Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications
Abstract
1. Introduction
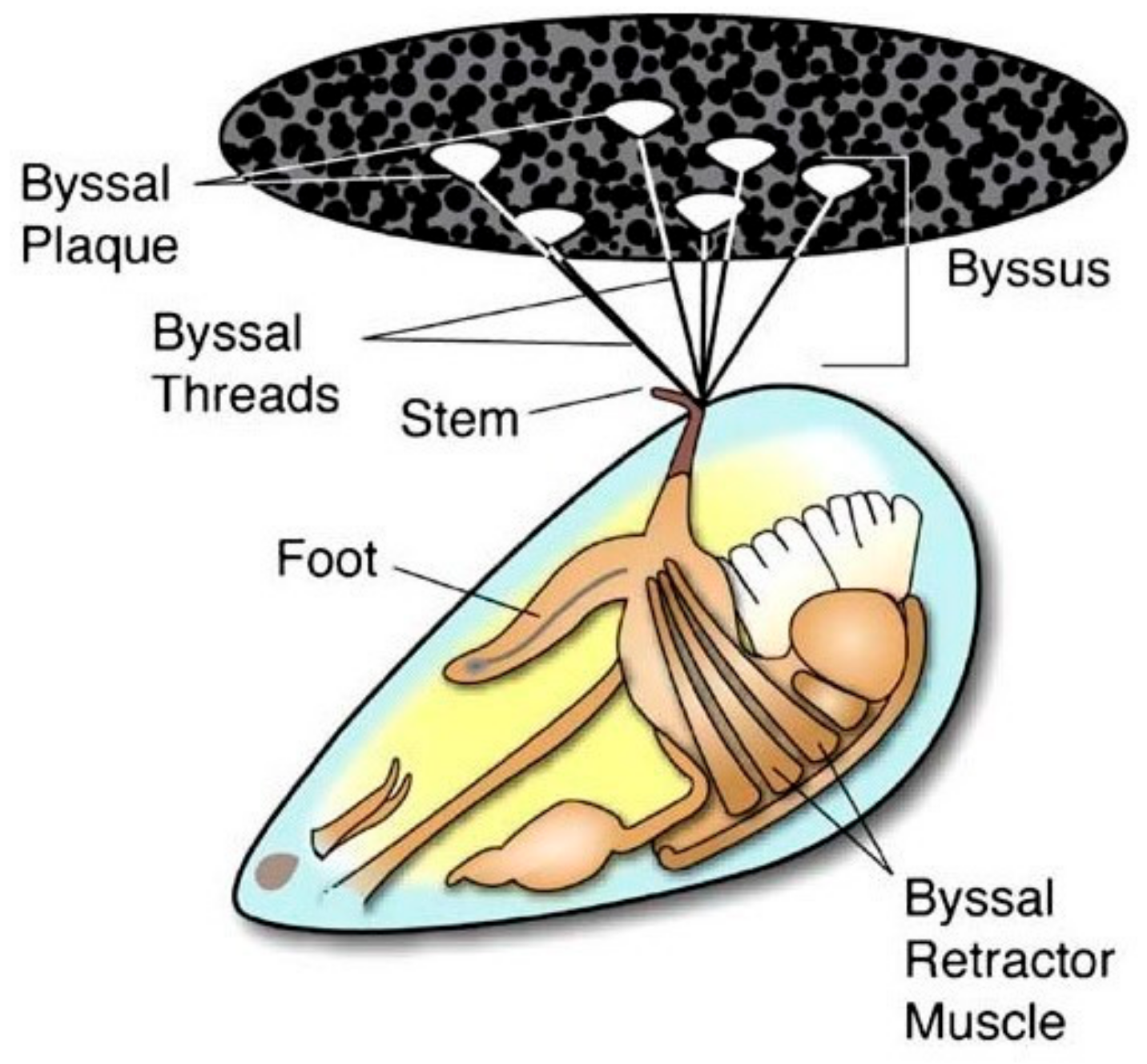
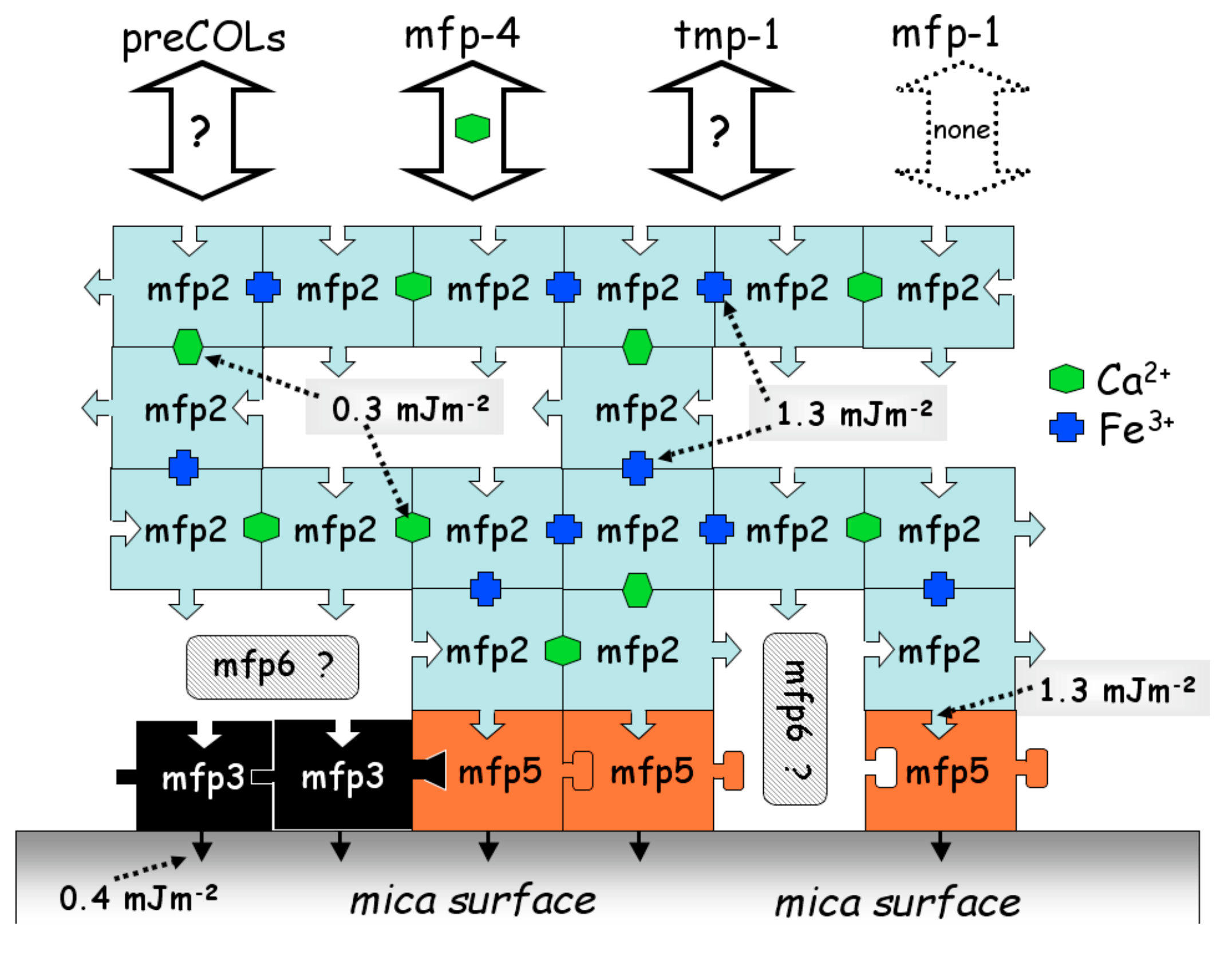
2. Mussel Adhesive Proteins
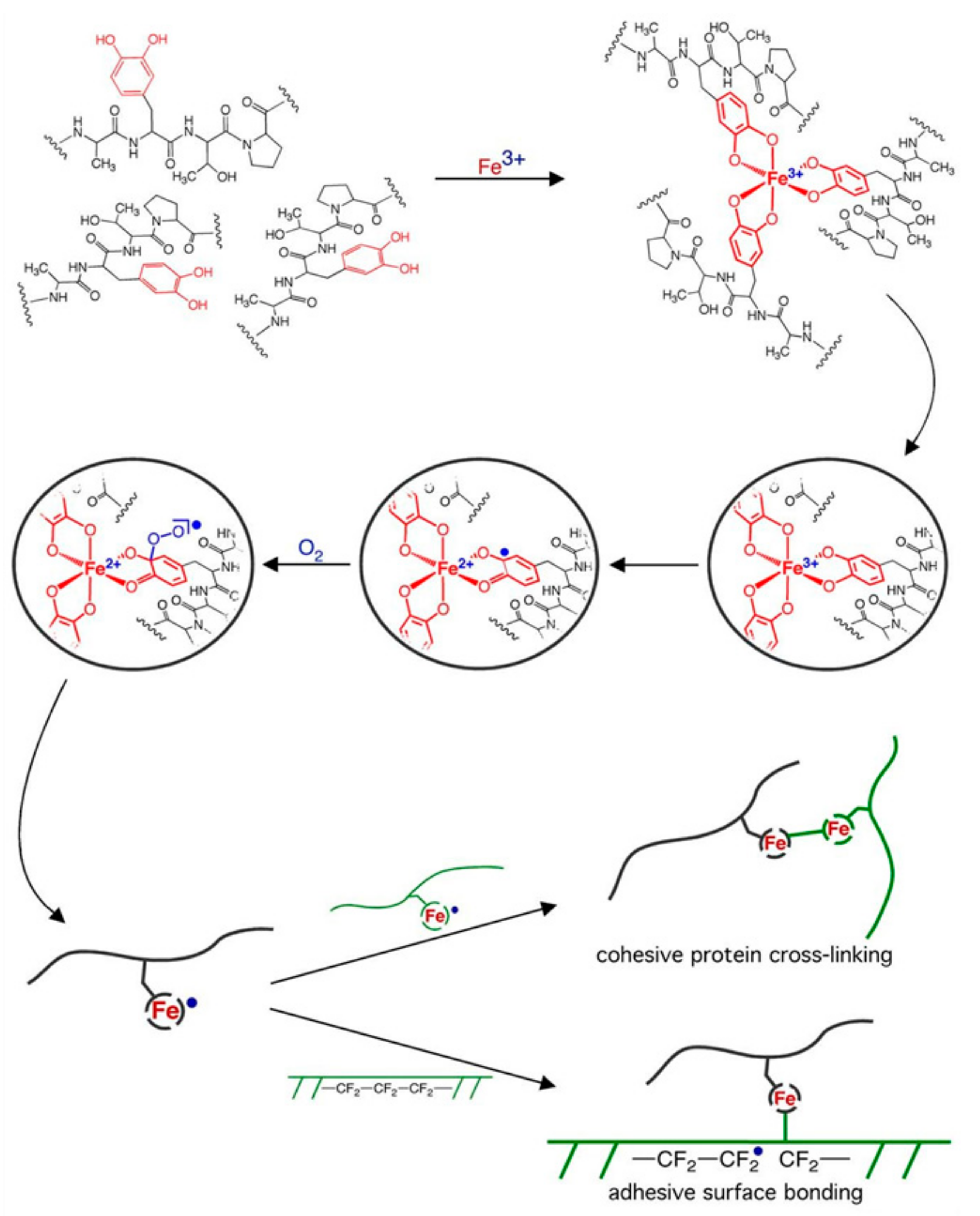
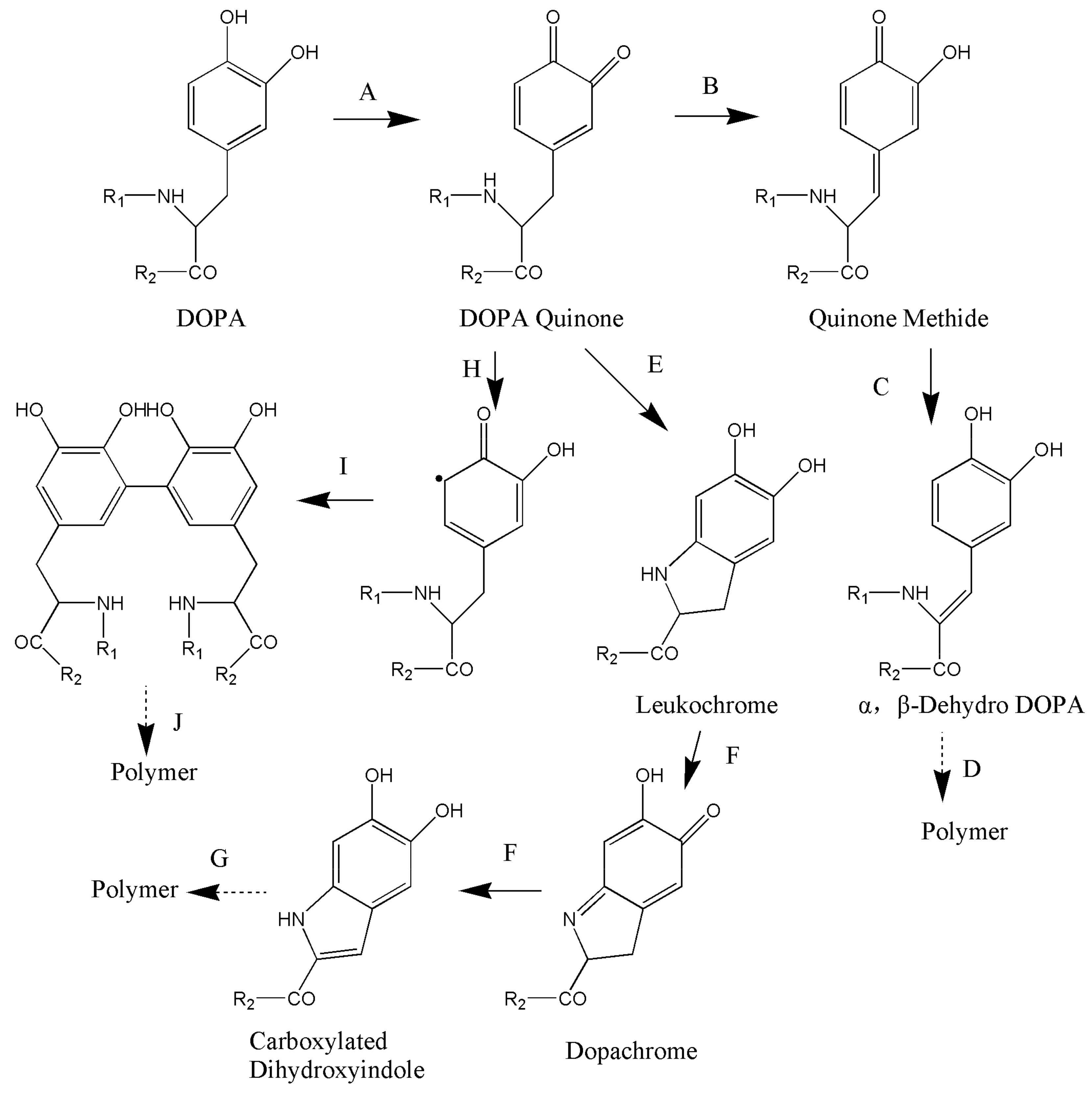
3. Formation Mechanisms of Catechol-Based Hydrogels
4. Types of Catechol-Based Hydrogels
4.1. Non-Functional Hydrogels
4.2. Nanocomposite Hydrogels
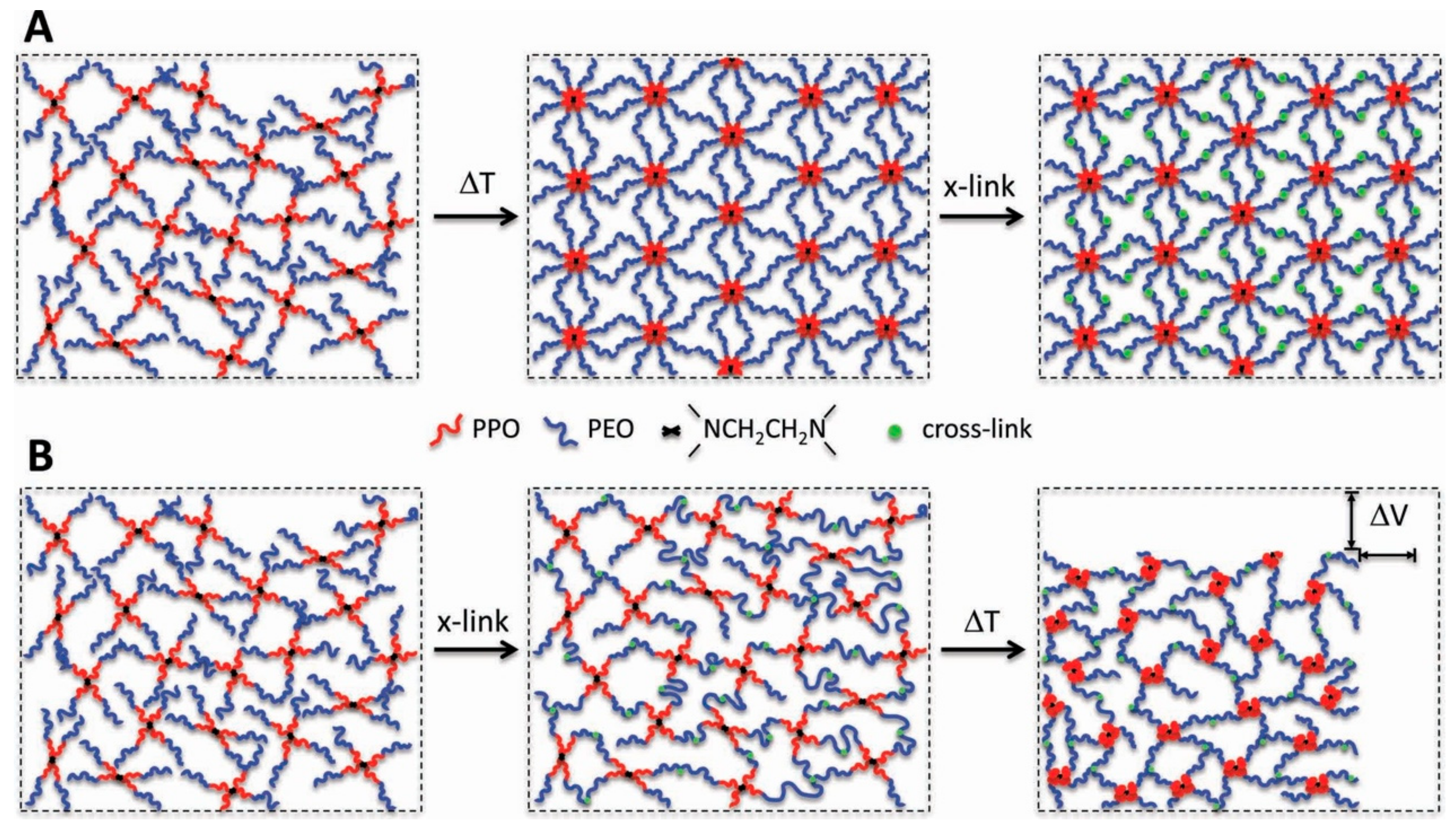
4.3. Thermosensitive Hydrogels
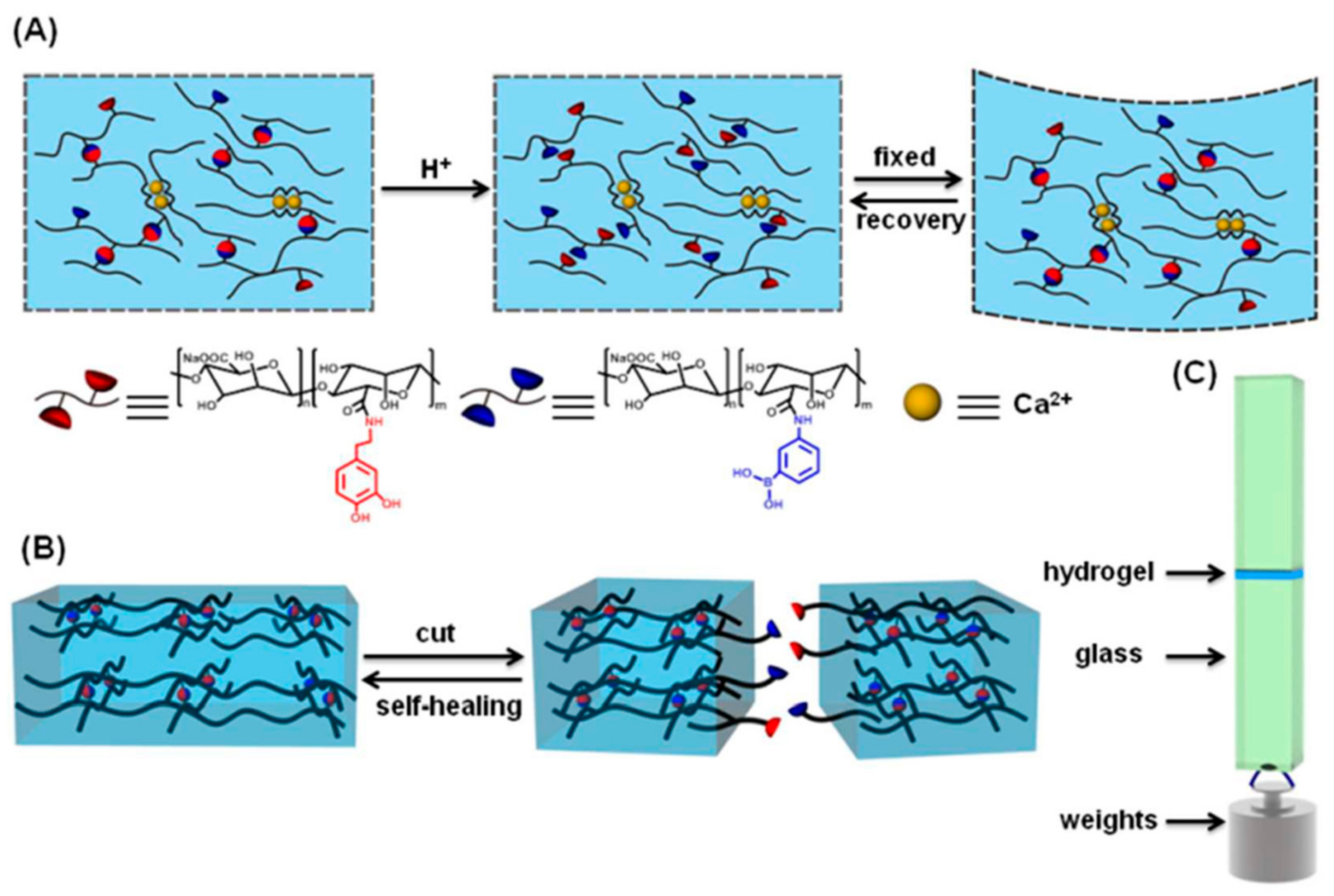
4.4. pH-Responsive Hydrogels
5. Interactions of Catechol-Functionalized Hydrogels on the Surface of Biological Tissues
5.1. Effect of Oxidation State on Hydrogel Adhesion
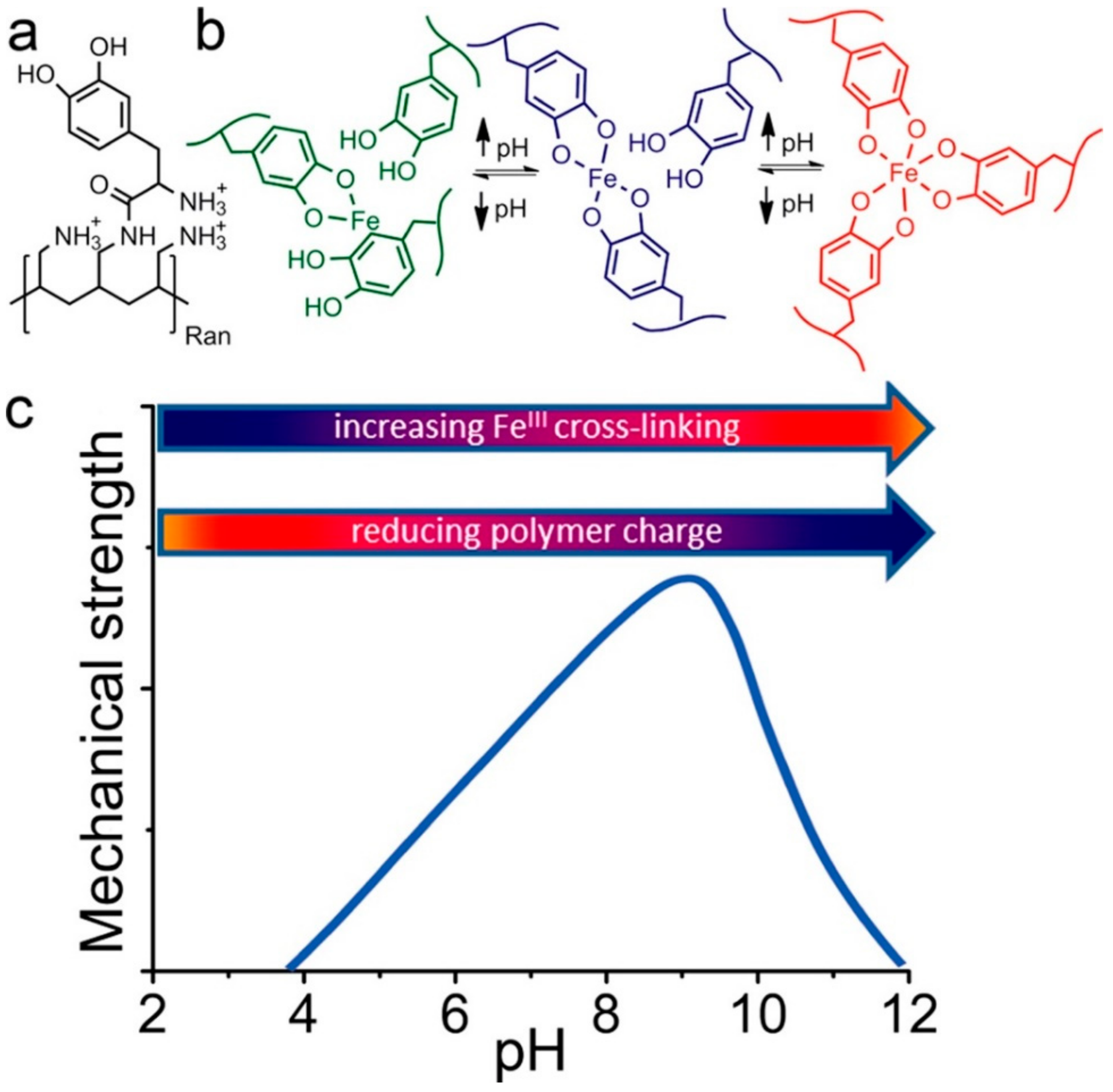
5.2. Effect of pH on Hydrogel Adhesion
5.3. Effect of Molecular Weight on Hydrogel Adhesive Properties
6. Medical Applications of Catechol-Functionalized Hydrogels
6.1. Tissue Adhesion, Hemostasis, and Healing
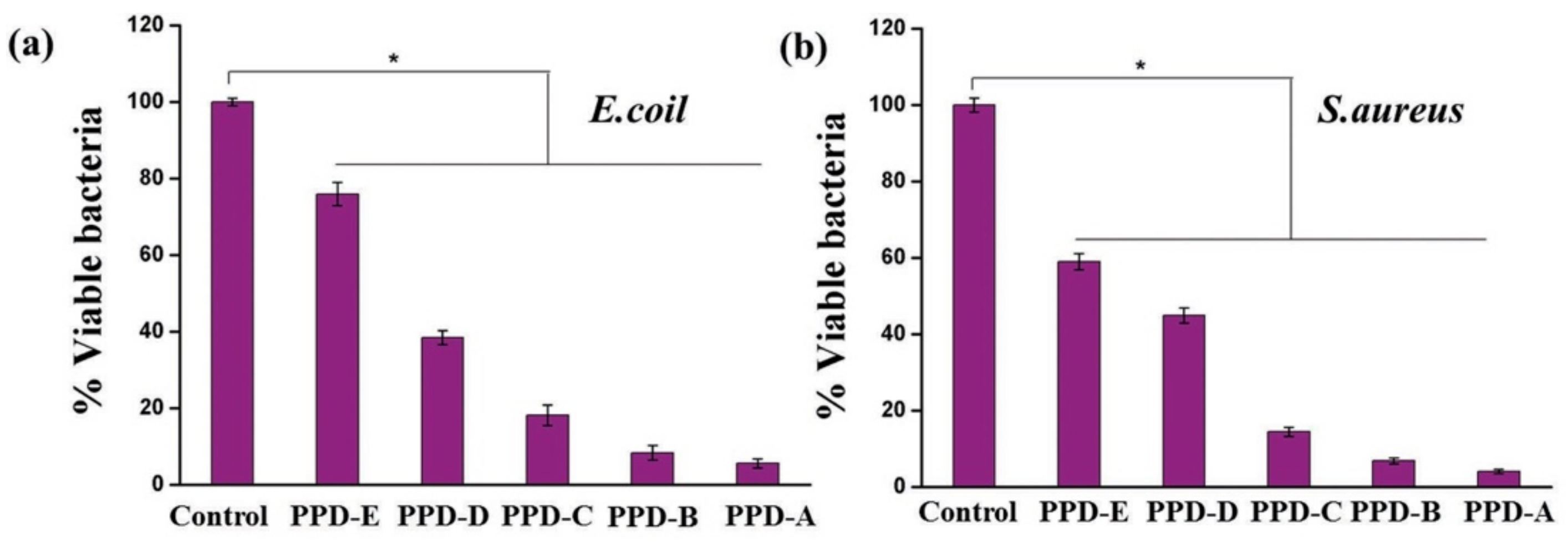
6.2. Antimicrobial and Anti-Infective Applications
6.3. Biomedical Coatings
6.3.1. Bone Regeneration and Orthopedic Implant Coatings
6.3.2. Heparin-Mimetic Coatings
6.3.3. Antimicrobial Coatings
6.4. Drug Delivery
6.5. Tissue Engineering Scaffolds
6.6. Islet Transplantation
7. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warner, S.C.; Waite, J.H. Expression of multiple forms of an adhesive plaque protein in an individual mussel, Mytilus edulis. Mar. Biol. 1999, 134, 729–734. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef]
- Zhao, H.; Waite, J.H. Linking adhesive and structural proteins in the attachment plaque of Mytilus californianus. J. Biol. Chem. 2006, 281, 26150–26158. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.D.; John, M.E.; Sjoestroem, S.; Fenster, A.; Waite, J.H. A cohort of new adhesive proteins identified from transcriptomic analysis of mussel foot glands. J. R. Soc. Interface 2017. [Google Scholar] [CrossRef]
- Yu, M.; Hwang, J.; Deming, T.J. Role of l-3,4-dihydroxyphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Danner, E.; Israelachvili, J.N.; Waite, J.H. Effects of interfacial redox in mussel adhesive protein films on mica. Adv. Mater. 2011, 23, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Mian, S.A.; Gao, X.; Nagase, S.; Jang, J. Adsorption of catechol on a wet silica surface: density functional theory study. Teor. Chem. Acc. 2011, 130, 333–339. [Google Scholar] [CrossRef]
- Danner, E.W.; Kan, Y.; Hammer, M.U.; Israelachvili, J.N.; Waite, J.H. Adhesion of mussel foot protein Mefp-5 to mica: An underwater superglue. Biochemistry 2012, 51, 6511–6518. [Google Scholar] [CrossRef]
- Taylor, S.W.; Chase, D.B.; Emptage, M.H.; Nelson, M.J.; Waite, J.H. Ferric ion complexes of a DOPA-containing adhesive protein from Mytilus edulis. Inorg. Chem. 1996, 35, 7572–7577. [Google Scholar] [CrossRef]
- Hight, L.M.; Wilker, J.J. Synergistic effects of metals and oxidants in the curing of marine mussel adhesive. J. Mater. Sci. 2007, 42, 8934–8942. [Google Scholar] [CrossRef]
- Kummert, R.; Stumm, W. The surface complexation of organic acids on hydrous γ-Al2O3. J. Colloid. Interface Sci. 1980, 75, 373–385. [Google Scholar] [CrossRef]
- Lee, B.P.; Chao, C.-Y.; Nunalee, F.N.; Motan, E.; Shull, K.R.; Messersmith, P.B. Rapid gel formation and adhesion in photocurable and biodegradable block copolymers with high DOPA content. Macromolecules 2006, 39, 1740–1748. [Google Scholar] [CrossRef]
