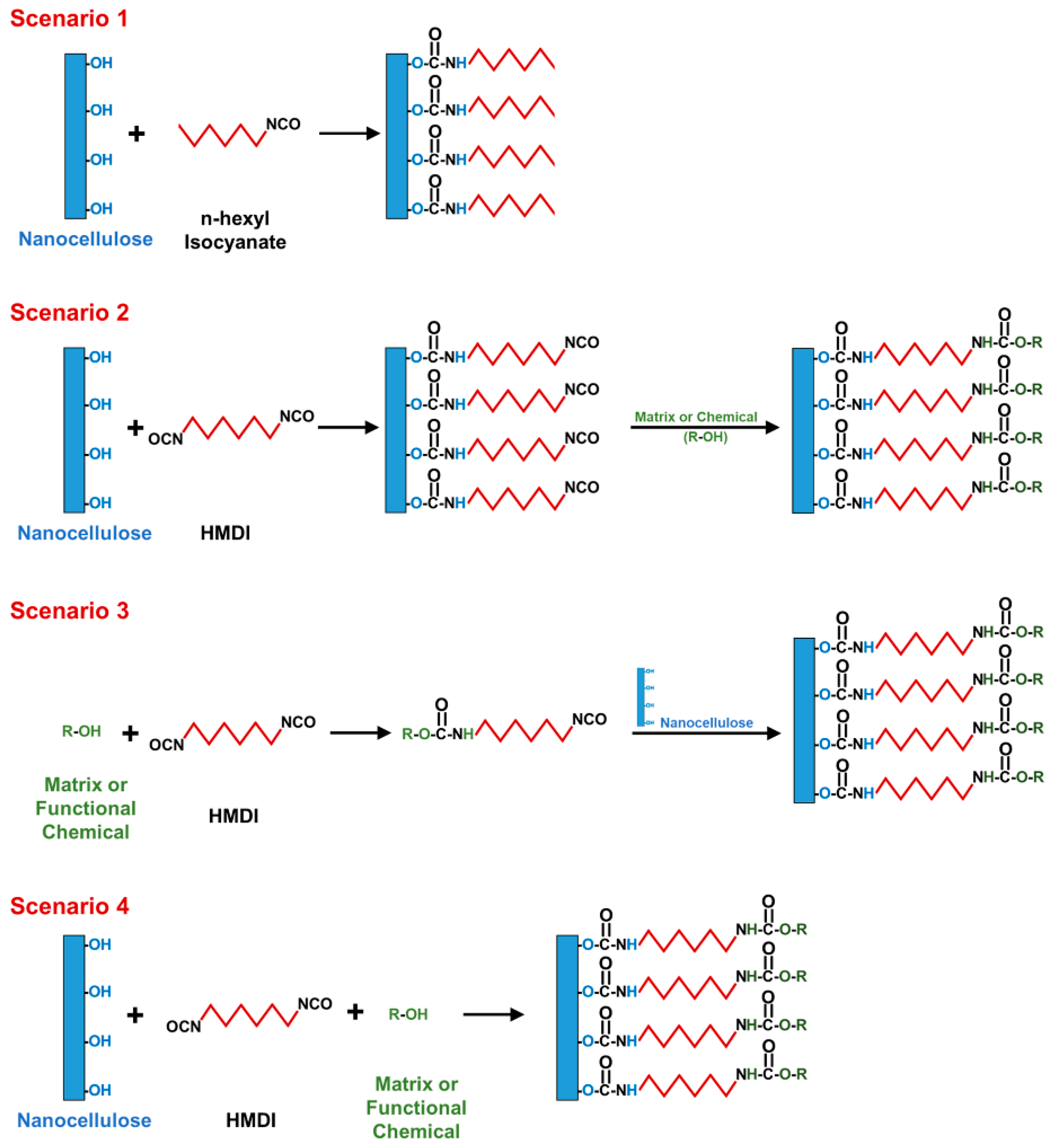
2. General Scenarios of Nanocellulose Modification Using Isocyanates
3. Cellulose Modification Using Aliphatic and Aromatic Isocyanates
3.1. Preparation of Functional Cellulose (Scenario 1 and 2)
3.2. Improving Cellulose Properties (Scenario 1 and 2)
3.3. Improving Cellulose Processing and Performance with Nonpolar Matrices (Scenario 1 and 3)
3.4. Cellulose/Matrix Cross-linking (Scenario 3 and 4)
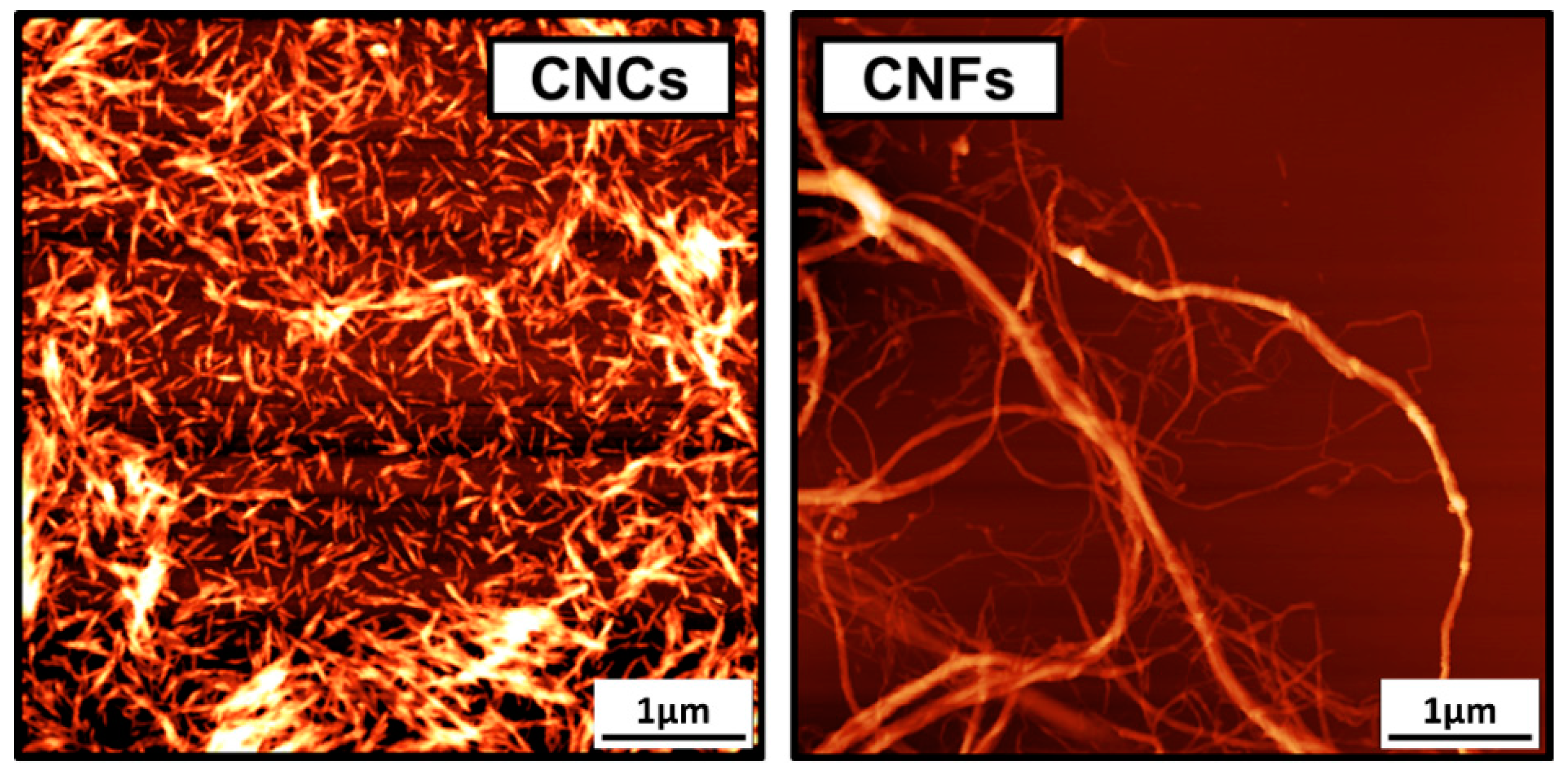
4. Nanocellulose Modification Using Aliphatic and Aromatic Isocyanates
4.1. Preparation of Functional Nanocellulose (Scenario 1 and 3)
4.2. Improving Nanocellulose Properties (Scenario 1,2, and 3)
4.3. Improving Nanocellulose Processing and Performance with Nonpolar Matrices (Scenario 1, 2, and 3)
4.4. Nanocellulose/Matrix Cross-Linking (Scenario 4)
5. Challenges
6. Conclusions
Funding
Conflicts of Interest
References
- Sun, R. Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels: Chemistry, Extractives, Lignins, Hemicelluloses and Cellulose, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications. Cellulose 2010, 17, 835–848. [Google Scholar] [CrossRef]
- Mao, J.; Abushammala, H.; Brown, N.; Laborie, M.-P. Comparative assessment of methods for producing cellulose I nanocrystals from cellulosic sources. In Nanocelluloses: Their Preparation, Properties, and Applications, ACS Symposium Series; ACS Publications: Washington, DC, USA, 2017; Volume 1251, pp. 19–53. [Google Scholar]
- Standard Terms and Their Definition for Cellulose Nanomaterial; International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171. [Google Scholar] [CrossRef]
- Abushammala, H.; Goldsztayn, R.; Leao, A.; Laborie, M.-P. Combining steam explosion with 1-ethyl-3-methylimidazlium acetate treatment of wood yields lignin-coated cellulose nanocrystals of high aspect ratio. Cellulose 2016, 23, 1813–1823. [Google Scholar] [CrossRef]
- Abushammala, H.; Krossing, I.; Laborie, M.-P. Ionic liquid-mediated technology to produce cellulose nanocrystals directly from wood. Carbohydr. Polym. 2015, 134, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.; Luong, J.H.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.; Rho, D. Cellulose nanocrystals from renewable biomass. U.S. Patent 8,900,706, 2 December 2014. [Google Scholar]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. Acs Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Kiziltas, A.; Erbas Kiziltas, E.; Boran, S.; Gardner, D.J. Micro-and nanocellulose composites for automotive applications. In Proceedings of the SPE Automotive Composites Conference and Exhibition (ACCE), Novi, MI, USA, 11–13 September 2013. [Google Scholar]
- Plackett, D.; Letchford, K.; Jackson, J.; Burt, H. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp. Pap. Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomed. 2013, 8, 287–298. [Google Scholar] [CrossRef]
- Khan, A.; Huq, T.; Khan, R.A.; Riedl, B.; Lacroix, M. Nanocellulose-based composites and bioactive agents for food packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 163–174. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Fraschini, C.; Chauve, G.; Bouchard, J. TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs). Cellulose 2017, 24, 2775–2790. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, J.; Gong, J.; Li, J.; Mo, L. Preparation, characterization and acetylation of cellulose nanocrystal allomorphs. Cellulose 2018, 25, 4905–4918. [Google Scholar] [CrossRef]
- Yuan, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Surface acylation of cellulose whiskers by drying aqueous emulsion. Biomacromolecules 2006, 7, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Salajková, M.; Berglund, L.A.; Zhou, Q. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 2012, 22, 19798–19805. [Google Scholar] [CrossRef]
- Song, Z.; Xiao, H.; Zhao, Y. Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper. Carbohydr. Polym. 2014, 111, 442–448. [Google Scholar] [CrossRef]
- Cervin, N.T.; Aulin, C.; Larsson, P.T.; Wågberg, L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2012, 19, 401–410. [Google Scholar] [CrossRef]
