2.1. Calculations
The density functional theory (DFT) calculations were performed using the Gaussian 09 [
19] program running on PC computer and Gauss View [
20] molecular visualization program. To calculate optimized geometrical structure of studied compounds the Lee-Yang-Parr Becke’s three parameter hybrid functional method (B3LYP) with 6-311+G(d,p) basis set was used. The optimized molecular geometry was characterized as minimum in the energy by the absence of imaginary wavenumbers.
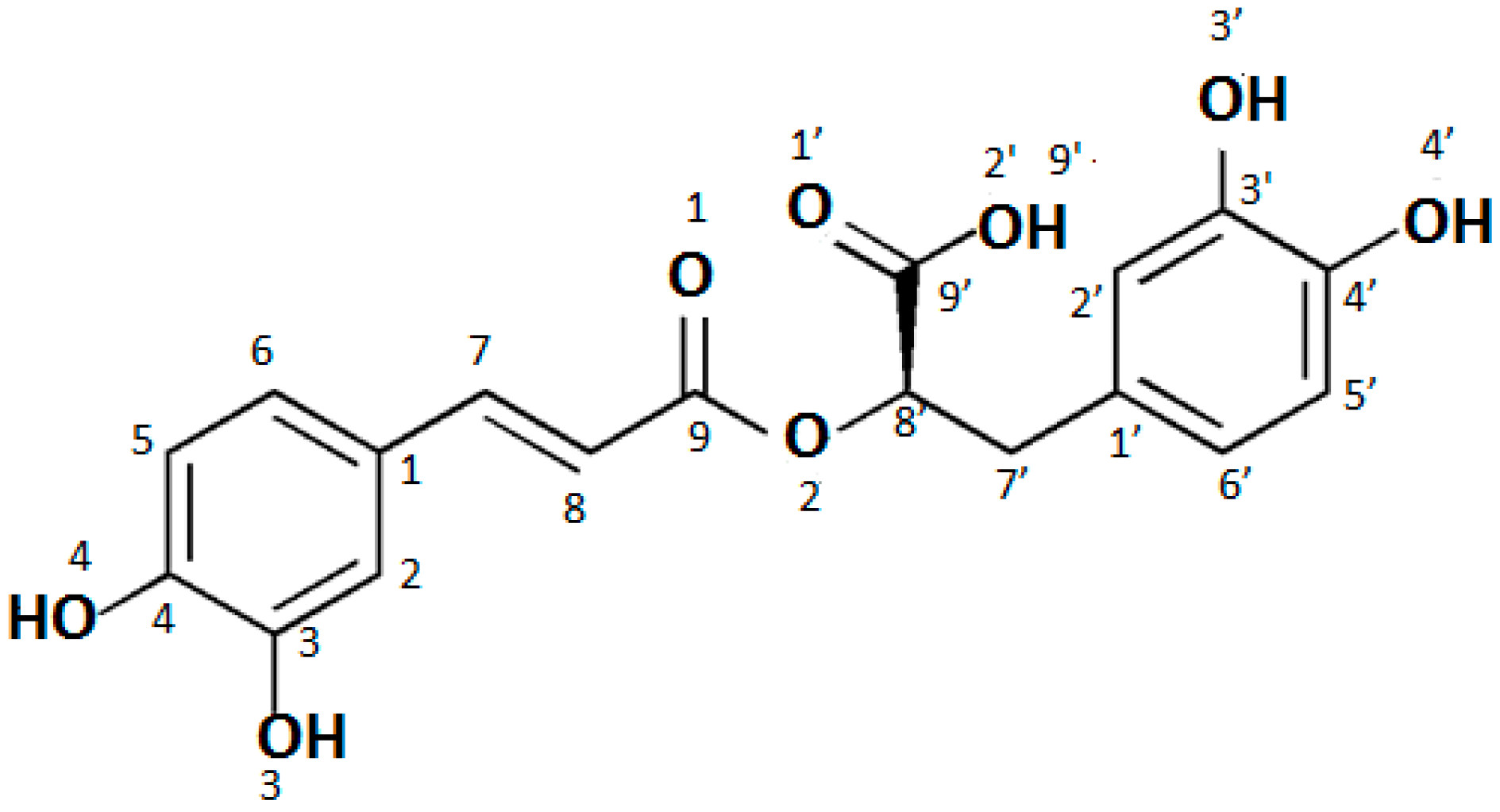
The calculated lengths of the bonds and size of the angles of the optimized molecules of rosmarinic acid (RA) as well as lithium (RA-Li), sodium (RA-Na), and potassium (RA-K) rosmarinates are shown in
Table 1. Results obtained for rosmarinic acid molecule were compared with calculated and experimental data found in the literature [
7,
21]. Good linear correlation was obtained for theoretical as well as experimental data. Correlation coefficients received for bonds’ lengths equal R = 0.9996 and 0.9997, respectively. Regarding the angles between bonds appropriate values are 0.9941 for the calculated and 0.9786 for the experimental one. Exemplary correlations are shown in
Figure S1.
Analysis of the obtained data shows that salt is formed by the substitution of the alkali metal ion in place of the hydrogen cation in the carboxyl group of the rosmarinic acid molecule, since the biggest alternations were found within this group. The bond length between the O2′-H/M atoms in the salt molecules increases in comparison to the rosmarinic acid molecule from 0.894 Å to 1.562 Å (the numbering of atoms in the acid molecule is shown in
Figure 1). Much smaller changes were noted in the bonds of C9′-O1′ (the increase by 0.056–0.062 Å) and C9′-O2′ (the decrease by 0.084–0.088 Å). In this way, these bonds in the COO group in salt molecules are almost equal. The lengths of bonds C9–O2, O2–C8′, and C8′-C9 change only by 0.004–0.017 Å. The remaining bonds in the studied molecules do not change at all. The size of the angles in the salt molecules also change a little compared to the acid molecule with the exception of the carboxyl group. The biggest changes were observed for C9′-O1′-H/M (the increase by 27.76–35.28°) and C9′-O2′-H/M (the decrease by 17.93–25.15°) angles. Much smaller changes were observed for the O1′-C9′-C8′ (the decrease by 6.94°), O1′-C9′-O2′ (2.09°) and C1′-C7′-C8′ (the increase by 0.97°) angles. The changes of angles around carbonyl group are about 1.2–1.3°. Other angles change only slightly (0.01–0.27°). This indicates, that the cation of metal is substituted instead of the hydrogen atom in the carboxyl group.