- Schnurrer, J.; Lehr, C.-M. Mucoadhesive properties of the mussel adhesive protein. Int. J. Pharmaceut. 1996, 141, 251–256. [Google Scholar] [CrossRef]
- Guvendiren, M.; Brass, D.A.; Messersmith, P.B.; Shull, K.R. Adhesion of DOPA-functionalized model membranes to hard and soft surfaces. J. Adhesion. 2009, 85, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.L.; Meredith, H.J.; Wiker, J.J. Molecular weight effects upon the adhesive bonding of a mussel mimetic polymer. ACS Appl. Mater. Interfaces 2013, 5, 5091–5096. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliver. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Srivastava, A. Robust, self-healing hydrogels synthesised from catechol rich polymers. J. Mater. Chem. B 2015, 3, 899–910. [Google Scholar] [CrossRef]
- Lee, Y.; Chung, H.J.; Yeo, S.; Ahn, C.-H.; Lee, H.; Messersmith, P.B.; Park, T.G. Thermo-sensitive, injectable, and tissue adhesive sol–gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010, 6, 977–983. [Google Scholar] [CrossRef]
- Fullenkamp, D.E.; Rivera, J.G.; Gong, Y.-k.; Lau, K.H.A.; He, L.; Varshney, R.; Messersmith, P.B. Mussel-inspired silver-releasing antibacterial hydrogels. Biomaterials 2012, 33, 3783–3791. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.-J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.-H.; Chung, H.-M.; et al. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, M.; Su, Z.; Xie, Y.; Chen, M.; Zong, L.; Gao, Y.; Li, H.; Qi, J.; Zhao, Q.; et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials 2014, 35, 7157–7171. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-H.; Lu, C.-H.; Guo, X.-C.; Chen, F.-R.; Yang, H.-H.; Wang, X.-R. Mussel-inspired molecularly imprinted polymer coating superparamagnetic nanoparticles for protein recognition. J. Mater. Chem. 2010, 20, 880–883. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.; Tan, X.; Sun, L.; Yu, R.; Yang, H.; Chen, G. Preparation of boronate-functionalized molecularly imprinted monolithic column with polydopamine coating for glycoprotein recognition and enrichment. J. Chromatogr. A 2013, 1319, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, R.; Shen, F.; Tang, L.; Wang, J.; Huang, N. Construction of mussel-inspired coating via the direct reaction of catechol and polyethyleneimine for efficient heparin immobilization. Appl. Surf. Sci. 2015, 328, 163–169. [Google Scholar] [CrossRef]
- Watanabe, H.; Fujimoto, A.; Nishida, J.; Ohishi, T.; Takahara, A. Biobased polymer coating using catechol derivative urushiol. Langmuir 2016, 32, 4619–4623. [Google Scholar] [CrossRef]
- Zhong, J.; Ji, H.; Duan, J.; Tu, H.; Zhang, A. Coating morphology and surface composition of acrylic terpolymers with pendant catechol, OEG and perfluoroalkyl groups in varying ratio and the effect on protein adsorption. Colloid. Surf. B 2016, 140, 254–261. [Google Scholar] [CrossRef]
- Oh, Y.J.; Cho, I.H.; Lee, H.; Park, K.-J.; Lee, H.; Park, S.Y. Bio-inspired catechol chemistry: A new way to develop a re-moldable and injectable coacervate hydrogel. Chem. Commun. 2012, 48, 11895–11897. [Google Scholar] [CrossRef]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.-W.; Lee, H. Hyaluronic acid catechol: A biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Park, H.-J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.-W. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.-N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.-W. Three-dimensional electroconductive hyaluronic acid hydrogels incorporated with carbon nanotubes and polypyrrole by catechol-mediated dispersion enhance neurogenesis of human neural stem cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef] [PubMed]