- Laitinen, O.; Hartmann, R.; Sirviö, J.A.; Liimatainen, H.; Rudolph, M.; Ämmälä, A.; Illikainen, M. Alkyl aminated nanocelluloses in selective flotation of aluminium oxide and quartz. Chem. Eng. Sci. 2016, 144, 260–266. [Google Scholar] [CrossRef]
- Morandi, G.; Thielemans, W. Synthesis of cellulose nanocrystals bearing photocleavable grafts by ATRP. Polym. Chem. 2012, 3, 1402–1407. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.; Jeyaratnam, N.; Yuvaraj, A. Polyurethane types, synthesis and applications–a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Zhang, C.; Gilbert, R.; Fornes, R. Preliminary studies of reduction of moisture absorption of cellulose using masked isocyanates. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1992; Volume 203. [Google Scholar]
- Chen, W.; Bin, Q.; Bai, Z.-W.; Zhou, X.-P.; Xie, X.-L. Partial carbamoylation of cellulose microspheres: A new method to prepare adsorbents for liquid chromatography. Chin. J. Polym. Sci. 2013, 31, 1725–1732. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, M.; Feng, Y.; Wu, J.; Gao, X.; Zhang, J.; He, J.; Zhang, J. Homogeneous synthesis of partially substituted cellulose phenylcarbamates aiming at chiral recognition. Polym. Int. 2015, 64, 1037–1044. [Google Scholar] [CrossRef]
- Okada, Y.; Yamamoto, C.; Kamigaito, M.; Gao, Y.; Shen, J.; Okamoto, Y. Enantioseparation using cellulose tris (3, 5-dimethylphenylcarbamate) as chiral stationary phase for HPLC: Influence of molecular weight of cellulose. Molecules 2016, 21, 1484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Dong, S.; Zhang, X.; Wu, Q.; Zhao, L.; Shi, Y. Nanocellulose 3, 5-Dimethylphenylcarbamate Derivative Coated Chiral Stationary Phase: Preparation and Enantioseparation Performance. Chirality 2016, 28, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Du, W.; Gao, Y.; Cao, Y.; Yin, Y. Cellulose nanocrystals as water-in-oil Pickering emulsifiers via intercalative modification. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 634–642. [Google Scholar] [CrossRef]
- Guo, Y.-H.; Guo, J.-J.; Li, S.-C.; Li, X.; Wang, G.-S.; Huang, Z. Properties and paper sizing application of waterborne polyurethane emulsions synthesized with TDI and IPDI. Colloids Surf. A Physicochem. Eng. Asp. 2013, 427, 53–61. [Google Scholar] [CrossRef]
- Mix, R.; Gähde, J.; Goering, H.; Schulz, G. Segmented polyurethanes with 4, 4′-bis-(6-hydroxyhexoxy) biphenyl as chain extender. Part 2. Synthesis and properties of MDI-polyurethanes in comparison with 2, 4-TDI-polyurethanes. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 33–44. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Quillerou, J.; Gandini, A. Urethanes and polyurethanes bearing furan moieties—3. Synthesis, characterization and comparative kinetics of the formation of diurethanes. Eur. Polym. J. 1993, 29, 1217–1224. [Google Scholar] [CrossRef]
- Semsarzadeh, M.; Navarchian, A. Kinetic Study of the Bulk Reaction Between TDI and PPG in Prescence of DBTDL and FEAA Catalysts Using Quantitative FTIR Spectroscopy. J. Polym. Eng. 2003, 23, 225–240. [Google Scholar] [CrossRef]
- Evans, R.; Wearne, R.H.; Wallis, A.F. Effect of amines on the carbanilation of cellulose with phenylisocyanate. J. Appl. Polym. Sci. 1991, 42, 813–820. [Google Scholar] [CrossRef]
- Morelli, C.L.; Belgacem, N.; Bretas, R.E.; Bras, J. Melt extruded nanocomposites of polybutylene adipate-co-terephthalate (PBAT) with phenylbutyl isocyanate modified cellulose nanocrystals. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Morelli, C.L.; Belgacem, M.N.; Branciforti, M.C.; Bretas, R.E.; Crisci, A.; Bras, J. Supramolecular aromatic interactions to enhance biodegradable film properties through incorporation of functionalized cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2016, 83, 80–88. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Tang, B.; Yuan, L.; Wang, K.; Liu, X.; Zhu, X.; Li, J.; Ge, Z.; Chen, S. New insights into synergistic antimicrobial and antifouling cotton fabrics via dually finished with quaternary ammonium salt and zwitterionic sulfobetaine. Chem. Eng. J. 2018, 336, 123–132. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 2009, 26, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Follain, N.; Belbekhouche, S.; Marais, S.; Dufresne, A. Thermal and mechanical properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. Carbohydr. Polym. 2013, 91, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Ashori, A.; Nourbakhsh, A. Polypropylene cellulose-based composites: The effect of bagasse reinforcement and polybutadiene isocyanate treatment on the mechanical properties. J. Appl. Polym. Sci. 2009, 111, 1684–1689. [Google Scholar] [CrossRef]