The lengths of bonds within the hydroxyl groups practically do not change in the series: RA → RA-Li → RA-Na → RA-K. On the other hand, the presence of weak intramolecular hydrogen bonds between the ortho-position hydroxyl groups should be noted. The bonds in acid molecule are similar to those in its salts molecules, so it is difficult to say, on this basis, which molecules will have stronger antioxidant properties, acid whether its salts. Antioxidant action of phenolic compound is based on free radical quenching. The free radical scavenging mechanism depends on hydrogen atom transfer (HAT), a single electron transfer (SET), or the combination of both HAT and SET, which may be described as ArOH → ArO
• + e
− + H
+ [
21]. Thus, antioxidant activity depends on (i) H-abstraction and (ii) stability of the formed free radical. Cao et al. [
21] studied the geometry structures of ground state of rosmarinic acid and its free radicals formed on O3, O4, O3′, and O4′ atoms. They found that stronger intramolecular hydrogen bonds (1.97–1.98 Å) are in more radical form than in the ground state of acid molecule, which stabilizes the free radical forms and makes the abstraction of hydrogen atom from the hydroxyl group occur easily. The ability to create resonance semiquinone even quinone structures additionally results in the stability of the radicals.
By comparing two aromatic rings in studied molecules, it was found that the ring A’ has more equalized bonds lengths (ΔC′C′ = 0.013
) than the A ring (ΔCC = 0.023
) in acid as well as in salt molecules. This conclusion is confirmed when one compares the differentiation of angles. In the case of angles in the aromatic rings the variation in the size of the angles in the salt molecules (2.38° < ΔCCC < 2.55° and 2.55° < ΔC′C′C′ < 2.62°) is greater than in rosmarinic acid molecule (ΔCCC = 2.21° and ΔC′C′C′ = 2.40°). This is also visible in the values of aromaticity indices (
Table 2) [
22,
23,
24]. The ring A has probably more disturbed aromaticity because of the resonance semiquinone structures. On the other hand, A’ ring aromaticity is higher in salts molecules than in rosmarinic acid molecule, perhaps for the reason that the symmetry of the molecules increase.
The values of dipole moment, energy, and aromatic indices calculated for rosmarinic acid and its lithium, sodium, and potassium salts are shown in
Table 2. The dipole moment values of rosmarinic acid and its salt molecules increase in the series: Rosmarinic acid < Li < Na < K salt. It indicates that polarity of studied compounds increase. The absolute energy of the tested molecules decreases in the same series. The values of the energy variation (ΔE) associated to the substitution reaction of the carboxylic hydrogen atom by an alkali metal atom have been calculated and included in
