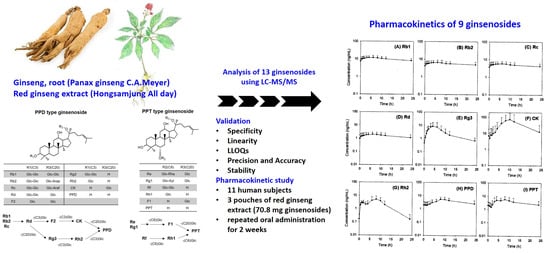
Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract
Abstract
1. Introduction
2. Results
2.1. MS/MS Analysis
2.2. Sample Praperations
2.3. Analytical Method Validation
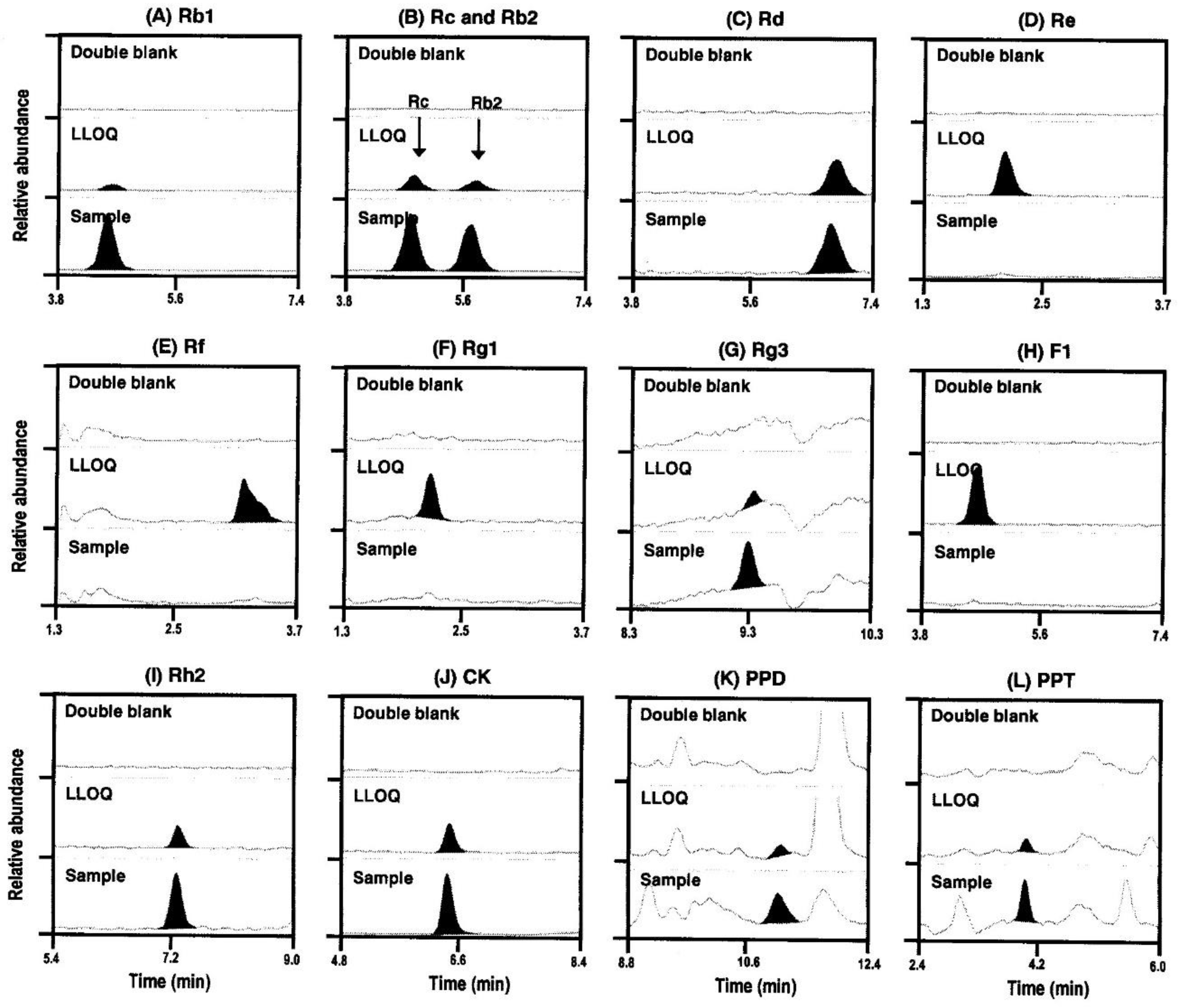
2.3.1. Specificity
2.3.2. Linearity and LLOQ
2.3.3. Precision and Accuracy
2.3.4. Extraction Recovery and Matrix Effect
2.3.5. Stability
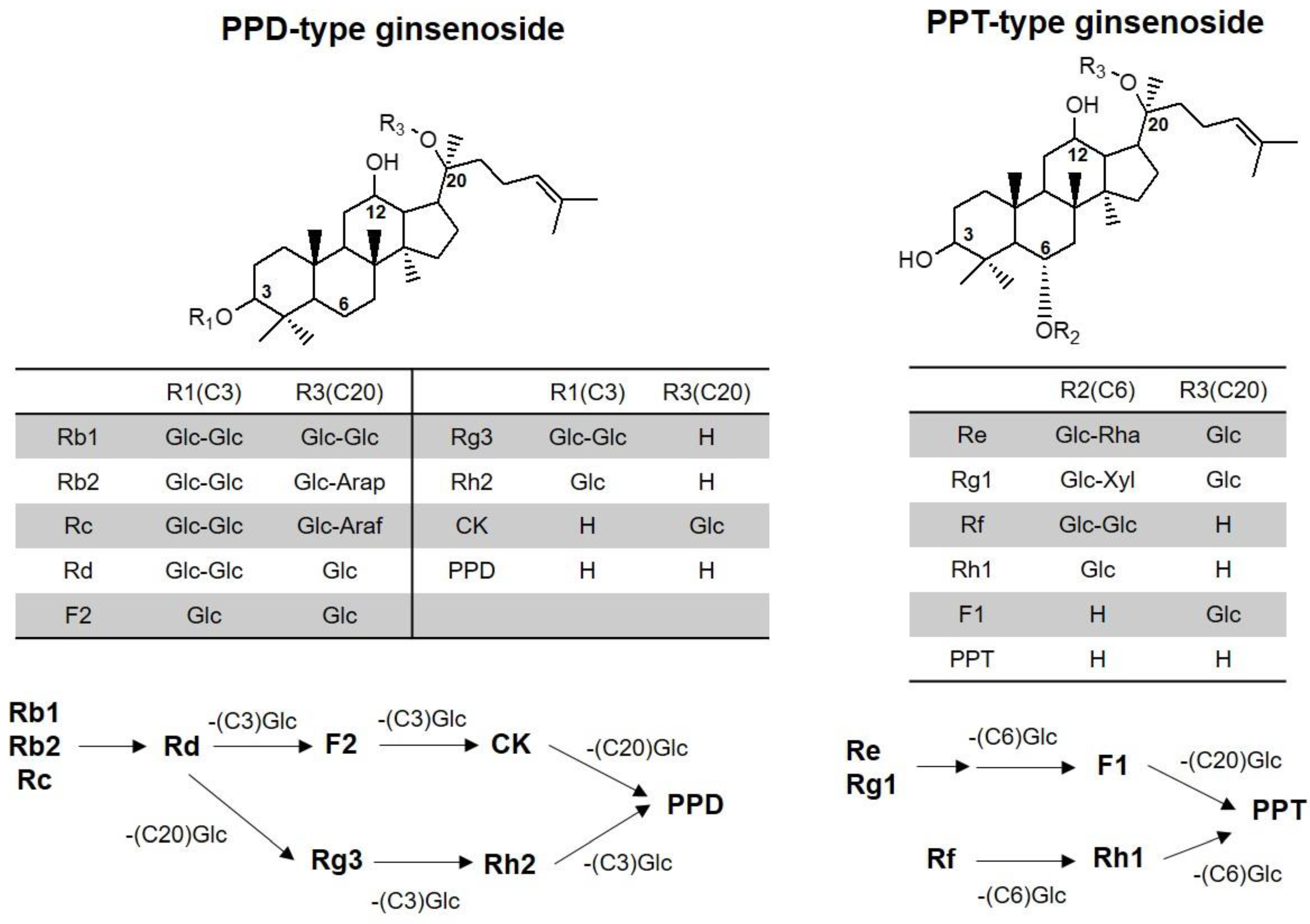
2.4. Contents of Ginsenosides in Red Ginseng Extract
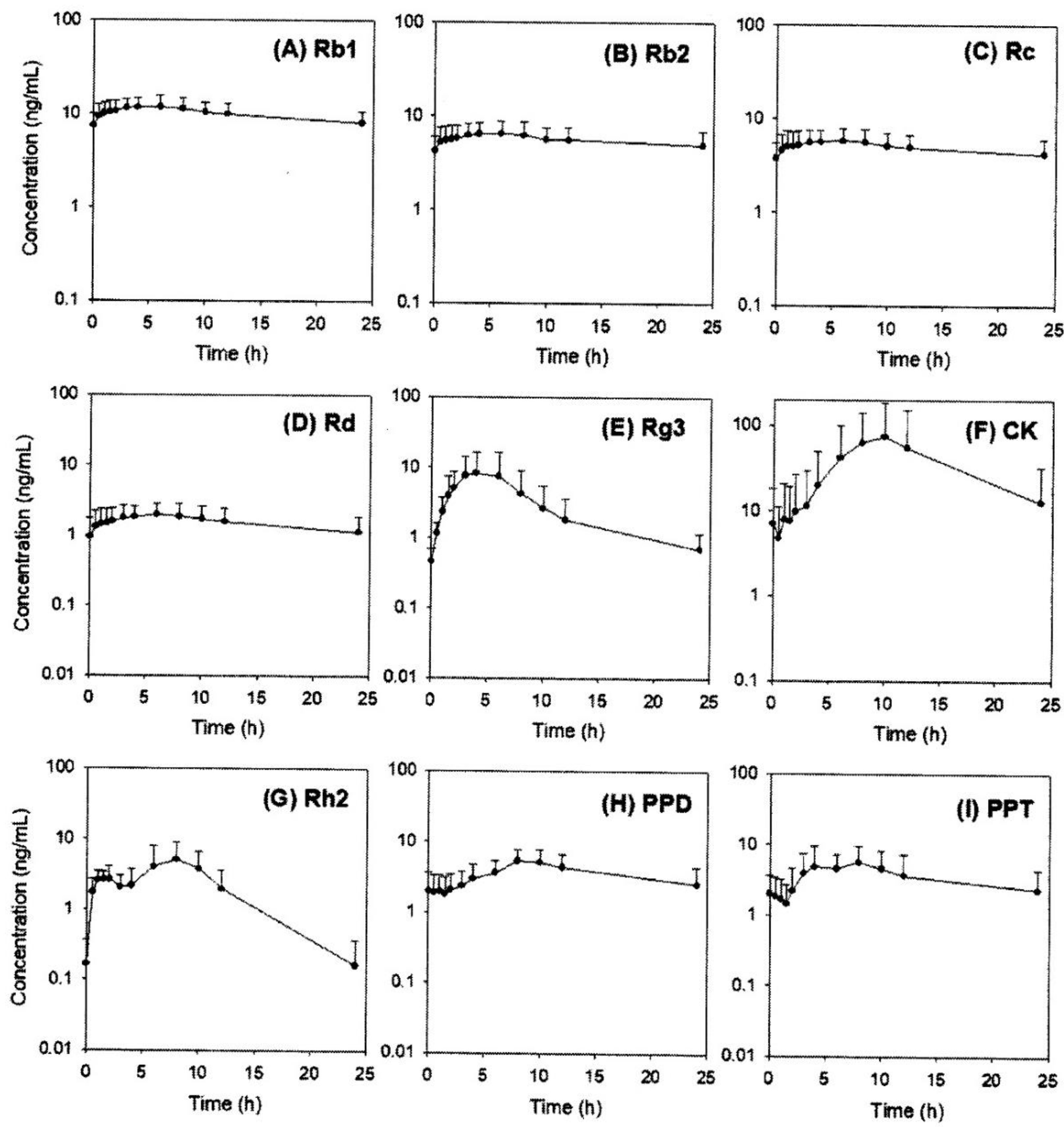
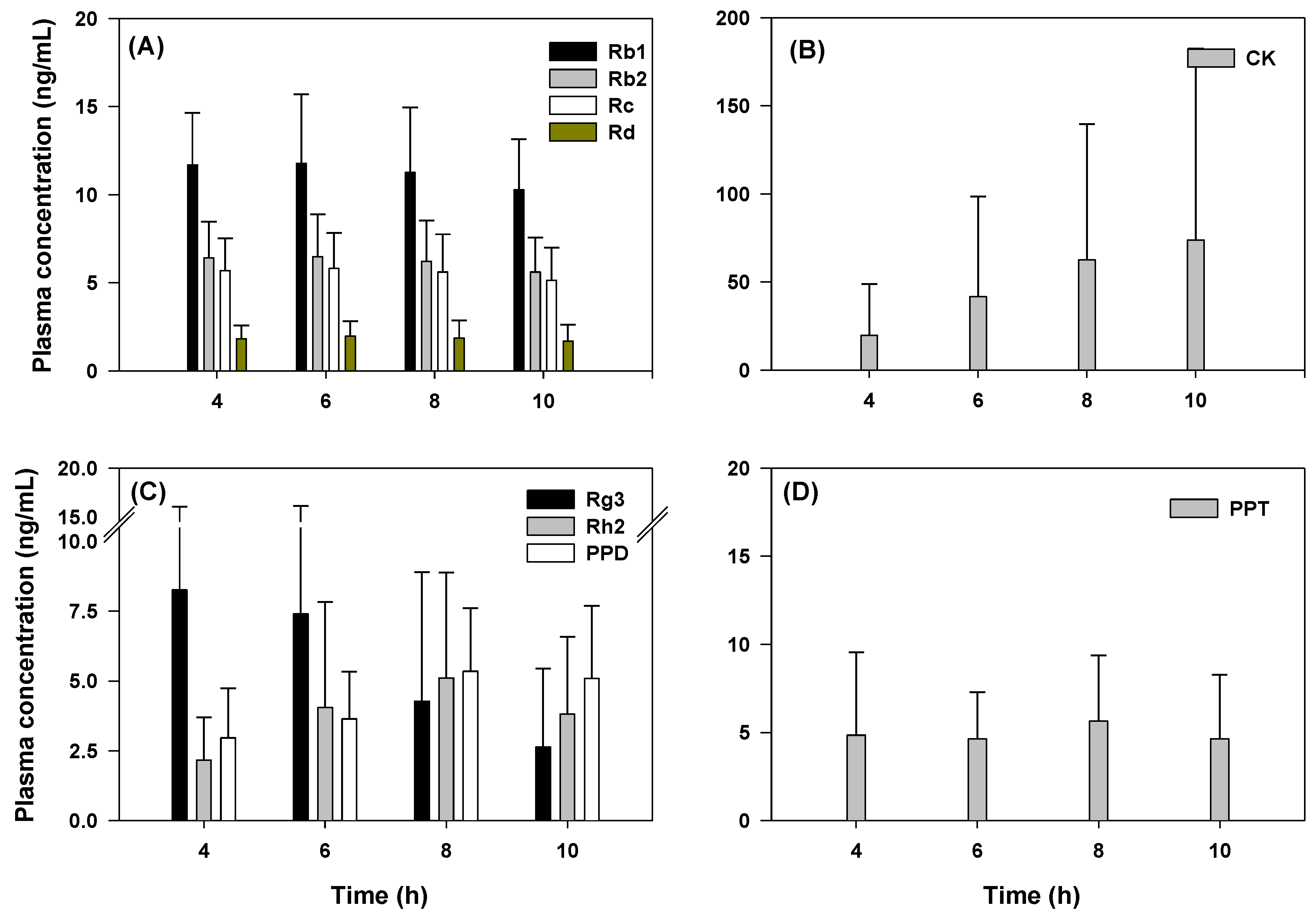
2.5. Pharmacokinetics of Rb1, Rb2, Rc, Rd, Rg3, Rh2, CK, PPD, and PPT Following 2 Weeks-Repeated Administration of Red Ginseng Extract
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. LC-MS/MS Analysis
4.2.1. Instrument
4.2.2. HPLC Condition
4.2.3. Preparation of Stock, Working, and Quality Control (QC) Solutions
4.2.4. Preparation of Calibration Curve and QC Samples
4.3. Method Validation
4.3.1. Specificity
4.3.2. Linearity
4.3.3. Precision and Accuracy
4.3.4. Extraction Recovery and Matrix Effect
4.3.5. Stability
4.4. Pharmacokinetic Study
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Won, H.J.; Kim, H.I.; Park, T.; Kim, H.; Jo, K.; Jeon, H.; Ha, S.J.; Hyun, J.M.; Jung, A.; Kim, J.S.; et al. Non-clinical pharmacokinetic behavior of ginsenosides. J. Ginseng Res. 2018, 43, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Ru, W.; Wang, D.; Xu, Y.; He, X.; Sun, Y.E.; Qian, L.; Zhou, X.; Qin, Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.). Drug