- Gwon, J.-G.; Cho, H.-J.; Chun, S.-J.; Lee, S.; Wu, Q.; Lee, S.-Y. Physiochemical, optical and mechanical properties of poly (lactic acid) nanocomposites filled with toluene diisocyanate grafted cellulose nanocrystals. RSC Adv. 2016, 6, 9438–9445. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Peresin, M.S.; Habibi, Y.; Venditti, R.A.; Rojas, O.J. Reinforcing poly (ε-caprolactone) nanofibers with cellulose nanocrystals. ACS Appl. Mater. Interfaces 2009, 1, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Dierov, Z.K.; Tsiomenko, A.; Davranov, K.; Kulaev, I. Hydrophobic chromatography and characterization of lipases secreted by the fungus rhizopus-microporous UZLT-4B. Biochem. -Mosc. 1993, 58, 677–683. [Google Scholar]
- Shang, W.; Huang, J.; Luo, H.; Chang, P.R.; Feng, J.; Xie, G. Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil. Cellulose 2013, 20, 179–190. [Google Scholar] [CrossRef]
- Abushammala, H. A Simple Method for the Quantification of Free Isocyanates on the Surface of Cellulose Nanocrystals upon Carbamation using Toluene Diisocyanate. Surface 2019, 2, 444–454. [Google Scholar] [CrossRef]
- Abushammala, H. On the Para/Ortho Reactivity of Isocyanate Groups during the Carbamation of Cellulose Nanocrystals Using 2,4-Toluene Diisocyanate. Polymer 2019, 11, 1164. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Ragauskas, A.J. Rigid polyurethane foam/cellulose whisker nanocomposites: Preparation, characterization, and properties. J. Nanosci. Nanotechnol. 2011, 11, 6904–6911. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Ethanol organosolv lignin-based rigid polyurethane foam reinforced with cellulose nanowhiskers. RSC Adv. 2012, 2, 3347–3351. [Google Scholar] [CrossRef]
- Charles, G.P.E. Manufacture of new products derived from cellulose. U.S. Patent 1,357,450, 2 November 1920. [Google Scholar]
- Welch, C.M. Process for the reaction of isocyanates with cellulose in the presence of organic phosphites. U.S. Patent 2,993,888, 25 July 1961. [Google Scholar]
- George, M. Structural element made from paper and like sheets. U.S. Patent 2,428,979, 14 November 1947. [Google Scholar]
- Ellzey, S., Jr.; Wade, C.P.; Mack, C.H. Part II: Textile Properties of Fabric Modified by Reaction with Phenyl Isocyanate. Text. Res. J. 1962, 32, 1029–1033. [Google Scholar] [CrossRef]
- Ellzey, S., Jr.; Mack, C.H. Reaction of Aryl Isocyanates with Cotton Cellulose: Part I: Variables in the Reaction Using Phenyl Isocyanate. Text. Res. J. 1962, 32, 1023–1029. [Google Scholar] [CrossRef]
- Ohno, Y.; Uchimoto, I. Studies on reaction of cellulose with isocyanate. 1. Reaction of cellulose with phenyl isocyanate. Kog Kagaku Zasshi 1970, 73, 2527–2530. [Google Scholar] [CrossRef]
- Ohno, Y.; Sato, T.; Miyamoto, K. Studies on reaction of cellulose with isocyanate. 3. Reaction of cellulose with 2, 4-diisocyanatotoluene in n, n-dimethylformamide. Nippon. Kagaku. Kaishi 1976, 3, 1300–1303. [Google Scholar] [CrossRef]
- Sato, T.; Ohno, Y.; Tamura, T. Studies on reaction of cellulose with isocyanate. 5. Reaction of cellulose with hexamethylene diisocyanate in n, n-dimethylformamide. Nippon. Kagaku. Kaishi 1978, 5, 760–764. [Google Scholar] [CrossRef]
- Gemeiner, P.; Augustin, J.; Drobnica, L. Reactions of cellulose isothiocyanates with thiol and amino compounds. Carbohydr. Res. 1977, 53, 217–222. [Google Scholar] [CrossRef]
- Chen, L.F.; Tsao, G.T. Chemical procedures for enzyme immobilization on porous cellulose beads. Biotechnol. Bioeng. 1977, 19, 1463–1473. [Google Scholar] [CrossRef]
- Saraji, M.; Farajmand, B. Chemically modified cellulose paper as a thin film microextraction phase. J. Chromatogr. A 2013, 1314, 24–30. [Google Scholar] [CrossRef]
- Pend, X.; Sato, M.; Kawase, T.; Ikeno, K.; Sawada, H.; Hamada, N.; Wada, K.; Takahashi, Y.; Yoshimura, T. Synthesis and soil repellent, antibacterial and antifungal properties of blocked isocyanate co-oligomers having cation segments. Sen-I Gakkaishi 2002, 58, 163–169. [Google Scholar]