Table 2.
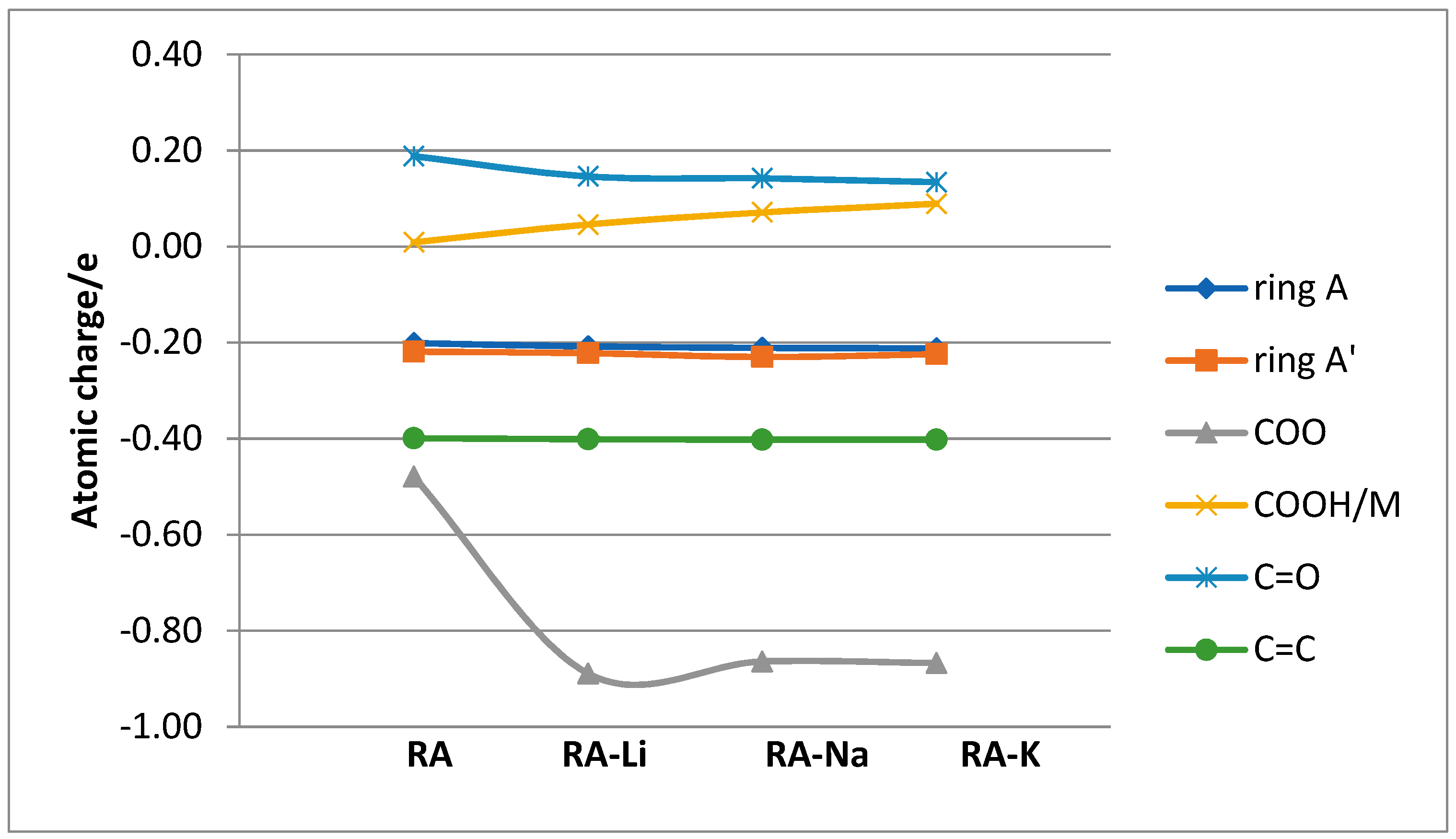
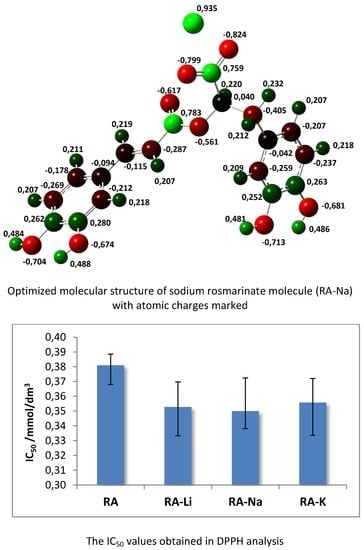
The atomic charge distribution in studied molecules was calculated by the natural bond orbital method (NBO) using B3LYP/6-311+G(d,p). Data obtained for rosmarinic acid and its sodium salt are presented in
Figure 2. In the case of lithium and potassium rosmarinates, the values of atomic charges are very similar to sodium salt. The atomic charges on most of the atoms in molecules of a lithium and potassium salts only slightly differ relative to the sodium rosmarinate molecule, they are in the range 0.000–0.007e. The exception are oxygen atoms of carboxylate group: O1′ (−0.813, −0.799 and −0.803e), O2′(−0.836, −0.824 and −0.822), as well as alkali metal ion (0.935, 0.935, and 0.956 e, for RA-Li, RA-Na, and RA-K, respectively). Systematic changes in the series: RA > RA-Li > RA-Na > RA-K is noted for atomic charges of C7, C8, and O1 atoms. When it comes to comparing salt molecules with an acid molecule, the largest changes are observed for H9′/M atom (atomic charge increases by 0.468e. In the case of O1′ and O2′ atoms, negative atomic charges significantly increase for lithium salts (by 0.231 and 0.144e, respectively) in comparison to acid, but in the Li > Na > K salts series they slightly decrease (0.014 and 0.012e). Much smaller changes (0.037e) in the series RA > RA-Li > RA-Na > RA-K occur for the charges on C9′ atom of carboxylate group. The summary charges calculated for the aromatic rings, double bond, and carbonyl and carboxylic/carboxylate groups were calculated and are shown in
Figure 3. In summary, the biggest changes in comparison to acid molecule are observed for COO group. However, very good linear correlation was found between the summary charge calculated for the COOH/M group and electron affinity, the ionic potential, and the atomic radius of the hydrogen/metal atom. Corresponding correlation coefficients are 0.9998, 0.9918, and 0.9891, respectively.
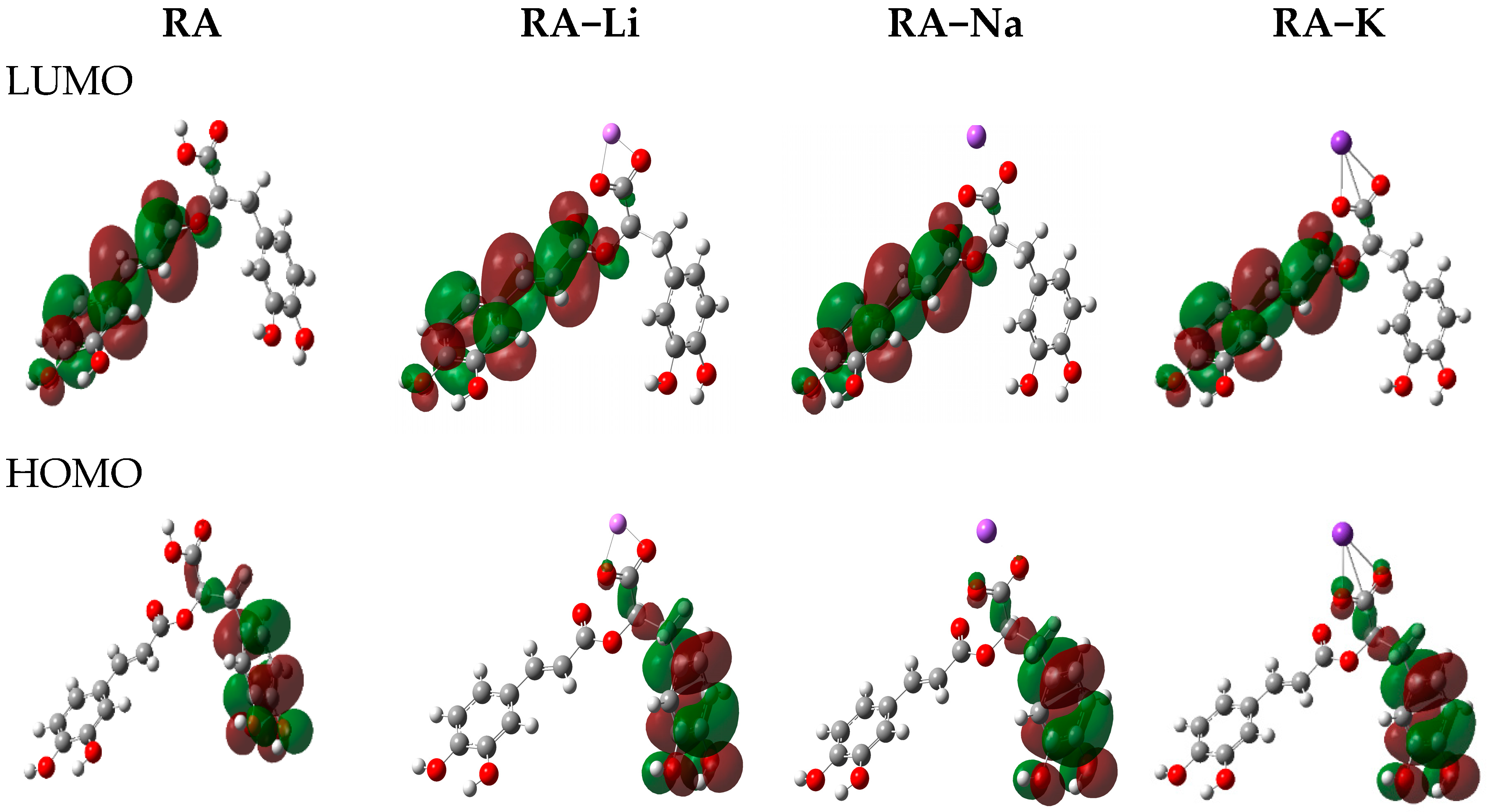
The frontier orbital energies are the important parameters of the molecular electron structure. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energies as well as other general reactivity descriptors [
25,
26,
27,
28] such as ionization potential (I), electron affinity (A), electronegativity (
χ), chemical hardness (
η), softness (s), chemical potential (