Dis. Ther. 2015, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.K.; Choi, S.Y.; Yun, H.Y. Epidemiological study on cancer prevention by ginseng: Are all kinds of cancers preventable by ginseng? J. Korean Med. Sci. 2001, 16, S19–S27. [Google Scholar] [CrossRef]
- Gui, Q.F.; Xu, Z.R.; Xu, K.Y.; Yang, Y.M. The efficacy of ginseng-related therapies in type 2 Diabetes mellitus: An updated systematic review and meta-analysis. Medicine (Baltimore) 2016, 95, e2584. [Google Scholar] [CrossRef]
- Park, T.Y.; Hong, M.; Sung, H.; Kim, S.; Suk, K.T. Effect of Korean Red Ginseng in chronic liver disease. J. Ginseng Res. 2017, 41, 450–455. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, M.; Choi, M.K.; Song, I.S. Effects of red ginseng extract on the pharmacokinetics and elimination of methotrexate via Mrp2 regulation. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Seong, S.J.; Kang, W.Y.; Heo, J.K.; Jo, J.; Choi, W.G.; Liu, K.H.; Lee, S.; Choi, M.K.; Han, Y.H.; Lee, H.S.; et al. A comprehensive in vivo and in vitro assessment of the drug interaction potential of red ginseng. Clin. Ther. 2018, 40, 1322–1337. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, B.S.; Park, H.W.; Ahn, N.G.; Cho, B.G.; Cho, Y.L.; Kwak, Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef]