- Sato, T.; Karatsu, K.; Kitamura, H.; Ohno, Y. Synthesis of cellulose derivatives containing amino acid residues and their adsorption of metal ions. Sen’i Gakkaishi 1983, 39, T519–T524. [Google Scholar] [CrossRef]
- Sato, T.; Motomura, S.; Ohno, Y. Adsorption and desorption of metal ions by systems based on cellulose derivatives that contain amino acid residues. Sen’i Gakkaishi 1985, 41, T235–T240. [Google Scholar] [CrossRef]
- Tursi, A.; Beneduci, A.; Chidichimo, F.; De Vietro, N.; Chidichimo, G. Remediation of hydrocarbons polluted water by hydrophobic functionalized cellulose. Chemosphere 2018, 201, 530–539. [Google Scholar] [CrossRef]
- Sato, J.; Sugimura, K.; Teramoto, Y.; Nishio, Y. Preparation and chiroptical properties of cellulose chlorophenylcarbamate–silica hybrids having a chiral nematic mesomorphic structure. Polymer 2019, 173, 172–181. [Google Scholar] [CrossRef]
- Peng, X.; Kawase, T.; Sato, M.; Ikeno, K.; Sawada, H. Surface modification of cellulose and polyester by oligomeric fluoroalkylating agents having oxime-blocked isocyanate groups. Sen-I Gakkaishi 2002, 58, 91–97. [Google Scholar] [CrossRef][Green Version]
- Rajkumar, S.; Tjong, J.; Nayak, S.; Sain, M. Wetting behavior of soy-based resin and unsaturated polyester on surface-modified sisal fiber mat. J. Reinf. Plast. Compos. 2015, 34, 807–818. [Google Scholar] [CrossRef]
- Botaro, V.R.; Gandini, A. Chemical modification of the surface of cellulosic fibres. 2. Introduction of alkenyl moieties via condensation reactions involving isocyanate functions. Cellulose 1998, 5, 65–78. [Google Scholar] [CrossRef]
- Botaro, V.R.; Gandini, A.; Belgacem, M.N. Heterogeneous chemical modification of cellulose for composite materials. J. Thermoplast. Compos. Mater. 2005, 18, 107–117. [Google Scholar] [CrossRef]
- Trejo-O’Reilly, J.; Cavaille, J.Y.; Gandini, A. Cationic copolymerization of styrenes with an isocyanate-bearing homologue. React. Funct. Polym. 1997, 32, 9–19. [Google Scholar] [CrossRef]
- Trejo-O’reilly, J.-A.; Cavaille, J.-Y.; Gandini, A. The surface chemical modification of cellulosic fibres in view of their use in composite materials. Cellulose 1997, 4, 305–320. [Google Scholar] [CrossRef]
- Badanova, A.K.; Taussarova, B.R.; Kutzhanova, A.Z. Hydrophobic finishing of cellulosic textile material. World. Appl. Sci. J. 2014, 30, 1409–1416. [Google Scholar]
- Yuan, J.; Zhang, J.; Zang, X.; Shen, J.; Lin, S. Improvement of blood compatibility on cellulose membrane surface by grafting betaines. Colloids. Surf. B Biointerfaces 2003, 30, 147–155. [Google Scholar] [CrossRef]
- Furuzono, T.; Ishihara, K.; Nakabayashi, N.; Tamada, Y. Chemical modification of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine. II. Graft-polymerization onto fabric through 2-methacryloyloxyethyl isocyanate and interaction between fabric and platelets. Biomaterials 2000, 21, 327–333. [Google Scholar] [CrossRef]
- Ghatge, N.; Sabne, M.; Gujar, K.; Mahajan, S. Modification of cellulose acetate by aliphatic isocyanates for reverse osmosis studies. Int. J. Polym. Mater. 1984, 10, 281–291. [Google Scholar] [CrossRef]
- Mahajan, S.; Sabne, M.; Gujar, K.; Ghatge, N. Selectivity of isocyanate modified cellulose acetate membranes to sugars. Int. J. Polym. Mater. 1985, 11, 39–45. [Google Scholar] [CrossRef]
- Maldas, D.; Kokta, B.V. Effect of Fiber Treatment on the Mechanical Properties of Hybrid Fiber-Reinforced Polystyrene Composites: I. Use of Mica and Wood Pulp as Hybrid Filler. J. Compos. Technol. Res. 1990, 12, 217–221. [Google Scholar]
- Maldas, D.; Kokta, B. Effect of fiber treatment on the mechanical properties of hybrid fiber reinforced polystyrene composites: IV. Use of glass fiber and sawdust as hybrid fiber. J. Compos. Mater. 1991, 25, 375–390. [Google Scholar] [CrossRef]
- Girones, J.; Pimenta, M.; Vilaseca, F.; De Carvalho, A.; Mutje, P.; Curvelo, A. Blocked isocyanates as coupling agents for cellulose-based composites. Carbohydr. Polym. 2007, 68, 537–543. [Google Scholar] [CrossRef]
- Joly, C.; Kofman, M.; Gauthier, R. Polypropylene/cellulosic fiber composites chemical treatment of the cellulose assuming compatibilization between the two materials. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 1981–1996. [Google Scholar] [CrossRef]