μ), electrophilicity (
ω), and nucleophilicity (N) indexes calculated on the basis of the HOMO and LUMO orbital’s energy are gathered in
Table 3. Reactivity descriptors were calculated as follows: I = −E
HOMO; A = −E
LUMO;
χ = (I + A/2);
η = (I − A)/2; s = 1/2
η;
μ = −(I + A)/2;
ω =
μ2/2
η; N = E
HOMO(nuclefil)−E
HOMO(TCNE) (nucleofilicity index N is referred to tetracyanoethylene TCE [
28). The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) play an important role for predicting the charge transfer within the molecule, chemical reactivity, bioactivity, and stability of the compound [
25,
29]. The higher the HOMO energy is, the stronger electron donor molecule is, while LUMO energy reflects the ability to accept the electron. The distribution of HOMO electron density in a phenolic molecule may qualitatively indicate the active site of scavenging free radicals, because the reaction of H-abstraction is associated with the transfer of electron [
21]. The HOMO and LUMO electron densities for rosmarinic acid and its lithium, sodium, and potassium salts are shown in
Figure 4. The HOMO electron density in both acid and its salt molecules is mainly distributed over carbon atoms in A’ ring and O3′, O4′ oxygen atoms of ortho-hydroxyl groups, which can donate electrons easily during the H-abstraction. The LUMO electron density is distributed over ring A, double bond, carbonyl and carboxylic groups forming a conjugating system. This is beneficial for electron scattering and has some effects on free radicals such as O
2− [
21]. The HOMO-LUMO energy gap is a useful descriptor of chemical and biological activity of molecules. The smaller gap value is, the more chemically active the molecule is, and it is termed a soft molecule. The HOMO-LUMO gap values decrease in the following order: RA-Li > RA-Na > RA-K > RA, indicating the increase in the reactivity of studied molecules in above series. In this way rosmarinic acid should be chemically more active then salts molecules. The electrophilic or nucleophilic index is related to molecule ability to exchange electron density during a reaction [
30,
31]. The electron affinity, electronegativity and electrophilicity indexes decrease in series: rosmarinic acid > its lithium > sodium > potassium salts, while the values of chemical potential increase in the above series. The ionization potential decreases in order RA-Li > RA > RA-Na > RA-K, whereas the values of nucleophilicity increase in the above series. It indicates that the sodium and potassium salts have higher electron transfer ability than rosmarinic acid.