- Jin, S.; Lee, S.; Jeon, J.H.; Kim, H.; Choi, M.K.; Song, I.S. Enhanced Intestinal permeability and plasma concentration of metformin in rats by the repeated administration of Red Ginseng extract. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, J.; Hu, X.; Li, K.; Sun, C. Simultaneous determination of ginsenoside (G-Re, G-Rg1, G-Rg2, G-F1, G-Rh1) and protopanaxatriol in human plasma and urine by LC-MS/MS and its application in a pharmacokinetics study of G-Re in volunteers. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Kim, K.E.; Du, G.J.; Qi, L.W.; Wen, X.D.; Li, P.; Bauer, B.A.; Bissonnette, M.B.; Musch, M.W.; Chang, E.B.; et al. Ultra-performance liquid chromatography and time-of-flight mass spectrometry analysis of ginsenoside metabolites in human plasma. Am. J. Chin. Med. 2011, 39, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.Y.; Lee, H.W.; Kim, H.K.; Kim, H.H.; Chang, S.G.; Sohn, D.H.; Kim, J.; Lee, H.S. Simultaneous determination of ginsenoside Rb1 and Rg1 in human plasma by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2004, 35, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.D.; Ryu, J.H.; Lee, D.E.; Lee, M.H.; Shim, J.J.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; Shim, W.S.; Yim, S.V.; et al. Enhanced absorption study of ginsenoside compound K (20-O-beta-(d-Glucopyranosyl)-20(S)-protopanaxadiol) after oral administration of fermented red ginseng extract (HYFRG) in healthy Korean volunteers and rats. Evid. Based Complement. Alternat. Med. 2016, 2016, 3908142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Han, J.; Yu, W.; Deng, L.; Fawcett, J.P.; Liu, Z.; Gu, J. Rapid and sensitive LC-MS/MS assay for the quantitation of 20(S)-protopanaxadiol