- Moulay, S. Dopa/catechol- tethered polymers: Bioadhesives and biomimetic adhesive materials. Polym. Rev. 2014, 54, 436–513. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Pol. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.Y.; Newland, B.; Liu, W.; Wang, W.; Wang, W.X. Catechol functionalized hyperbranched polymers as biomedical materials. Prog. Polym. Sci. 2018, 78, 47–55. [Google Scholar] [CrossRef]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C.J. Mussel adhesion: Finding the tricks worth mimicking. J. Adhesion 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Nicklisch, S.C.T.; Waite, J.H. Mini-review: The role of redox in Dopa-mediated marine adhesion. Biofouling 2012, 28, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Zeng, H.; Wu, J.P. Marine mussel adhesion: biochemistry, mechanisms, and biomimetics. J. Adhes. Sci. Technol. 2013, 27, 2139–2162. [Google Scholar] [CrossRef]
- Ahn, B.K. Perspectives on mussel-inspired wet adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Waite, J.H.; Tanzer, M.L. Polyphenolic substance of Mytilus edulis: Novel adhesive containing l-dopa and hydroxyproline. Science 1981. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E. Seasonal variation in the attachment strength of blue mussels: Causes and consequences. Limnol. Oceanogr. 2002, 47, 1723–1733. [Google Scholar] [CrossRef]
- Yamamoto, H. Adhesive studies of synthetic polypeptides: A model for marine adhesive proteins. J. Adhes. Sci. Technol. 1987, 1, 177–183. [Google Scholar] [CrossRef]
- Waite, J.H. Adhesion à la moule. Integr. Comp. Biol. 2002, 42, 1172–1180. [Google Scholar] [CrossRef]
- Haemers, S.; Koper, G.J.M.; Frens, G. Effect of oxidation rate on cross-linking of mussel adhesive proteins. Biomacromolecules 2003, 4, 632–640. [Google Scholar] [CrossRef]
- Sever, M.J.; Weisser, J.T.; Monahan, J.; Srinivasan, S.; Wilker, J.J. Metal-Mediated Cross-Linking in the Generation of a Marine-Mussel Adhesive. Angew. Chem. Int. Ed. 2004, 43, 448–450. [Google Scholar] [CrossRef]
- Hwang, D.S.; Zeng, H.; Masic, A.; Harrington, M.J.; Israelachvili, J.N.; Waite, J.H. Protein- and metal-dependent interactions of a prominent protein in mussel adhesive plaques. J. Biol. Chem. 2010, 285, 25850–25858. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Hwang, D.S.; Israelachvili, J.N.; Waite, J.H. Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc. Natl. Acad. Sci. USA 2010. [Google Scholar] [CrossRef] [PubMed]
- Wilker, J.J. Marine bioinorganic materials: mussels pumping iron. Curr. Opin. Chem. Biol. 2010, 14, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H.; Lin, G. Solving the riddle of the intradiol and extradiol catechol dioxygenases: How do enzymes control hydroperoxide rearrangements? Chem. Commun. 2001. [Google Scholar] [CrossRef]
- Fullenkamp, D.E.; Barrett, D.G.; Miller, D.R.; Kurutz, J.W.; Messersmith, P.B. pH-dependent cross-linking of catechols through oxidation via Fe3+ and potential implications for mussel adhesion. RSC Adv. 2014, 4, 25127–25134. [Google Scholar] [CrossRef] [PubMed]