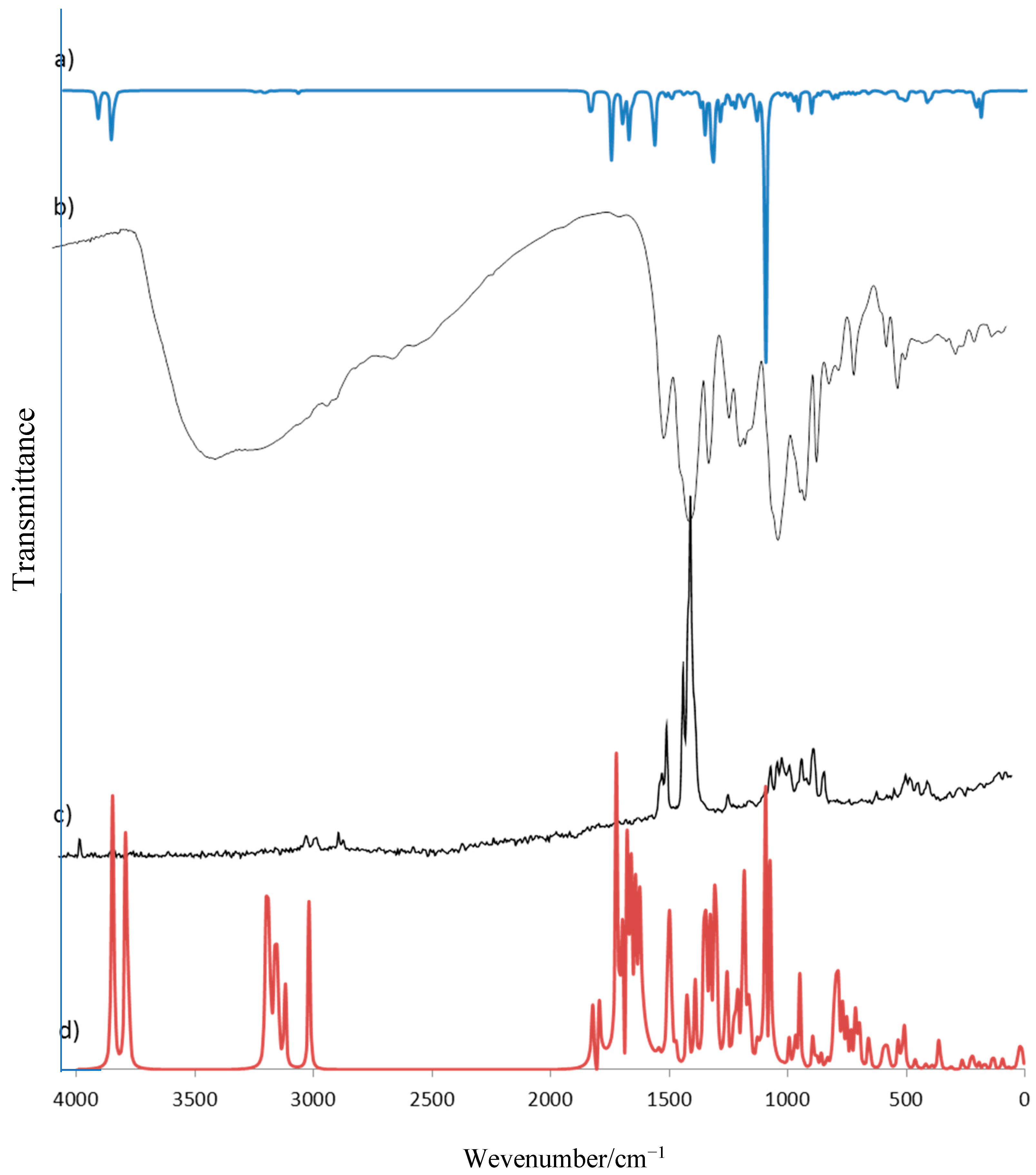
The wavenumbers and intensities of bands in vibrational spectra for the optimized structures of rosmarinic acid and its lithium, sodium, and potassium salt molecules were also calculated and presented in
Table 4.
2.2. Vibrational Spectra
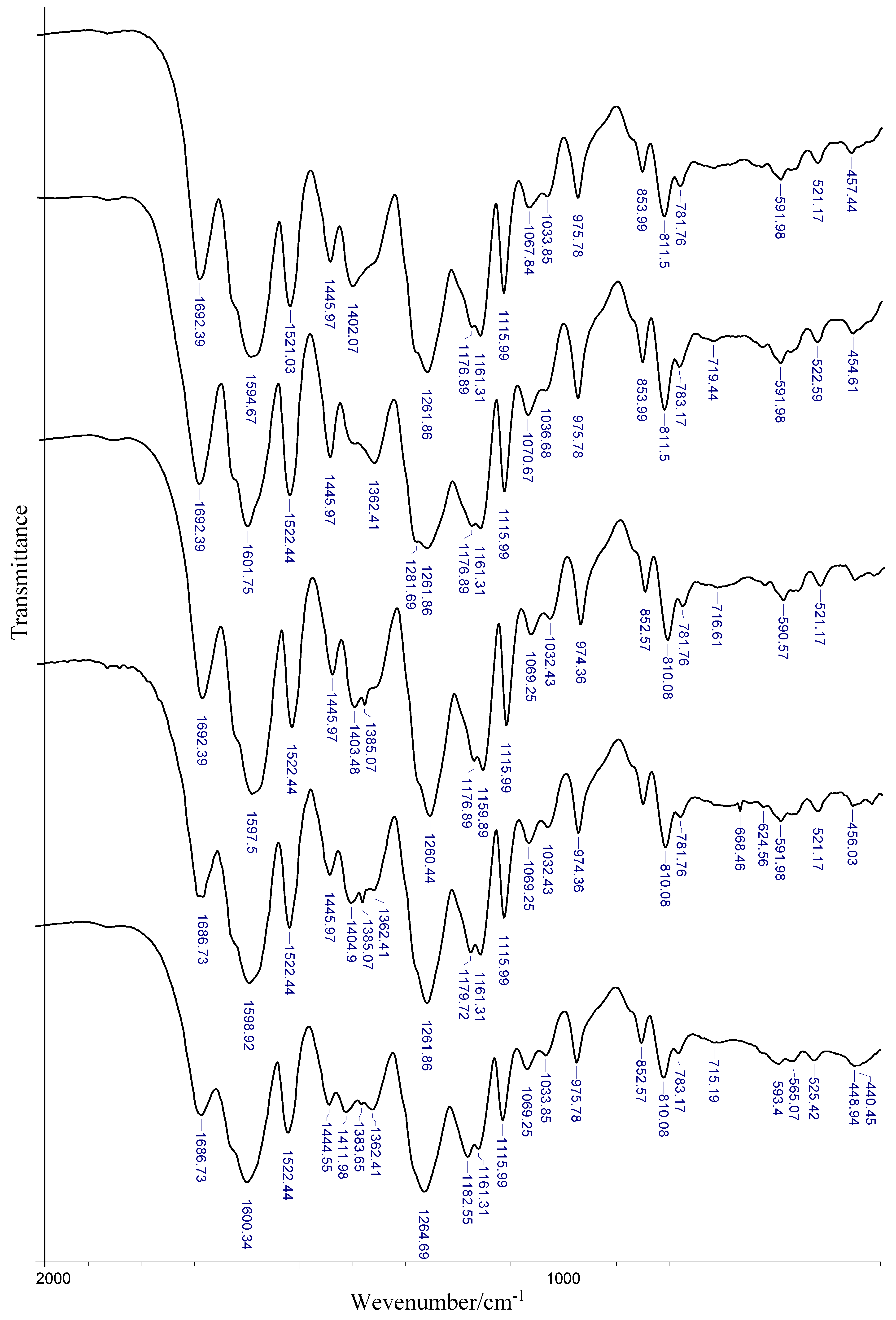
Experimental data of IR and Raman spectra of rosmarinic acid and its alkali metal salts are gathered in
Table 5 and visualized in
Figure 5. The assignment of bands is based on the literature, quantum-mechanical calculations, and personal experience. The bands are numbered along with the notation used by Varsányi [
32].