in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Jin, S.J.; Jeon, J.H.; Kang, W.Y.; Seong, S.J.; Yoon, Y.R.; Han, Y.H.; Song, I.S. Tolerability and pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administration of red ginseng extract in human beings. J. Ginseng Res. 2018. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Du, F.; Gao, X.; Ma, X.; Huang, Y.; Xu, F.; Niu, W.; Wang, F.; Mao, Y.; et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab. Dispos. 2009, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Yu, Y.; Long, J.; Li, Y.; Jiang, J.; Wang, L.; Xu, C.; Duan, G. Tentative identification of 20(S)-protopanaxadiol metabolites in human plasma and urine using ultra-performance liquid chromatography coupled with triple quadrupole time-of-flight mass spectrometry. J. Ginseng Res. 2018. [Google Scholar] [CrossRef]
- Kadian, N.; Raju, K.S.; Rashid, M.; Malik, M.Y.; Taneja, I.; Wahajuddin, M. Comparative assessment of bioanalytical method validation guidelines for pharmaceutical industry. J. Pharm. Biomed. Anal. 2016, 126, 83–97. [Google Scholar] [CrossRef]
- FDA. FDA guidance for industry: Bioanalytical method validation. 2018. Available online: https://www.gmp-compliance.org/guidelines/gmp-guideline/fda-guidance-for-industry-bioanalytical-method-validation (accessed on 17 July 2019).
- EMA. EMA Guideline on bioanalytical method validation. 2011. Available online: https://www.therqa.com/forum/good-laboratory-practice-discussion-forum/thread/3049/ (accessed on 17 July 2019).