- Canche-Escamilla, G.; Cauich-Cupul, J.; Mendizabal, E.; Puig, J.; Vazquez-Torres, H.; Herrera-Franco, P. Mechanical properties of acrylate-grafted henequen cellulose fibers and their application in composites. Compos. Part A Appl. Sci. Manuf. 1999, 30, 349–359. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, F.; Endo, T.; Hirotsu, T. Isocyanate as a compatibilizing agent on the properties of highly crystalline cellulose/polypropylene composites. J. Mater. Sci. 2005, 40, 3607–3614. [Google Scholar] [CrossRef]
- Darie, R.N.; Vlad, S.; Anghel, N.; Doroftei, F.; Tamminen, T.; Spiridon, I. New PP/PLA/cellulose composites: Effect of cellulose functionalization on accelerated weathering behavior. Polym. Adv. Technol. 2015, 26, 941–952. [Google Scholar] [CrossRef]
- Suwanruji, P.; Tuechart, T.; Smitthipong, W.; Chollakup, R. Modification of pineapple leaf fiber surfaces with silane and isocyanate for reinforcing thermoplastic. J. Thermoplast. Compos. Mater. 2017, 30, 1344–1360. [Google Scholar] [CrossRef]
- George, J.; Bhagawan, S.; Thomas, S. Improved interactions in chemically modified pineapple leaf fiber reinforced polyethylene composites. Compos. Interfaces 1997, 5, 201–223. [Google Scholar] [CrossRef]
- George, J.; Bhagawan, S.; Thomas, S. Thermogravimetric and dynamic mechanical thermal analysis of pineapple fibre reinforced polyethylene composites. J. Therm. Anal. Calorim. 1996, 47, 1121–1140. [Google Scholar] [CrossRef]
- Joseph, K.; Thomas, S.; Pavithran, C. Effect of chemical treatment on the tensile properties of short sisal fibre-reinforced polyethylene composites. Polymer 1996, 37, 5139–5149. [Google Scholar] [CrossRef]
- Joseph, P.; Rabello, M.S.; Mattoso, L.; Joseph, K.; Thomas, S. Environmental effects on the degradation behaviour of sisal fibre reinforced polypropylene composites. Compos. Sci. Technol. 2002, 62, 1357–1372. [Google Scholar] [CrossRef]
- Girones, J.; Pimenta, M.; Vilaseca, F.; Carvalho, A.J.d.; Mutje, P.; Curvelo, A. Blocked diisocyanates as reactive coupling agents: Application to pine fiber–polypropylene composites. Carbohydr. Polym. 2008, 74, 106–113. [Google Scholar] [CrossRef]
- Ly, B.; Thielemans, W.; Dufresne, A.; Chaussy, D.; Belgacem, M. Surface functionalization of cellulose fibres and their incorporation in renewable polymeric matrices. Compos. Sci. Technol. 2008, 68, 3193–3201. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, C.; Dong, Y.; Yan, Y.; Li, J.; Shi, S.Q.; Cai, L. Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Ind. Crop. Prod. 2016, 80, 207–213. [Google Scholar] [CrossRef]
- Liu, W.; Chen, T.; Qiu, R. Effect of fiber modification with 3-isopropenyl-dimethylbenzyl isocyanate (TMI) on the mechanical properties and water absorption of hemp-unsaturated polyester (UPE) composites. Holzforschung 2014, 68, 265–271. [Google Scholar] [CrossRef]
- Reulier, M.; Perrin, R.; Avérous, L. Biocomposites based on chemically modified cellulose fibers with renewable fatty-acid-based thermoplastic systems: Effect of different fiber treatments. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. Effect of kenaf fibre modification on morphology and mechanical properties of thermoplastic polyurethane materials. Ind. Crop. Prod. 2015, 74, 566–576. [Google Scholar] [CrossRef]
- Gallego, R.; Arteaga, J.; Valencia, C.; Franco, J. Thickening properties of several NCO-functionalized cellulose derivatives in castor oil. Chem. Eng. Sci. 2015, 134, 260–268. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Preparation, characterization and mechanical properties of bio-based polyurethane adhesives from isocyanate-functionalized cellulose acetate and castor oil for bonding wood. Polymer 2017, 9, 132. [Google Scholar] [CrossRef]
- Tonoli, G.H.D.; Mendes, R.F.; Siqueira, G.; Bras, J.; Belgacem, M.N.; Savastano, H. Isocyanate-treated cellulose pulp and its effect on the alkali resistance and performance of fiber cement composites. Holzforschung 2013, 67, 853–861. [Google Scholar] [CrossRef]
- Tonoli, G.H.D.; Belgacem, M.N.; Siqueira, G.; Bras, J.; Savastano Jr, H.; Lahr, F.R. Processing and dimensional changes of cement based composites reinforced with surface-treated cellulose fibres. Cem. Concr. Compos. 2013, 37, 68–75. [Google Scholar] [CrossRef]