In the IR spectrum of rosmarinic acid, there is a very intense band originating from the stretching vibration of the carboxyl group νC=O at 1708 cm−1, while in Raman there are two bands of νC=O at 1708 and 1727 cm−1 (weak and medium intensity, respectively). In the rosmarinic acid spectrum the bands associated with in plane βC=O at 790 cm−1 (IR) and 792 cm−1 (Raman) as well as out of plane γC=O at 682 cm−1 (IR) deformations are observed. Some bands are connected with vibrations of hydroxyl groups, they are weak and of medium intensity bands coming from the stretching vibrations νOH at 3419, 3456 and 3399 cm−1 (IR) and deformations βOH at 1478 cm−1 and γOH at 917 cm−1 (Raman). In addition, a number of bands associated with vibrations of the aromatic ring can be seen in the ligand spectrum.
The substitution of the alkali metal atom in place of the hydrogen atom in the carboxylic group causes the disappearance of some characteristic for acid bands in the salt spectra, i.e., the bands coming from stretching and deformation vibrations of carboxylic group. Instead, there are very intense bands derived from the asymmetric stretching vibrations of carboxylate anion νas(COO−) lying in the range 1608–1606 cm−1 (Raman) and 1600–1598 cm−1 (IR) and symmetrical stretching vibration bands νs(COO−) in the range 141–1403 cm−1 (IR spectra). The less intense bands derived from symmetrical in plane deformations βs(COO−) appear at 738–736 cm−1 in IR spectra (751–728 cm−1. In Raman) and asymmetrical deformations βas(COO−) in the range 565–564 cm−1 (IR) and 573–568 cm−1 (Raman) as well as out of plane deformations γs(COO−) bands (715–718 and 724–713 cm−1, respectively in IR and Raman spectra). There are also low-intensity bands in Raman spectra, for example bands of symmetrical stretching vibrations νs(COO−) in the range 1417–1407 cm−1. The Raman spectra of alkali metal rosmarinates show a decrease in the wavenumbers and a reduction in the intensity of the bands coming from the vibrations of the aromatic ring, e.g., νas(CH2), β(CH), 19b (βOH, νCC), 16b (ϕCC), and 6b (αCCC) in comparison to the acid spectrum. In addition, the wavenumbers of some bands change regularly in the series of Li → Cs salts. The bands of βs(COO−) in Raman spectra, νs(COO−) and νas(COO−) in IR spectra are shifted to lower wavenumbers in Li > Na > K > Rb > Cs rosmarinates series but the bands of β(CH) deformation in the range 1075–1068 cm−1 and 5 (γCH) in Raman spectra show an increasing tendency in the above series. However a systematic decrease of the wavenumbers of the bands of asymmetric and symmetric stretching vibrations of carboxylate anions in the IR spectra was observed in the Li > Na > K > Rb > Cs salt series, the values of difference Δν = νas(COO−) − νs(COO−) increase in the order: Li > Cs > Na > K > Rb, correspondingly 188, 193, 194, 195, and 203 cm−1. It indicates an increasing share of metal-oxygen ionic binding.
The very good linear correlation was observed between the values of wavenumbers of chosen bands (νs(COO), βs(COO), β(CH), 5) and the parameters characterizing metals, i.e., electronegativity, atomic radius, electron affinity, and ionic potential (defined as the ratio of the ion charge to its radius). For electronegativity, ionic potential, and atomic radius, high correlation coefficients (0.875–0.987) were obtained. In addition, it was found that the wavenumbers of νs(COO−) band very well linearly correlates with the lengths of bonds (C9′-O1′ and C9′-O2′) and sizes of angles (O1′-C9′-O2′, C9′-O1′-M and C9′-O1′-M) of carboxyl group (correlation coefficients are in the range 0.991–0.999).
Experimental data have been compared to calculated wavenumbers and the good linear correlation is found (correlation coefficient R = 0.998, 0.999, 0.998, and 0.999, respectively for RA, RA-Li, RA-Na, and RA-K). Experimental and calculated vibrational spectra are presented exemplary for rosmarinic acid in
Figure 6.
2.3. NMR Spectra
The experimental and theoretical chemical shifts of protons and carbons in NMR spectra of rosmarinic acid and its alkali metal salts in DMSO saturated solution are gathered in
Table 6. Literature data [
8] of experimentally obtained chemical shifts in
13C and
1H NMR spectra of rosmarinic acid are also presented. The good correlation between literature and the obtained data in this work is found (R = 0.999 for
13C as well as
1H NMR).
The good linear correlation is also obtained between our experimental and calculated data connecting NMR spectra obtained for rosmarinic acid and its lithium, sodium, and potassium salts is obtained. The correlation coefficients equal 0.857, 0.879, 0.880 and 0.874 for 13C NMR, for 1H NMR corresponding values are: 0.987, 0.976, 0.979, and 0.938.