- Park, S.E.; Na, C.S.; Yoo, S.A.; Seo, S.H.; Son, H.S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J. Ginseng Res. 2017, 41, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Yang, Y.Y.; Ouyang, D.S.; Yang, G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng. Res. 2018, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the ginsenoside Rc and Rg3 are available from the authors. |
| Sample Preparation Method | Compound | Precursor Ion (m/z) | Product Ion (m/z) | Retention Time (min) | Fragmentor Voltage (V) a | Collision Energy (V) |
|---|---|---|---|---|---|---|
| Protein precipitation | Rb1 | 1131.6 | 365.1 | 4.6 | 165 | 65 |
| Rb2 | 1101.6 | 335.1 | 5.7 | 185 | 60 | |
| Rc | 1101.6 | 335.1 | 4.8 | 185 | 60 | |
| Rd | 969.9 | 789.5 | 6.8 | 170 | 50 | |
| Re | 969.9 | 789.5 | 2.1 | 170 | 50 | |
| Rf | 823.5 | 365.1 | 3.3 | 135 | 55 | |
| Rg1 | 824.0 | 643.6 | 2.2 | 135 | 40 | |
| Rg3 | 807.5 | 365.2 | 9.3 | 165 | 60 | |
| F1 | 661.5 | 203.1 | 4.6 | 185 | 40 | |
| F2 | 807.5 | 627.5 | 9.4 | 135 | 40 | |
| Berberine (IS) | 336.1 | 320.0 | 4.5 | 135 | 30 | |
| LLE | Rh1 | 603.4 | 423.4 | 2.9 | 135 | 10 |
| Rh2 | 587.4 | 407.4 | 4.5 | 135 | 15 | |
| CK | 645.5 | 203.1 | 6.4 | 160 | 35 | |
| PPD | 425.3 | 109.1 | 11.0 | 125 | 25 | |
| PPT | 441.3 | 109.1 | 4.0 | 130 | 30 | |
| 13C-caffeine (IS) | 198 | 140 | 2.9 | 120 | 20 |
| Analyte | Linear Range (ng/mL) | Slope ± SD a | Intercept ± SD a | Correlation Coefficient a |
|---|---|---|---|---|
| Rb1 | 0.5–200 | 0.0485 ± 0.0205 | 0.0007 ± 0.0019 | 0.997 |
| Rb2 | 0.5–200 | 0.1069 ± 0.0394 | −0.0003 ± 0.0041 | 0.997 |
| Rc | 0.5–200 | 0.1408 ± 0.0393 | 0.0003 ± 0.0047 | 0.997 |
| Rd | 0.5–200 | 0.2597 ± 0.0536 | −0.0388 ± 0.0903 | 0.996 |
| Re | 0.5–200 | 0.2509 ± 0.0290 | −0.0048 ± 0.0095 | 0.997 |
| Rf | 0.5–200 | 0.1980 ± 0.0308 | −0.0056 ± 0.0095 | 0.995 |
| Rg1 | 0.5–200 | 0.0648 ± 0.0081 | −0.0010 ± 0.0071 | 0.994 |
| Rg3 | 0.5–200 | 0.0687 ± 0.0092 | 0.0008 ± 0.0069 | 0.987 |
| F1 | 0.5–200 | 0.8728 ± 0.2221 | −0.0337 ± 0.0437 | 0.995 |
| Rh2 | 0.5–200 | 0.0146 ± 0.0035 | −0.0006 ± 0.0009 | 0.996 |
| CK | 0.5–200 | 0.0860 ± 0.0442 | −0.0071 ± 0.0076 | 0.990 |
| PPD | 0.5–200 | 0.0476 ± 0.0120 | 0.0004 ± 0.0024 | 0.995 |
| PPT | 0.5–200 | 0.0221 ± 0.0022 | −0.0019 ± 0.0037 | 0.996 |
| Analyte | QC (ng/mL) | Inter-day (n = 5) | Intra-day (n = 6) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Measured (ng/mL) | SD | CV (%) | RE (%) | Measured (ng/mL) | SD | CV (%) | RE (%) | ||
| Rb1 | 1.5 | 1.5 | 0.1 | 6.1 | 0.9 | 1.5 | 0.1 | 7.7 | 1.9 |
| 15 | 15.1 | 0.7 | 4.5 | 0.5 | 15.0 | 0.3 | 1.9 | 0.2 | |
| 150 | 150.3 | 8.8 | 5.9 | 0.2 | 154.8 | 5.8 | 3.7 | 3.2 | |
| Rb2 | 1.5 | 1.5 | 0.1 | 4.7 | 2.5 | 1.5 | 0.1 | 5.2 | 1.4 |
| 15 | 15.0 | 0.6 | 4.2 | −0.1 | 15.2 | 0.3 | 2.2 | 1.4 | |
| 150 | 154.5 | 10.3 | 6.6 | 3.0 | 162.6 | 6.6 | 4.1 | 8.4 | |
| Rc | 1.5 | 1.5 | 0.1 | 4.4 | −0.8 | 1.5 | 0.1 | 5.0 | 0.3 |
| 15 | 14.9 | 0.8 | 5.0 | −0.4 | 14.8 | 0.5 | 3.2 | −1.3 | |
| 150 | 154.1 | 11.9 | 7.7 | 2.7 | 160.2 | 7.6 | 4.7 | 6.8 | |
| Rd | 1.5 | 1.5 | 0.1 | 6.4 | 2.0 | 1.6 | 0.1 | 5.8 | 6.3 |
| 15 | 14.9 | 0.7 | 5.0 | −0.8 | 15.2 | 0.3 | 2.1 | 1.2 | |
| 150 | 155.8 | 10.7 | 6.9 | 3.9 | 164.4 | 6.4 | 3.9 | 9.6 | |