- Paquet, O.; Krouit, M.; Bras, J.; Thielemans, W.; Belgacem, M.N. Surface modification of cellulose by PCL grafts. Acta Mater. 2010, 58, 792–801. [Google Scholar] [CrossRef]
- Wang, D.; Xuan, Y.; Huang, Y.; Shen, J. Synthesis and properties of graft copolymer of cellulose diacetate with poly (caprolactone monoacrylate). J. Appl. Polym. Sci. 2003, 89, 85–90. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, X. Preparation and characterization of cellulose diacetate-graft-poly (butylene glycol adipate) copolymers. Russ. J. Appl. Chem. 2014, 87, 1763–1772. [Google Scholar] [CrossRef]
- Miao, S.D.; Liu, Y.Y.; Wang, P.; Zhang, S.P. Castor oil and microcrystalline cellulose based polymer composites with high tensile strength. Adv. Mater. Res. 2012, 399–401, 1531–1535. [Google Scholar] [CrossRef]
- Cardamone, J.M. Reacting cotton cellulose with lignin-based polyurethane. Text. Res. J. 1992, 62, 371–381. [Google Scholar] [CrossRef]
- Habibi, Y.; Dufresne, A. Highly filled bionanocomposites from functionalized polysaccharide nanocrystals. Biomacromolecules 2008, 9, 1974–1980. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W.; Dufresne, A. Polymer grafting onto starch nanocrystals. Biomacromolecules 2007, 8, 2916–2927. [Google Scholar] [CrossRef]
- Thielemans, W.; Belgacem, M.N.; Dufresne, A. Starch nanocrystals with large chain surface modifications. Langmuir 2006, 22, 4804–4810. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M.; Kaiser, E.Q.; Archibald, D.D. Quantification of cellulose nanowhiskers sulfate esterification levels. Carbohydr. Polym. 2013, 92, 1809–1816. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Verlhac, C.; Dedier, J.; Chanzy, H. Availability of surface hydroxyl groups in Valonia and bacterial cellulose. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 1171–1177. [Google Scholar] [CrossRef]
- Missoum, K.; Bras, J.; Belgacem, M.N. Organization of aliphatic chains grafted on nanofibrillated cellulose and influence on final properties. Cellulose 2012, 19, 1957–1973. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qin, Z.-Y. Surface grafting of cellulose nanocrystals with poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Carbohydr. Polym. 2014, 101, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar] [CrossRef]
- Verdolotti, L.; Stanzione, M.; Khlebnikov, O.; Silant’ev, V.; Postnova, I.; Lavorgna, M.; Shchipunov, Y. Dimensionally Stable Cellulose Aerogel Strengthened by Polyurethane Synthesized in Situ. Macromol. Chem. Phys. 2019, 220, 1800372. [Google Scholar] [CrossRef]
- Hassan, M.L.; Bras, J.; Hassan, E.A.; Fadel, S.M.; Dufresne, A. Polycaprolactone/modified bagasse whisker nanocomposites with improved moisture-barrier and biodegradability properties. J. Appl. Polym. Sci. 2012, 125, E10–E19. [Google Scholar] [CrossRef]
- Follain, N.; Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Marais, S.; Dufresne, A. Tunable gas barrier properties of filled-PCL film by forming percolating cellulose network. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 26–30. [Google Scholar] [CrossRef]
- Pinheiro, I.; Ferreira, F.; Souza, D.; Gouveia, R.; Lona, L.; Morales, A.; Mei, L. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur. Polym. J. 2017, 97, 356–365. [Google Scholar] [CrossRef]
- Pinheiro, I.; Ferreira, F.; Alves, G.; Rodolfo, A.; Morales, A.; Mei, L. Biodegradable PBAT-Based Nanocomposites Reinforced with Functionalized Cellulose Nanocrystals from Pseudobombax munguba: Rheological, Thermal, Mechanical and Biodegradability Properties. J. Polym. Environ. 2019, 27, 757–766. [Google Scholar] [CrossRef]
- Espino-Pérez, E.; Bras, J.; Ducruet, V.; Guinault, A.; Dufresne, A.; Domenek, S. Influence of chemical surface modification of cellulose nanowhiskers on thermal, mechanical, and barrier properties of poly (lactide) based bionanocomposites. Eur. Polym. J. 2013, 49, 3144–3154. [Google Scholar] [CrossRef]
- Rueda, L.; d’Arlas, B.F.; Zhou, Q.; Berglund, L.A.; Corcuera, M.; Mondragon, I.; Eceiza, A. Isocyanate-rich cellulose nanocrystals and their selective insertion in elastomeric polyurethane. Compos. Sci. Technol. 2011, 71, 1953–1960. [Google Scholar] [CrossRef]