In 13C NMR spectra there are peaks connecting carbons of aromatic rings (C1–C6) and (C1′-C6′), the double bond (C7–C8), the ester group C9 and carboxyl group C9′. 13C NMR spectra of rosmarinic acid and its alkali metal salts shows the highest values of chemical shifts for C9′ carbon atom (170.8–173.1 ppm), slightly lower values of chemical shifts are observed for C9 atom (165.9–166.3 ppm). The chemical shifts observed for C3, C4, and C3′, C4′ atoms (the places of hydroxy groups substitution) as well as for C7 atom (of double bond) are in the range 143.7–148.9 ppm. The lowest chemical shift is observed for C7′ atom of CH2 group (36.1–37.3ppm). The chemical shifts for C8′ carbon atom are 72.8–76.2 ppm. The highest changes in salt spectra in comparison to acid spectrum is noted for C8′ and C1′ (127.3–129.9 ppm) carbon atoms. The increase in chemical shifts in salts spectra in comparison to acid spectrum is observed in the case of C2, C9, C1′, C2′, C7′, C8′, and C9′ atoms. In comparison, a decrease is noted for C6, C7, C3′, and C4′ atoms. There are no regular changes in the series of alkali metal rosmarinates in the experimental 13C NMR spectra. In theoretical spectra the decrease in chemical shifts is noted for C2, C3, C4, C5, C6, C9, C3′, C5′, C6′, and C9′, but only an increase for C1 and C1′ atoms.
In calculated
1H NMR spectra of rosmarinic acid and its alkali metal salts the chemical shifts for H3, H4, and H3′, H4′ protons are in the range 4.3–5.5 ppm. The signals for above atoms in experimental spectrum of acid are in the range 8.7–9.6 ppm, but in salts spectra corresponding signals are not found. The phenolic proton signals is usually a sharp singlet in the range of 7.5–4.0 ppm. The presence of two groups relative to each other in the ortho position strongly influences the chemical shift of phenolic protons (signals are in the range of 12–10 ppm). This is due to the formation of intramolecular hydrogen bonds [
33]. In calculated spectra, the peak assigned for H9′ proton of carboxylic group occurs at 6.0 ppm, whereas in experimental spectra it is found at 13.0 ppm. Similar results were obtained in our previous investigations [
27]. In experimental
1H NMR spectra of rosmarinates, the decrease in chemical shifts in comparison to acid spectrum is observed in the case of H6, H7, H8, H5′, H6′, H7′, and H8′ protons, while the increase is only noted for H7′. In theoretical spectra the decrease is observed for H3, H4, H3′, H4′, H8, H2′, H5′, and H6′ protons, and the increase for H7″ and H7 atoms. When it comes to comparing spectra of alkali metal rosmarinates, in experimental spectra, only slight changes are noted, while in theoretical spectra, the decrease in the series Li > Na > K salts is observed for the most protons, the increasing tendency is only observed for H7′.
The linear correlation was found between chemical shifts of C9′ atom in experimental NMR spectra and some metal parameters such as electronegativity (R = 0.773), ionic potential (0.766), atomic radius (0.762), and electron affinity (0.708).
2.4. Antioxidant Activity
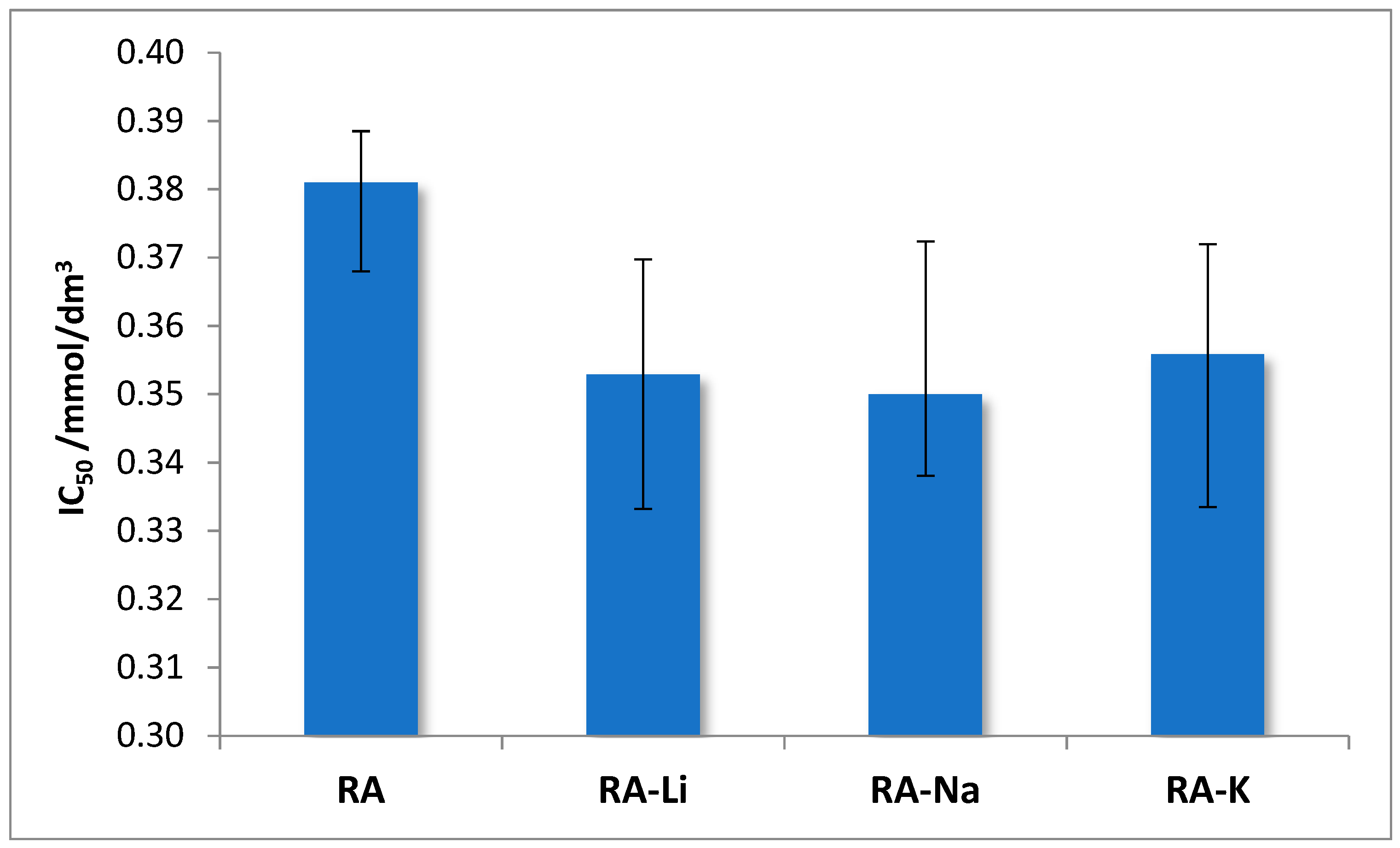
The antioxidant properties of rosmarinic acid and its salts with lithium, sodium, and potassium were examined. To assess the antioxidant activity, two methods were used that consist of (i) scavenging of free, stable DPPH* radicals and (ii) reduction of Fe
3+ ions (FRAP method). The obtained results connecting the DPPH radical scavenging activity of studied compounds are expressed as IC
50. The lower IC
50 value, the better DPPH radical neutralizing properties represented by the test samples. Studies show that alkali metal rosmarinates have better antioxidant properties than rosmarinic acid, IC
50 values obtained for salts are very similar (
Figure 7).