| Re | 1.5 | 1.5 | 0.1 | 3.7 | −0.7 | 1.6 | 0.1 | 3.9 | 6.1 |
| 15 | 15.1 | 0.6 | 4.2 | 0.5 | 15.4 | 0.5 | 3.1 | 2.8 | |
| 150 | 150.1 | 9.8 | 6.5 | 0.1 | 158.6 | 6.0 | 3.8 | 5.7 | |
| Rf | 1.5 | 1.5 | 0.1 | 6.0 | 0.4 | 1.6 | 0.1 | 5.2 | 4.4 |
| 15 | 15.0 | 0.6 | 3.7 | −0.3 | 15.3 | 0.9 | 5.6 | 2.2 | |
| 150 | 156.7 | 11.4 | 7.3 | 4.5 | 166.3 | 7.9 | 4.8 | 10.8 | |
| Rg1 | 1.5 | 1.6 | 0.1 | 6.1 | 3.9 | 1.6 | 0.1 | 5.9 | 6.2 |
| 15 | 15.2 | 0.9 | 5.8 | 1.5 | 15.8 | 0.5 | 2.9 | 5.1 | |
| 150 | 151.1 | 7.7 | 5.1 | 0.7 | 155.5 | 5.6 | 3.6 | 3.7 | |
| Rg3 | 1.5 | 1.4 | 0.2 | 10.3 | −5.9 | 1.6 | 0.1 | 5.9 | 8.5 |
| 15 | 15.8 | 1.6 | 10.2 | 5.2 | 15.9 | 1.2 | 7.5 | 5.9 | |
| 150 | 159.2 | 16.0 | 10.1 | 6.1 | 166.4 | 11.7 | 7.0 | 11.0 | |
| F1 | 1.5 | 1.5 | 0.1 | 6.5 | −3.3 | 1.5 | 0.1 | 3.7 | −0.3 |
| 15 | 14.9 | 0.8 | 5.5 | −1.0 | 14.9 | 0.3 | 2.2 | −0.7 | |
| 150 | 154.8 | 10.9 | 7.1 | 3.2 | 160.8 | 7.4 | 4.6 | 7.2 | |
| Rh2 | 1.5 | 1.4 | 0.1 | 8.2 | −4.9 | 1.3 | 0.1 | 9.5 | −11.8 |
| 15 | 15.2 | 0.4 | 2.6 | 1.5 | 13.6 | 1.2 | 8.6 | −9.2 | |
| 150 | 151.5 | 7.5 | 5.0 | 1.0 | 151.2 | 16.9 | 11.2 | 0.8 | |
| CK | 1.5 | 1.4 | 0.2 | 10.6 | −4.9 | 1.5 | 0.1 | 6.5 | −3.4 |
| 15 | 14.5 | 1.9 | 13.0 | −3.5 | 12.8 | 1.4 | 10.8 | −15.0 | |
| 150 | 163.2 | 12.0 | 7.3 | 8.8 | 141.1 | 10.0 | 7.1 | −6.0 | |
| PPD | 1.5 | 1.5 | 0.2 | 11.7 | −1.1 | 1.5 | 0.1 | 5.1 | 3.0 |
| 15 | 14.9 | 0.5 | 3.5 | −0.5 | 15.0 | 0.5 | 3.2 | −0.1 | |
| 150 | 166.4 | 17.2 | 10.3 | 10.9 | 155.4 | 6.3 | 4.1 | 3.6 | |
| PPT | 1.5 | 1.5 | 0.1 | 5.0 | 2.4 | 1.5 | 0.1 | 4.2 | 1.4 |
| 15 | 14.8 | 0.4 | 2.8 | −1.5 | 15.7 | 0.4 | 2.4 | 4.6 | |
| 150 | 153.8 | 2.0 | 1.3 | 2.5 | 156.8 | 2.3 | 1.5 | 4.5 | |
| Analyte | QC (ng/mL) | Recovery (%) | Matrix Effect (%) | Analyte | QC (ng/mL) | Recovery (%) | Matrix Effect (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery | CV | Matrix Effect | CV | Recovery | CV | Matrix Effect | CV | ||||
| Rb1 | 1.5 | 93.5 | 8.5 | 74.5 | 13.6 | Rg3 | 1.5 | 91.9 | 14.9 | 92.2 | 12.2 |
| 15 | 85.5 | 8.1 | 78.3 | 7.0 | 15 | 90.3 | 6.4 | 70.3 | 6.8 | ||
| 150 | 89.3 | 4.0 | 75.8 | 5.8 | 150 | 94.2 | 10.2 | 67.9 | 4.2 | ||
| Rb2 | 1.5 | 96.0 | 6.5 | 79.4 | 8.5 | F1 | 1.5 | 96.9 | 7.2 | 56.6 | 4.0 |
| 15 | 86.0 | 5.9 | 82.1 | 6.1 | 15 | 90.1 | 6.5 | 57.9 | 3.2 | ||
| 150 | 89.3 | 2.9 | 78.5 | 6.3 | 150 | 93.1 | 3.3 | 57.1 | 3.2 | ||
| Rc | 1.5 | 93.8 | 6.7 | 69.8 | 12.5 | Rh2 | 1.5 | 64.9 | 11.8 | 99.7 | 3.2 |
| 15 | 87.7 | 6.4 | 70.4 | 8.9 | 15 | 64.9 | 3.7 | 95.6 | 2.4 | ||
| 150 | 91.3 | 4.3 | 67.8 | 8.4 | 150 | 65.4 | 3.6 | 98.5 | 2.6 | ||
| Rd | 1.5 | 96.4 | 7.3 | 73.9 | 8.0 | CK | 1.5 | 60.0 | 14.9 | 88.6 | 7.3 |
| 15 | 88.7 | 5.2 | 75.5 | 8.4 | 15 | 64.0 | 14.4 | 93.1 | 6.6 | ||
| 150 | 90.8 | 3.8 | 72.4 | 6.8 | 150 | 56.3 | 12.4 | 77.0 | 5.1 | ||
| Re | 1.5 | 99.2 | 13.1 | 9.5 | 7.4 | PPD | 1.5 | 79.9 | 5.0 | 98.4 | 14.9 |
| 15 | 93.5 | 5.8 | 7.0 | 5.5 | 15 | 70.7 | 5.4 | 96.7 | 4.1 | ||
| 150 | 95.5 | 3.1 | 7.9 | 6.6 | 150 | 71.6 | 5.2 | 100.1 | 5.7 | ||
| Rf | 1.5 | 93.0 | 4.2 | 19.5 | 13.6 | PPT | 1.5 | 81.7 | 7.2 | 77.8 | 11.8 |
| 15 | 88.1 | 6.7 | 16.4 | 9.9 | 15 | 77.5 | 4.3 | 77.3 | 5.3 | ||
| 150 | 94.0 | 7.1 | 18.2 | 10.9 | 150 | 81.9 | 6.8 | 76.6 | 5.4 | ||
| Rg1 | 1.5 | 97.5 | 13.4 | 9.9 | 5.8 | ||||||