- Akemi Ooka, A.; Garrell, R.L. Surface-enhanced Raman spectroscopy of DOPA-containing peptides related to adhesive protein of marine mussel, Mytilus edulis. Biopolymers 2000, 57, 92–102. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Danner, E.; Ashley, R.K.; Israelachvili, J.N.; Waite, J.H. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 2011, 7, 588–590. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef]
- Waite, J.H. The phylogeny and chemical diversity of quinone-tanned glues and varnishes. Comp. Biochem. Physiol. Part. B Comp. Biochem. 1990, 97, 19–29. [Google Scholar] [CrossRef]
- Rzepecki, L.M.; Nagafuchi, T.; Waite, J.H. α, β-Dehydro-3,4-dihydroxyphenylalanine derivatives: Potential schlerotization intermediates in natural composite materials. Arch. Biochem. Biophys. 1991, 285, 17–26. [Google Scholar] [CrossRef]
- Baty, A.M.; Leavitt, P.K.; Siedlecki, C.A.; Tyler, B.J.; Suci, P.A.; Marchant, R.E.; Geesey, G.G. Adsorption of adhesive proteins from the marine mussel, Mytilus edulis, on polymer films in the hydrated state using Angle Dependent X-ray Photoelectron Spectroscopy and Atomic Force Microscopy. Langmuir 1997, 13, 5702–5710. [Google Scholar] [CrossRef]
- Waite, J.H. Nature’s underwater adhesive specialist. Int. J. Adhes. Adhes. 1987, 7, 9–14. [Google Scholar] [CrossRef]
- Sánchez-Cortés, S.; Francioso, O.; García-Ramos, J.V.; Ciavatta, C.; Gessa, C. Catechol polymerization in the presence of silver surface. Colloids Surf. A 2001, 176, 177–184. [Google Scholar] [CrossRef]
- Gallivan, J.P.; Dougherty, D.A. A Computational study of cation−π interactions vs salt bridges in aqueous media: Implications for protein engineering. J. Am. Chem. Soc. 2000, 122, 870–874. [Google Scholar] [CrossRef]
- Lu, Q.; Oh, D.X.; Lee, Y.; Jho, Y.; Hwang, D.S.; Zeng, H. Nanomechanics of cation–π interactions in aqueous solution. Angew. Chem. 2013, 125, 4036–4040. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. UK 2013, 1, 783–790. [Google Scholar] [CrossRef]
- Neto, A.I.; Cibrão, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured polymeric coatings based on chitosan and dopamine-modified hyaluronic acid for biomedical applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef]
- Huang, K.; Lee, B.P.; Ingram, D.R.; Messersmith, P.B. Synthesis and characterization of self-assembling block copolymers containing bioadhesive end groups. Biomacromolecules 2002, 3, 397–406. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.-A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, W.; Li, X.; Ren, J.; Yun, W.; Zhang, K.; Li, G.; Yin, J. Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties. J. Mater. Chem. B. 2018, 6, 6377–6390. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, H.; Konst, S.; Sarmiento, R.; Rajachar, R.; Lee, B.P. Injectable Dopamine-modified poly (ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity. ACS Appl. Mater. Interfaces 2014, 6, 16982–16992. [Google Scholar] [CrossRef] [PubMed]
- Skelton, S.; Bostwick, M.; O’Connor, K.; Konst, S.; Casey, S.; Lee, B.P. Biomimetic adhesive containing nanocomposite hydrogel with enhanced materials properties. Soft Matter. 2013, 9, 3825–3833. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, H.; Qian, Z.C.; Fan, N.; Choi, W.Y.; Zhao, F.; Lee, B.P. A moldable nanocomposite hydrogel composed of a mussel-inspired polymer and a nanosilicate as a fit-to-shape tissue sealant. Angew. Chem. Int. Ed. 2017, 56, 4224–4228. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, K.; Ullah, W.; Ji, Y.; Ma, J. Chitin nanocrystal enhanced wet adhesion performance of mussel-inspired citrate-based soft-tissue adhesive. Carbohyd. Polym. 2018, 190, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Gonzalez-Lopez, J.; Fernandez-Tarrio, M.; Sandez-Macho, I.; Concheiro, A. Tetronic micellization, gelation and drug solubilization: Influence of pH and ionic strength. Eur. J. Pharm. Biopharm. 2007, 66, 244–252. [Google Scholar] [CrossRef]