- Faruk, O.; Sain, M.; Farnood, R.; Pan, Y.; Xiao, H. Development of lignin and nanocellulose enhanced bio PU foams for automotive parts. J. Polym. Environ. 2014, 22, 279–288. [Google Scholar] [CrossRef]
- Cordero, A.I.; Amalvy, J.I.; Fortunati, E.; Kenny, J.M.; Chiacchiarelli, L.M. The role of nanocrystalline cellulose on the microstructure of foamed castor-oil polyurethane nanocomposites. Carbohydr. Polym. 2015, 134, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Girouard, N.M.; Xu, S.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Site-selective modification of cellulose nanocrystals with isophorone diisocyanate and formation of polyurethane-CNC composites. ACS Appl. Mater. Interfaces 2016, 8, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Ikhwan, F.; Ilmiati, S.; Adi, H.K.; Arumsari, R.; Chalid, M. Novel route of synthesis for cellulose fiber-based hybrid polyurethane. In Proceedings of the Innovation in Polymer Science and Technology, Medan, Indonesia, 7–10 November 2016; p. 12019. [Google Scholar]
- Gimenez, R.B.; Leonardi, L.; Cerrutti, P.; Amalvy, J.; Chiacchiarelli, L.M. Improved specific thermomechanical properties of polyurethane nanocomposite foams based on castor oil and bacterial nanocellulose. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Leng, W.; Li, J.; Cai, Z. Synthesis and characterization of cellulose nanofibril-reinforced polyurethane foam. Polymers 2017, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wolodko, J.; Zhao, L.; Curtis, J.M. The preparation and characterization of polyurethane reinforced with a low fraction of cellulose nanocrystals. Prog. Org. Coat. 2018, 125, 207–214. [Google Scholar] [CrossRef]
- Hubmann, M.; Kong, X.; Curtis, J.M. Kinetic stabilization of cellulose nanocrystals in a photocurable prepolymer for application as an adhesion promoter in UV-curable coatings. Prog. Org. Coat. 2019, 129, 101–115. [Google Scholar] [CrossRef]
- Musk, A.W.; Peters, J.M.; Wegman, D.H. Isocyanates and respiratory disease: Current status. Am. J. Ind. Med. 1988, 13, 331–349. [Google Scholar] [CrossRef]
- Bengtström, L.; Salden, M.; Stec, A.A. The role of isocyanates in fire toxicity. Fire. Sci. Rev. 2016, 5, 1–23. [Google Scholar] [CrossRef]
- Marx-Figini, M. Studies on the ultrasonic degradation of cellulose macromolecular properties. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1997, 250, 85–92. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Williams, R.J. Kinetic and statistical aspects of the formation of polyurethanes from toluene diisocyanate. Polymer 1986, 27, 425–430. [Google Scholar] [CrossRef]
- Buckles, R.E.; McGrew, L. A kinetic study of the dimerization of phenyl isocyanate. J. Am. Chem. Soc. 1966, 88, 3582–3586. [Google Scholar] [CrossRef]
- Schwetlick, K.; Noack, R. Kinetics and catalysis of consecutive isocyanate reactions. Formation of carbamates, allophanates and isocyanurates. J. Chem. Soc. Perkin Trans. 2 1995, 2, 395–402. [Google Scholar] [CrossRef]
- Guo, J.; He, Y.; Xie, D.; Zhang, X. Process investigating and modelling for the self-polymerization of toluene diisocyanate (TDI)-based polyurethane prepolymer. J. Mater. Sci. 2015, 50, 5844–5855. [Google Scholar] [CrossRef]

| Category | Cellulose | Isocyanate | Matrix/Chemical | Ref |
|---|---|---|---|---|
| Functional Cellulose | Whatman Powder | 2,4-TDI | - | [58] |
| Cellulose Beads | 2,4-TDI or HMDI | - | [59] | |
| MCC | 2,4-TDI | n-Butanol | [44] | |
| Whatman Paper | Cyclohexyl Isocyanate or PI | - | [60] | |
| Cellulose | Phosphonium-containing Isocyanate | - | [61] | |
| Cotton Cellulose | Sulfopropylbetaine or Quaternary Ammonium Salt with a Reactive Isocyanate | - | [38] | |
| MCC | 2,4-TDI | Amino acids | [62,63] | |
| Plant Cellulose | MDI | - | [64] | |
| Cotton Spheres | HMDI, 2,4-TDI, 1,4-PDI | - | [26] | |
| MCC | Substituted PI | - | [27] | |
| Cellulose Oligomers | 3,5-Dimethylphenyl Isocyanate | - | [28] | |
| Cellulose | 3-Chlorophenyl or 4-Chlorophenyl Isocyanate | Silica | [65] | |
| Improving Cellulose Properties | Cellulose | PI, Cyclohexyl Isocyanate, or HMDI | - | [25] |
| Cellulose | Oxime-blocked Isocyanate Oligomers | - | [66] | |
| Sisal Fibers | MDI | Soy-based Resin | [67] | |
| Six Celluloses | Alkenyl Isocyanates | Styrene or Methylacrylate | [68,69] | |