| 15 | 97.6 | 8.5 | 7.2 | 3.4 | |||||||
| 150 | 96.2 | 5.5 | 7.7 | 4.2 | |||||||
| Analyte | Short-Term Stability (6 h, 25 °C) | Analyte | Short-Term Stability (6 h, 25 °C) | ||||||
| QC (ng/mL) | Measured (ng/mL) | CV (%) | RE (%) | QC (ng/mL) | Measured (ng/mL) | CV (%) | RE (%) | ||
| Rb1 | 1.5 | 1.6 | 5.3 | 3.9 | Rg3 | 1.5 | 1.4 | 13.5 | -7.9 |
| 15 | 13.5 | 3.3 | −9.9 | 15 | 13.7 | 4.7 | −8.7 | ||
| 150 | 137.0 | 6.8 | −8.7 | 150 | 133.7 | 4.2 | −10.8 | ||
| Rb2 | 1.5 | 1.5 | 1.6 | −2.9 | F1 | 1.5 | 1.5 | 2.3 | −2.9 |
| 15 | 13.3 | 4.7 | −11.5 | 15 | 13.3 | 4.5 | −11.1 | ||
| 150 | 137.5 | 8.7 | −8.3 | 150 | 138.9 | 6.4 | −7.4 | ||
| Rc | 1.5 | 1.4 | 2.8 | −4.3 | Rh2 | 1.5 | 1.5 | 5.1 | 0.2 |
| 15 | 13.4 | 3.4 | −10.6 | 15 | 14.9 | 2.9 | −0.8 | ||
| 150 | 138.5 | 8.2 | −7.6 | 150 | 149.7 | 4.3 | −0.2 | ||
| Rd | 1.5 | 1.5 | 4.0 | −3.5 | CK | 1.5 | 1.4 | 4.7 | −6.5 |
| 15 | 13.2 | 4.2 | −12.2 | 15 | 13.4 | 1.5 | −10.6 | ||
| 150 | 139.7 | 8.0 | −6.9 | 150 | 143.7 | 7.9 | −4.2 | ||
| Re | 1.5 | 1.5 | 1.8 | −1.3 | PPD | 1.5 | 1.6 | 5.0 | 5.4 |
| 15 | 14.1 | 2.0 | −5.8 | 15 | 15.3 | 2.2 | 2.0 | ||
| 150 | 143.4 | 3.7 | −4.4 | 150 | 146.2 | 5.6 | −2.5 | ||
| Rf | 1.5 | 1.4 | 3.0 | −7.6 | PPT | 1.5 | 1.5 | 3.0 | 1.0 |
| 15 | 13.6 | 3.4 | −9.1 | 15 | 15.0 | 2.0 | −0.3 | ||
| 150 | 150.3 | 9.2 | 0.2 | 150 | 146.0 | 5.0 | −2.7 | ||
| Rg1 | 1.5 | 1.5 | 5.6 | 2.0 | |||||
| 15 | 14.0 | 3.1 | −6.9 | ||||||
| 150 | 144.9 | 4.7 | −3.4 | ||||||
| Analyte | Post-Preparative Stability (24 h, 8 °C) | Analyte | Post-Preparative Stability (24 h, 8 °C) | ||||||
| QC (ng/mL) | Measured (ng/mL) | CV (%) | RE (%) | QC (ng/mL) | Measured (ng/mL) | CV (%) | RE (%) | ||
| Rb1 | 1.5 | 1.5 | 14.9 | −0.3 | Rg3 | 1.5 | 1.4 | 9.7 | −8.7 |
| 15 | 13.9 | 5.3 | −7.5 | 15 | 14.3 | 5.3 | −4.5 | ||
| 150 | 133.3 | 2.9 | −11.1 | 150 | 131.2 | 3.1 | −12.5 | ||
| Rb2 | 1.5 | 1.4 | 10.1 | −9.3 | F1 | 1.5 | 1.3 | 4.9 | −12.4 |
| 15 | 13.6 | 7.7 | −9.5 | 15 | 13.5 | 6.7 | −10.1 | ||
| 150 | 135.3 | 3.4 | −9.8 | 150 | 134.9 | 1.8 | −10.1 | ||
| Rc | 1.5 | 1.4 | 9.7 | −5.5 | Rh2 | 1.5 | 1.5 | 5.2 | 2.0 |
| 15 | 13.6 | 6.1 | −9.7 | 15 | 15.6 | 2.5 | 3.7 | ||
| 150 | 135.4 | 2.8 | −9.8 | 150 | 164.8 | 3.1 | 9.9 | ||
| Rd | 1.5 | 1.3 | 5.4 | −11.3 | CK | 1.5 | 1.4 | 12.4 | −5.9 |
| 15 | 13.4 | 7.0 | −10.7 | 15 | 13.7 | 4.9 | −8.8 | ||
| 150 | 136.2 | 3.0 | −9.2 | 150 | 160.7 | 3.3 | 7.2 | ||
| Re | 1.5 | 1.5 | 5.1 | −2.6 | PPD | 1.5 | 1.6 | 2.0 | 5.0 |
| 15 | 14.2 | 4.8 | −5.4 | 15 | 15.5 | 3.0 | 3.1 | ||
| 150 | 141.7 | 0.6 | −5.5 | 150 | 156.9 | 1.8 | 4.6 | ||
| Rf | 1.5 | 1.7 | 1.9 | 14.6 | PPT | 1.5 | 1.7 | 4.5 | 10.8 |
| 15 | 14.8 | 2.7 | −1.2 | 15 | 15.7 | 2.7 | 4.5 | ||
| 150 | 154.2 | 7.3 | 2.8 | 150 | 158.2 | 2.7 | 5.5 | ||
| Rg1 | 1.5 | 1.4 | 2.5 | −4.0 | |||||
| 15 | 14.2 | 9.7 | −5.3 | ||||||
| 150 | 142.8 | 3.3 | −4.8 | ||||||
| Analyte | Freeze-Thaw Stability (3 Cycles) | Analyte | Freeze-Thaw Stability (3 Cycles) | ||||||
| QC (ng/mL) | Measured (ng/mL) | CV (%) | RE (%) | QC (ng/mL) | Measured (ng/mL) | CV (%) | RE (%) | ||
| Rb1 | 1.5 | 1.5 | 2.3 | −2.6 | Rg3 | 1.5 | 1.6 | 2.7 | 9.2 |
| 15 | 13.6 | 3.2 | −9.5 | 15 | 13.6 | 1.4 | −9.2 | ||
| 150 | 143.6 | 5.4 | −4.3 | 150 | 140.1 | 4.7 | −6.6 | ||