- Go, D.H.; Joung, Y.K.; Lee, S.Y.; Lee, M.C.; Park, K.D. Tetronic–oligolactide–heparin hydrogel as a multi-functional scaffold for tissue regeneration. Macromol. Biosci. 2008, 8, 1152–1160. [Google Scholar] [CrossRef]
- Garty, S.; Kimelman-Bleich, N.; Hayouka, Z.; Cohn, D.; Friedler, A.; Pelled, G.; Gazit, D. Peptide-modified “smart” hydrogels and genetically engineered stem cells for skeletal tissue engineering. Biomacromolecules 2010, 11, 1516–1526. [Google Scholar] [CrossRef]
- Barrett, D.G.; Bushnell, G.G.; Messersmith, P.B. Mechanically robust, negative-swelling, mussel-inspired tissue adhesives. Adv. Healthc. Mater. 2013, 2, 745–755. [Google Scholar] [CrossRef]
- Xu, J.; Ge, Z.; Zhu, Z.; Luo, S.; Liu, H.; Liu, S. Synthesis and micellization properties of double hydrophilic A2BA2 and A4BA4 non-linear block copolymers. Macromolecules 2006, 39, 8178–8185. [Google Scholar] [CrossRef]
- Cellesi, F.; Tirelli, N.; Hubbell, J.A. Materials for cell encapsulation via a new tandem approach combining reverse thermal gelation and covalent crosslinking. Macromol. Chem. Phys. 2002, 203, 1466–1472. [Google Scholar] [CrossRef]
- Cellesi, F.; Tirelli, N.; Hubbell, J.A. Towards a fully-synthetic substitute of alginate: development of a new process using thermal gelation and chemical cross-linking. Biomaterials 2004, 25, 5115–5124. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- He, L.; Fullenkamp, D.E.; Rivera, J.G.; Messersmith, P.B. pH responsive self-healing hydrogels formed by boronate–catechol complexation. Chem. Commun. 2011, 47, 7497–7499. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y. Boronic acid-containing hydrogels: Synthesis and their applications. Chem. Soc. Rev. 2013, 42, 8106–8121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, Y.; Zhang, Y. Dynamically bonded layer-by-layer films for self-regulated insulin release. J. Mater. Chem. 2012, 22, 16299–16305. [Google Scholar] [CrossRef]
- Li, Z.; Lu, W.; Ngai, T.; Le, X.; Zheng, J.; Zhao, N.; Huang, Y.; Wen, X.; Zhang, J.; Chen, T. Mussel-inspired multifunctional supramolecular hydrogels with self-healing, shape memory and adhesive properties. Polym. Chem. UK 2016, 7, 5343–5346. [Google Scholar] [CrossRef]
- Narkar, A.R.; Lee, B.P. Incorporation of anionic monomer to tune the reversible catechol-boronate complex for pH-responsive, reversible adhesion. Langmuir 2018, 34, 9410–9417. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.J.; Miserez, A.; Waite, J.H. Chapter 3 - Diverse strategies of protein sclerotization in marine invertebrates: Structure–property relationships in natural biomaterials. In Advances in Insect Physiology; Casas, J., Simpson, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 38, pp. 75–133. [Google Scholar]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Peng, Y.; Han, B.; Liu, W.; Zhang, F.; Linhardt, R.J. IO4-stimulated crosslinking of catechol-conjugated hydroxyethyl chitosan as a tissue adhesive. J. Biomed. Mater. Res. B 2019, 107, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Soliman, G.M.; Barralet, J.; Cerruti, M. Mollusk glue inspired mucoadhesives for biomedical applications. Langmuir 2012, 28, 14010–14017. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Oh, D.X.; Kim, S.; Seo, J.H.; Hwang, D.S.; Masic, A.; Han, D.K.; Cha, H.J. Mussel-mimetic protein-based adhesive hydrogel. Biomacromolecules 2014, 15, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, R.; Xu, T.; Ma, X.; Yao, Z.; Chi, B.; Xu, H. A mussel-inspired poly(γ-glutamic acid) tissue adhesive with high wet strength for wound closure. J. Mater. Chem. B 2017, 5, 5668–5678. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Menyo, M.S.; Masic, A.; Waite, J.H.; Israelachvili, J.N. Adhesion of mussel foot protein-3 to TiO surfaces the effect of pH. Biomacromolecules 2013, 14, 1072–1077. [Google Scholar] [CrossRef]
- Guvendiren, M.; Messersmith, P.B.; Shull, K.R. Self-assembly and adhesion of DOPA-modified methacrylic triblock hydrogels. Biomacromolecules 2008, 9, 122–128. [Google Scholar] [CrossRef]
- Nicklisch, S.C.T.; Spahn, J.E.; Zhou, H.J.; Gruian, C.M.; Waite, J.H. Redox capacity of an extracellular matrix protein associated with adhesion in Mytilus Californianus. Biochemistry 2016, 55, 2022–2030. [Google Scholar] [CrossRef]
- Wei, W.; Yu, J.; Broomell, C.; Israelachvili, J.N.; Waite, J.H. Hydrophobic enhancement of Dopa-mediated adhesion in a mussel foot protein. J. Am. Chem. Soc. 2013, 135, 377–383. [Google Scholar] [CrossRef]
- White, J.D.; Wilker, J.J. Underwater bonding with charged polymer mimics of marine mussel adhesive proteins. Macromolecules 2011, 44, 5085–5088. [Google Scholar] [CrossRef]
- Maier, G.P.; Rapp, M.V.; Waite, J.H.; Israelachvili, J.N.; Butler, A. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 2015, 349, 628–632. [Google Scholar] [CrossRef]
- Clancy, S.K.; Sodano, A.; Cunningham, D.J.; Huang, S.S.; Zalicki, P.J.; Shin, S.; Ahn, B.K. Marine bioinspired underwater contact adhesion. Biomacromolecules 2016, 17, 1869–1874. [Google Scholar] [CrossRef]
- Narkar, A.R.; Kelley, J.D.; Pinnaratip, R.; Lee, B.P. Effect of ionic functional groups on the oxidation state and interfacial binding property of catechol-based adhesive. Biomacromolecules 2018, 19, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Cencer, M.; Liu, Y.; Winter, A.; Murley, M.; Meng, H.; Lee, B.P. Effect of pH on the rate of curing and bioadhesive properties of dopamine functionalized poly (ethylene glycol) hydrogels. Biomacromolecules 2014, 15, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Deruelle, M.; Leger, L.; Tirrell, M. Adhesion at the solid-elastomer interface: Influence of the interfacial chains. Macromolecules 1995, 28, 7419–7428. [Google Scholar] [CrossRef]
- Choi, G.Y.; Zurawsky, W.; Ulman, A. Molecular weight effects in adhesion. Langmuir 1999, 15, 8447–8450. [Google Scholar] [CrossRef]
- Galliano, A.; Bistac, S.; Schultz, J. Adhesion and friction of PDMS networks: molecular weight effects. J. Colloid Interface Sci. 2003, 265, 372–379. [Google Scholar] [CrossRef]
- Wei, W.; Yu, J.; Gebbie, M.A.; Tan, Y.; Martinez Rodriguez, N.R.; Israelachvili, J.N.; Waite, J.H. Bridging adhesion of mussel-inspired peptides: Role of charge, chain length, and surface type. Langmuir 2015, 31, 1105–1112. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.; Yang, J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Chen, W.; Xu, T.; Yun, S.; Xu, Z.; Xu, Z.; Sato, T.; Chi, B.; Xu, H. A biomimetic mussel-inspired ε-poly-l-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv. Funct. Mater. 2017, 27, 1604894. [Google Scholar] [CrossRef]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohyd. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef]