| Four Celluloses | Isocyanate-containing Polystyrene | - | [70,71] | |
| Cotton Fabric | 2,4-TDI and PEG | - | [72] | |
| Cellophane Sheets | HMDI | Betaines | [73] | |
| Cellulose Fabric | 2-Methacryloyloxyethyl Isocyanate | 2-Methacryloyloxyethyl Phosphoryl Choline | [74] | |
| Cellulose Acetate | Phenyl, Propyl, or Butyl Isocyanate | - | [75,76] | |
| Cellulose Processing with Nonpolar Matrices | Aspen Pulp and Sawdust | Poly(methylene(polyphenyl isocyanate)) | PS | [77,78] |
| Pine Pulp | MDI | PS | [79] | |
| Different Celluloses | Alkyl Isocyanates | PP | [80] | |
| Sisal Fibers | 2,4-TDI | PP | [81] | |
| Whatman Fibers | HMDI | PP | [82] | |
| Bagasse Fibers | Polybutadiene Isocyanate | PP | [41] | |
| Birch Pulp | 2,4-TDI | PP/PLA | [83] | |
| Pineapple Leaf Fibers | Poly(methylene(polyphenyl Isocyanate)) or HMDI | PP and PE | [84] | |
| MCC and Fibers | OI | PE | [69] | |
| Pineapple Leaf Cellulose | Poly(methylene(polyphenyl isocyanate)) | PE | [85,86] | |
| Sisal Fibers | 2,4-TDI-g-Cardanol | PE | [87,88] | |
| Pine Pulp | Derivatives of MDI | PP | [89] | |
| MCC | MDI and PPDI | Natural Rubber | [90] | |
| MCC | OI | Epoxidized Soybean Oil Polymer | [91] | |
| Hemp Fibers | 3-Isopropenyl-dimethylbenzyl Isocyanate | Polyester | [92] | |
| Arbocell Fibers | MDI then Ethanol | Thermoplastic Urethane or a Polyamide | [93] | |
| Kenaf Fibers | Blocked MDI | Polyglycol Polyol, 1,4-Butanediol, and MDI | [94] | |
| Pulp Fibers | HMDI | Castor Oil | [95] | |
| Cellulose Acetate | HMDI | Castor Oil | [96] | |
| Eucalyptus Pulp | OI | Cement | [97,98] | |
| Cellulose/ Matrix Cross-Linking | MCC and Pulp | 2,4-TDI | PCL | [99] |
| Cellulose Diacetate | 2,4-TDI | Poly(caprolactone monoacrylate) | [100] | |
| Cellulose Diacetate | 2,4-TDI | Poly(butylene glycol adipate) | [101] | |
| MCC | MDI | Castor Oil/MDI | [102] | |
| Cotton Cloth | Blocked Isocyanate of 2,4-TDI and Phenol | Lignin | [103] |
| Category | CNCs/CNFs | Isocyanate | Matrix/Chemical | Ref |
|---|---|---|---|---|
| Functional Nanocellulose | CNCs | 3,5-Dimethylphenyl Isocyanate | - | [29] |
| CNCs | OI | - | [30] | |
| CNCs | 2,4-TDI | Photocleavable Polymer | [23] | |
| Improving Nanocellulose Properties | CNFs | OI | - | [110] |
| CNCs | 2,4-TDI | Castor Oil | [45] | |
| CNCs | 2,4-TDI | PHBV | [111] | |
| CNFs | HMDI | Alkyl Diamines | [112] | |
| CNFs | HMDI | - | [113] | |
| Nanocellulose Processing with Nonpolar Matrices | CNCs, CNFs | OI | PCL | [39,40] |
| CNCs | OI | PCL | [114] | |
| CNCs | OI | PCL | [115] | |
| CNCs | 2,4-TDI then PCL diol | PCL | [43] | |
| CNCs | PCL with 2,4-TDI | PCL | [104] | |
| CNCs | OI or 4-Phenylbutyl Isocyanate | PBAT | [36,37] | |
| CNCs | OI | PBAT | [116] | |
| CNCs | OI | PBAT | [117] | |
| CNCs | 2,4-TDI | PLA | [42] | |
| CNCs | OI | PLA | [118] | |
| Nanocellulose Matrix Cross-Linking | CNCs | MDI | Certain Polyols | [48,49] |
| CNCs | HMDI | Polyurethane | [119] | |
| CNFs | Polymeric MDI | Lignin-Soy Polyol with Polymer MDI | [120] | |
| CNCs | MDI | Castor Oil and MDI | [121] | |
| CNCs | Isophorone Diisocyanate | Isophorone Diisocyanate and a Trifunctional Polyether Alcohol | [122] | |
| CNFs | Methylenebis(Cyclohexyl Isocyanate) | Methylenebis(Cyclohexyl Isocyanate) with PEG | [123] | |
| CNFs | MDI | Castor Oil Polyol and MDI | [124] | |
| CNFs | Poly(phenyl Isocyanate) | PEG and Poly(methylene(polyphenyl isocyanate)) | [125] | |
| CNCs | Polymeric MDI | Polyether Polyol and Polymeric MDI | [126] | |
| CNCs | Photocurable Isocyanate (3-isopropenyl-α,α-dimethylbenzyl Isocyanate) | Polyether Polyol and 3-Isopropenyl-α,α-dimethylbenzyl Isocyanate | [127] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. https://doi.org/10.3390/molecules24152782
Abushammala H, Mao J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules. 2019; 24(15):2782. https://doi.org/10.3390/molecules24152782
Chicago/Turabian StyleAbushammala, Hatem, and Jia Mao. 2019. "A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates" Molecules 24, no. 15: 2782. https://doi.org/10.3390/molecules24152782
APA StyleAbushammala, H., & Mao, J. (2019). A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules, 24(15), 2782. https://doi.org/10.3390/molecules24152782