The clear linear correlation was found between differences of wavenumbers Δνas-s(COO) in IR spectra and IC50 values (effective concentration of the solution required to decrease the initial DPPH radicals by 50%). The correlation coefficient equals 0.989.
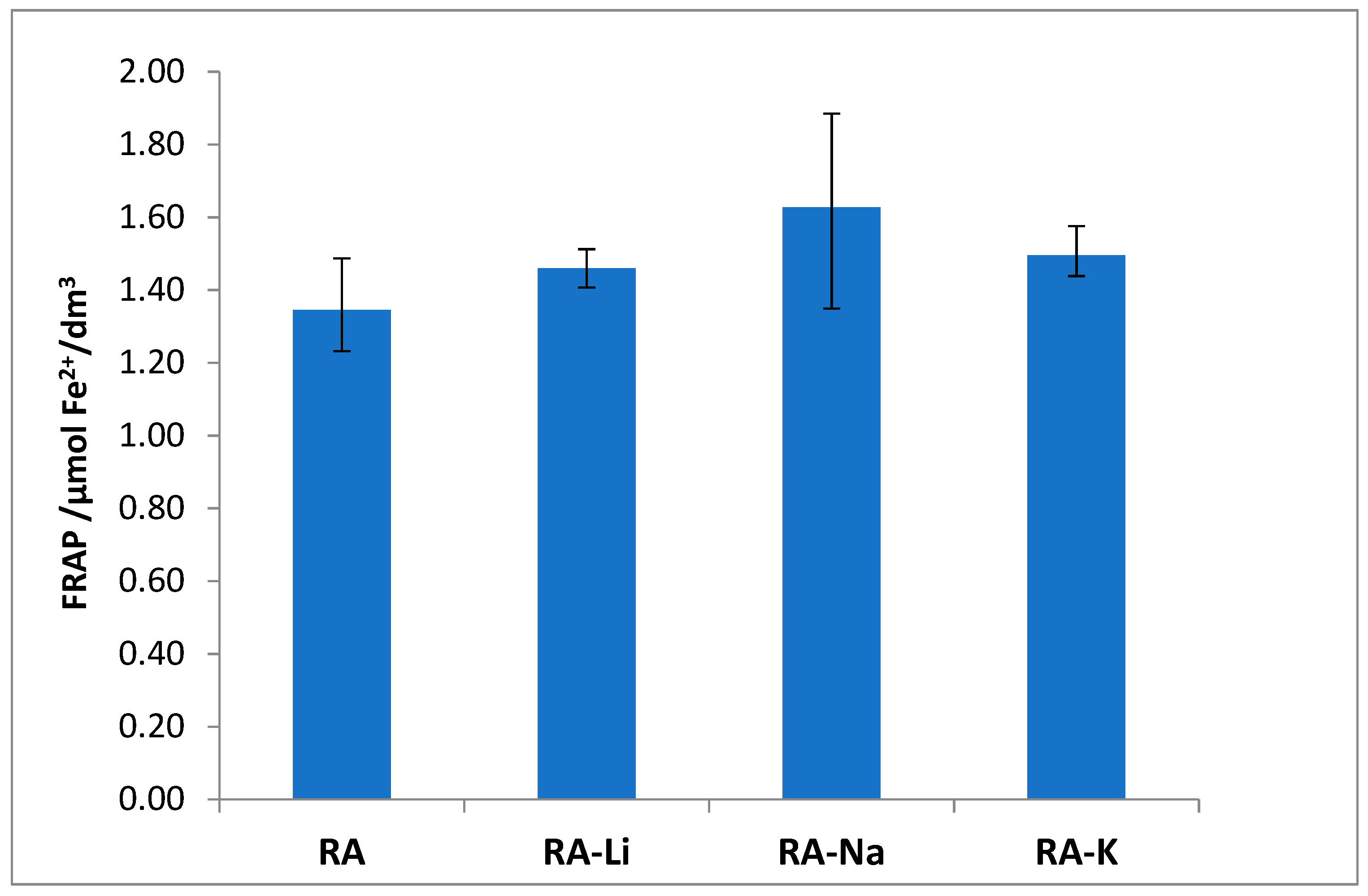
The studied salts have also higher ability to Fe
3+ reduction than rosmarinic acid (
Figure 8), and among them the sodium rosmarinate shows the highest antioxidant activity.
The antioxidant properties expressed through the Fe3+ ion reduction capability (FRAP method) linear correlate with the values of HOMO-LUMO energy gap. The correlation coefficient equals 0.7666.