- Guo, J.; Wang, W.; Hu, J.; Xie, D.; Gerhard, E.; Nisic, M.; Shan, D.; Qian, G.; Zheng, S.; Yang, J. Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives. Biomaterials 2016, 85, 204–217. [Google Scholar] [CrossRef]
- Guo, J.; Kim, G.B.; Shan, D.; Kim, J.P.; Hu, J.; Wang, W.; Hamad, F.G.; Qian, G.; Rizk, E.B.; Yang, J. Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives. Biomaterials 2017, 112, 275–286. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.T.; Faust, M.; Konst, S.; Lee, B.P. Hydrogen peroxide generation and biocompatibility of hydrogel-bound mussel adhesive moiety. Acta Biomater. 2015, 17, 160–169. [Google Scholar] [CrossRef]
- Meng, H.; Forooshani, P.K.; Joshi, P.U.; Osborne, J.; Mi, X.; Meingast, C.; Pinnaratip, R.; Kelley, J.; Narkar, A.; He, W.; et al. Biomimetic recyclable microgels for on-demand generation of hydrogen peroxide and antipathogenic application. Acta Biomater. 2019, 83, 109–118. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Cascio, R.L.; Crisafi, G.; Uccella, N.; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef]
- Hench, L.L. The story of bioglass®. J. Mater. Sci. Mater. M 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Alves, N.M.; Leonor, I.B.; Azevedo, H.S.; Reis, R.L.; Mano, J.F. Designing biomaterials based on biomineralization of bone. J. Mater. Chem. 2010, 20, 2911–2921. [Google Scholar] [CrossRef]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of scaffolds of poly (l-lactic acid) containing bioactive glass ceramic nanoparticles. Acta Biomater. 2008, 4, 1297–1306. [Google Scholar] [CrossRef]
- Couto, D.S.; Alves, N.M.; Mano, J.F. Nanostructured multilayer coatings combining chitosan with bioactive glass nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 1741–1748. [Google Scholar] [CrossRef]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J. Biomed. Mater. Res. A 2009, 88A, 304–313. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Widdows, K.L.; Erol, M.M.; Burch, C.W.; Sanz-Herrera, J.A.; Ochoa, I.; Stämpfli, R.; Roqan, I.S.; Gabe, S.; Ansari, T.; et al. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials 2011, 32, 4096–4108. [Google Scholar] [CrossRef]
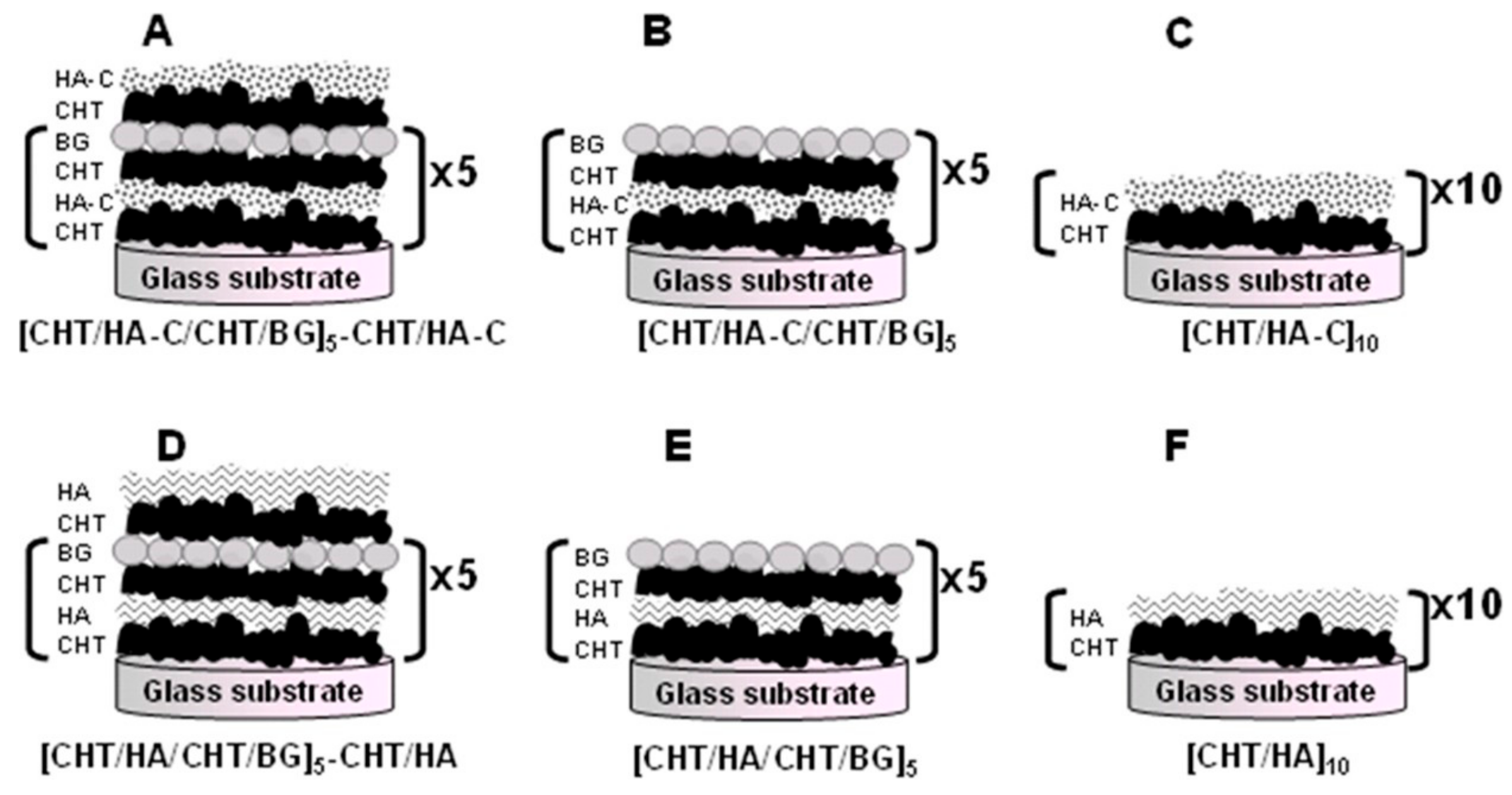
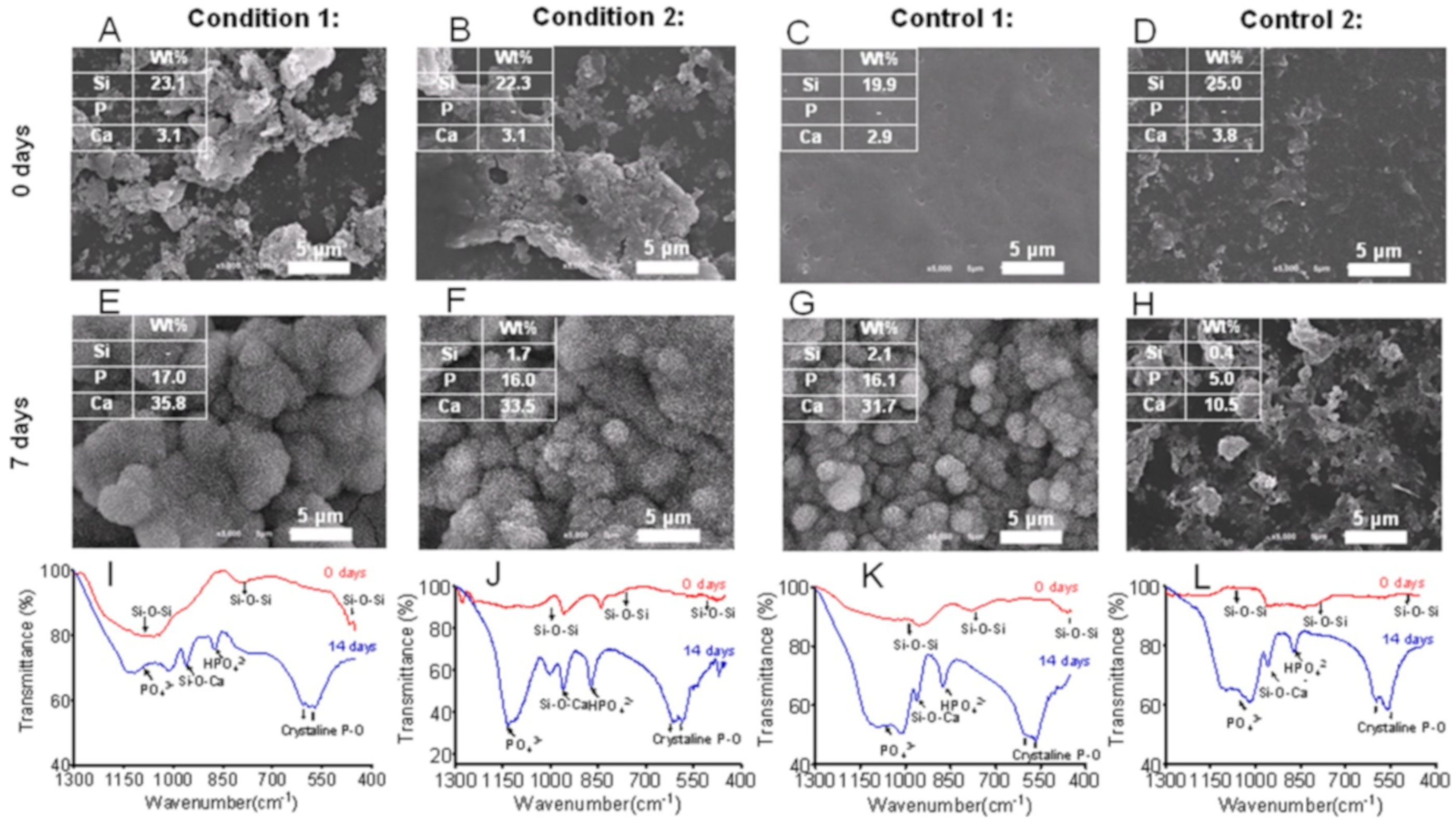
- Rego, S.J.; Vale, A.C.; Luz, G.M.; Mano, J.F.; Alves, N.M. Adhesive bioactive coatings inspired by sea life. Langmuir 2016, 32, 560–568. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Darabi, M.A.; Yuan, Q.; Xing, M. Mussel-inspired alginate gel promoting the osteogenic differentiation of mesenchymal stem cells and anti-infection. Mater. Sci. Eng. C 2016, 69, 496–504. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, Z.Y.; Yuan, X.B.; Cui, Z.D.; Yang, X.J. Fabrication of dopamine-modified hyaluronic acid/chitosan multilayers on titanium alloy by layer-by-layer self-assembly for promoting osteoblast growth. Appl. Surf. Sci. 2013, 284, 732–737. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Sheardown, H.; Brook, M.A. Immobilization of heparin on a silicone surface through a heterobifunctional PEG spacer. Biomaterials 2005, 26, 7418–7424. [Google Scholar] [CrossRef]
- Ran, F.; Nie, S.; Li, J.; Su, B.; Sun, S.; Zhao, C. Heparin-like macromolecules for the modification of anticoagulant biomaterials. Macromol. Biosci. 2012, 12, 116–125. [Google Scholar] [CrossRef]