| Rb2 | 1.5 | 1.4 | 4.3 | −4.7 | F1 | 1.5 | 1.5 | 3.1 | −0.7 |
| 15 | 13.3 | 2.2 | −11.1 | 15 | 13.4 | 1.4 | −10.4 | ||
| 150 | 145.2 | 5.4 | −3.2 | 150 | 145.8 | 5.0 | −2.8 | ||
| Rc | 1.5 | 1.5 | 1.7 | −0.3 | Rh2 | 1.5 | 1.5 | 4.0 | 0.4 |
| 15 | 13.5 | 2.1 | −10.3 | 15 | 15.6 | 4.7 | 3.7 | ||
| 150 | 147.8 | 5.8 | −1.5 | 150 | 152.3 | 2.1 | 1.5 | ||
| Rd | 1.5 | 1.4 | 2.0 | −5.7 | CK | 1.5 | 1.5 | 7.8 | −3.3 |
| 15 | 13.1 | 2.3 | −12.9 | 15 | 13.1 | 14.7 | −12.4 | ||
| 150 | 144.7 | 5.4 | −3.6 | 150 | 167.3 | 4.7 | 11.5 | ||
| Re | 1.5 | 1.5 | 2.4 | 0.4 | PPD | 1.5 | 1.6 | 6.0 | 6.6 |
| 15 | 13.9 | 2.4 | −7.1 | 15 | 15.7 | 3.7 | 4.5 | ||
| 150 | 144.5 | 5.8 | −3.7 | 150 | 160.4 | 2.2 | 7.0 | ||
| Rf | 1.5 | 1.4 | 1.3 | −5.5 | PPT | 1.5 | 1.4 | 5.6 | −3.5 |
| 15 | 14.0 | 2.9 | −6.8 | 15 | 15.4 | 1.6 | 2.8 | ||
| 150 | 156.8 | 7.7 | 4.5 | 150 | 158.7 | 1.7 | 5.8 | ||
| Rg1 | 1.5 | 1.5 | 3.1 | −2.5 | |||||
| 15 | 14.1 | 4.6 | −6.3 | ||||||
| 150 | 147.3 | 3.1 | −1.8 | ||||||
| Ginsenoside | mg/day | Ginsenoside | mg/day | ||
|---|---|---|---|---|---|
| PPD-type | Rb1 | 21.9 ± 2.1 | PPT-type | Re | 6.6 ± 1.3 |
| Rb2 | 10.4 ± 1.2 | Rg1 | 5.2 ± 0.6 | ||
| Rc | 12.9 ± 1.5 | F1 | 0.0 ± 0.0 | ||
| Rd | 5.9 ± 0.7 | PPT | 0.0 ± 0.0 | ||
| Rh2 | 0.0 ± 0.0 | ||||
| Rg3 | 7.9 ± 2.3 | ||||
| CK | 0.0 ± 0.0 | ||||
| PPD | 0.0 ± 0.0 | ||||
| Ginsenosides | PK Parameters | ||||
|---|---|---|---|---|---|
| AUC (ng∙h/mL) | Cmax (ng/mL) | Tmax (h) | MRT (h) | T1/2 (h) | |
| Rb1 | 227.6 ± 73.5 | 12.7 ± 3.6 | 4.5 ± 1.8 | 10.7 ± 1.5 | 42.9 ± 20.8 |
| Rb2 | 137.0 ± 48.8 | 6.9 ± 2.3 | 4.5 ± 2.3 | 11.8 ± 1.5 | 51.2 ± 22.8 |
| Rc | 123.0 ± 46.1 | 6.2 ± 2.1 | 4.3 ± 3.2 | 11.7 ± 1.5 | 34.5 ± 12.9 |
| Rd | 35.1 ± 19.5 | 2.2 ± 0.9 | 6.2 ± 2.1 | 10.4 ± 1.5 | 24.6 ± 8.0 |
| Re | ND | ND | ND | ND | ND |
| Rf | ND | ND | ND | ND | ND |
| Rg1 | ND | ND | ND | ND | ND |
| Rg3 | 68.0 ± 60.5 | 8.7 ± 8.9 | 3.6 ± 0.9 | 8.2 ± 1.4 | 9.4 ± 3.9 |
| F1 | ND | ND | ND | ND | ND |
| Rh2 | 49.9 ± 27.8 | 6.1 ± 3.5 | 6.0 ± 3.3 | 7.7 ± 1.5 | 3.1 ± 1.3 |
| CK | 873.0 ± 1236.0 | 81.6 ± 112.5 | 9.5 ± 1.6 | 10.6 ± 1.2 | 5.2 ± 1.1 |
| PPD | 85.1 ± 39.5 | 6.1 ± 2.3 | 8.7 ± 1.6 | 11.3 ± 1.9 | 12.6 ± 8.2 |
| PPT | 86.5 ± 49.8 | 7.9 ± 4.6 | 8.3 ± 6.2 | 11.2 ± 3.0 | 10.6 ± 8.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Jeon, J.-H.; Lee, S.; Kang, W.Y.; Seong, S.J.; Yoon, Y.-R.; Choi, M.-K.; Song, I.-S. Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract. Molecules 2019, 24, 2618. https://doi.org/10.3390/molecules24142618
Jin S, Jeon J-H, Lee S, Kang WY, Seong SJ, Yoon Y-R, Choi M-K, Song I-S. Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract. Molecules. 2019; 24(14):2618. https://doi.org/10.3390/molecules24142618
Chicago/Turabian StyleJin, Sojeong, Ji-Hyeon Jeon, Sowon Lee, Woo Youl Kang, Sook Jin Seong, Young-Ran Yoon, Min-Koo Choi, and Im-Sook Song. 2019. "Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract" Molecules 24, no. 14: 2618. https://doi.org/10.3390/molecules24142618
APA StyleJin, S., Jeon, J.-H., Lee, S., Kang, W. Y., Seong, S. J., Yoon, Y.-R., Choi, M.-K., & Song, I.-S. (2019). Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract. Molecules, 24(14), 2618. https://doi.org/10.3390/molecules24142618