- Ma, L.; Su, B.; Cheng, C.; Yin, Z.; Qin, H.; Zhao, J.; Sun, S.; Zhao, C. Toward highly blood compatible hemodialysis membranes via blending with heparin-mimicking polyurethane: Study in vitro and in vivo. J. Membr. Sci. 2014, 470, 90–101. [Google Scholar] [CrossRef]
- Mammadov, R.; Mammadov, B.; Toksoz, S.; Aydin, B.; Yagci, R.; Tekinay, A.B.; Guler, M.O. Heparin mimetic peptide nanofibers promote angiogenesis. Biomacromolecules 2011, 12, 3508–3519. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, S.-H.; Decker, C.G.; Wong, D.Y.; Loo, J.A.; Maynard, H.D. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat. Chem. 2013, 5, 221. [Google Scholar] [CrossRef]
- Pulsipher, A.; Griffin, M.E.; Stone, S.E.; Brown, J.M.; Hsieh-Wilson, L.C. Directing neuronal signaling through cell-surface glycan engineering. J. Am. Chem. Soc. 2014, 136, 6794–6797. [Google Scholar] [CrossRef]
- Bartneck, M.; Heffels, K.-H.; Pan, Y.; Bovi, M.; Zwadlo-Klarwasser, G.; Groll, J. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials 2012, 33, 4136–4146. [Google Scholar] [CrossRef]
- Fischer, M.; Vahdatzadeh, M.; Konradi, R.; Friedrichs, J.; Maitz, M.F.; Freudenberg, U.; Werner, C. Multilayer hydrogel coatings to combine hemocompatibility and antimicrobial activity. Biomaterials 2015, 56, 198–205. [Google Scholar] [CrossRef]
- Yin, P.; Huang, G.B.; Tse, W.H.; Bao, Y.G.; Denstedt, J.; Zhang, J. Nanocomposited silicone hydrogels with a laser-assisted surface modification for inhibiting the growth of bacterial biofilm. J. Mater. Chem. B 2015, 3, 3234–3241. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, C.; Nie, C.; He, C.; Deng, J.; Wang, L.; Xia, Y.; Zhao, C. Anticoagulant sodium alginate sulfates and their mussel-inspired heparin-mimetic coatings. J. Mater. Chem. B 2016, 4, 3203–3215. [Google Scholar] [CrossRef]
- You, I.; Kang, S.M.; Byun, Y.; Lee, H. Enhancement of blood compatibility of poly(urethane) substrates by mussel-inspired adhesive heparin coating. Bioconjug. Chem. 2011, 22, 1264–1269. [Google Scholar] [CrossRef]
- García-Fernández, L.; Cui, J.; Serrano, C.; Shafiq, Z.; Gropeanu, R.A.; Miguel, V.S.; Ramos, J.I.; Wang, M.; Auernhammer, G.K.; Ritz, S.; et al. Antibacterial strategies from the sea: Polymer-bound Cl-catechols for prevention of biofilm formation. Adv. Mater. 2013, 25, 529–533. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.-I.; Lee, H.; Cho, S.-W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Ryan, E.A.; Lakey, J.R.T.; Paty, B.W.; Imes, S.; Korbutt, G.S.; Kneteman, N.M.; Bigam, D.; Rajotte, R.V.; Shapiro, A.M.J. Successful islet transplantation. Diabetes 2002. [Google Scholar] [CrossRef]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.T.; Shapiro, A.M.J. Five-year follow-up after clinical islet transplantation. Diabetes 2005. [Google Scholar] [CrossRef]
- Brubaker, C.E.; Kissler, H.; Wang, L.-J.; Kaufman, D.B.; Messersmith, P.B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials 2010, 31, 420–427. [Google Scholar] [CrossRef]
- Tannock, L.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Ohman, H.; Vahlquist, A. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Derm. Venereol. 1994, 74, 375–379. [Google Scholar] [CrossRef]
- Soller, B.R.; Micheels, R.H.; Coen, J.; Parikh, B.; Chu, L.; His, C. Feasibility of non-invasive measurement of tissue pH using near-infrared reflectance spectroscopy. J. Clin. Monit. Comput. 1996, 12, 387–395. [Google Scholar] [CrossRef]
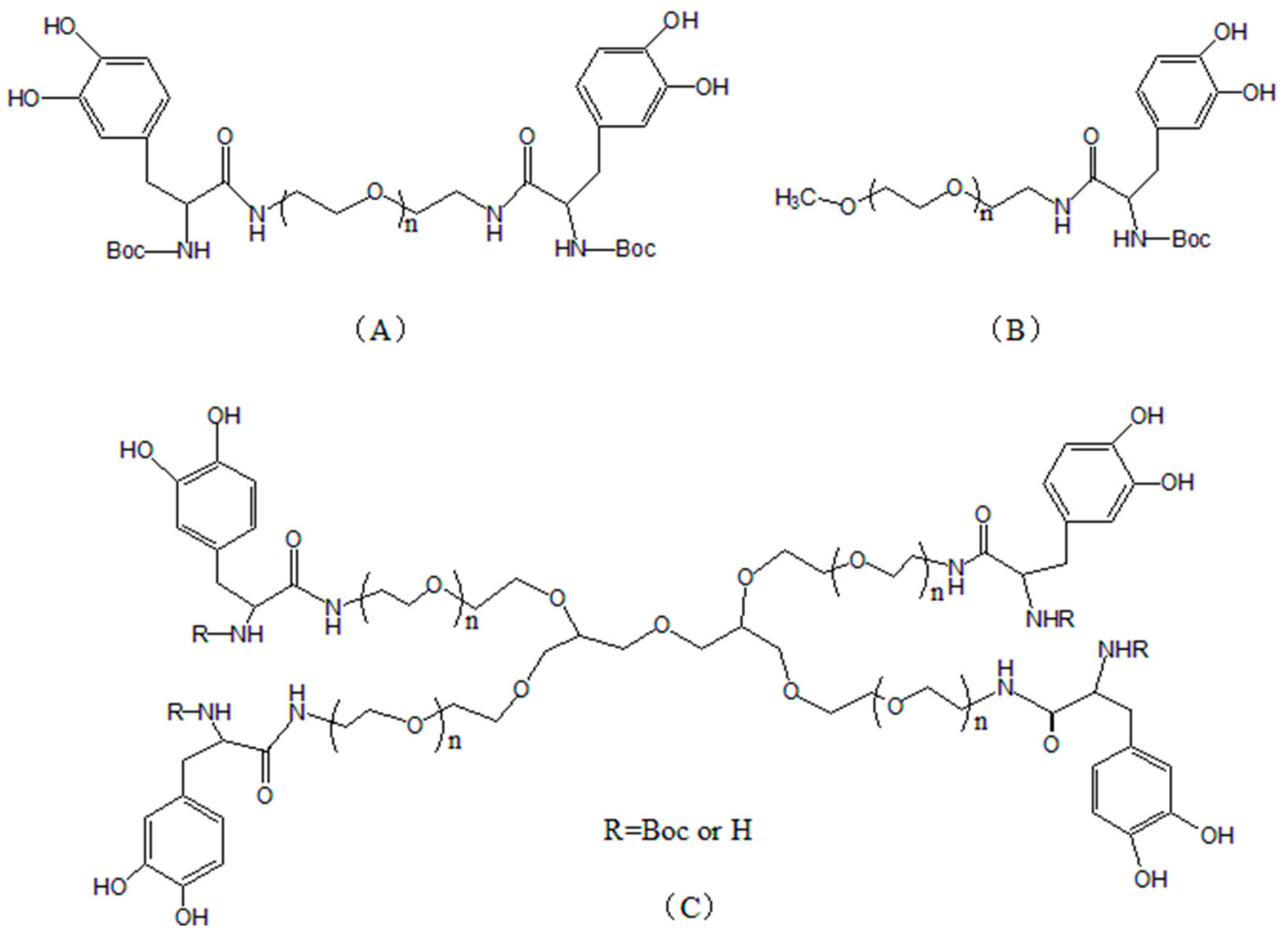
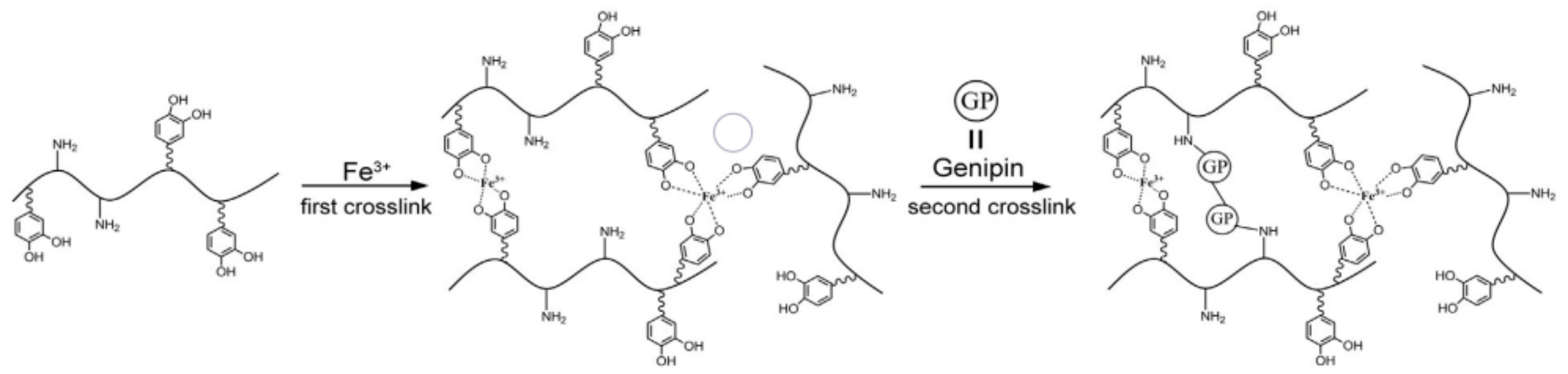
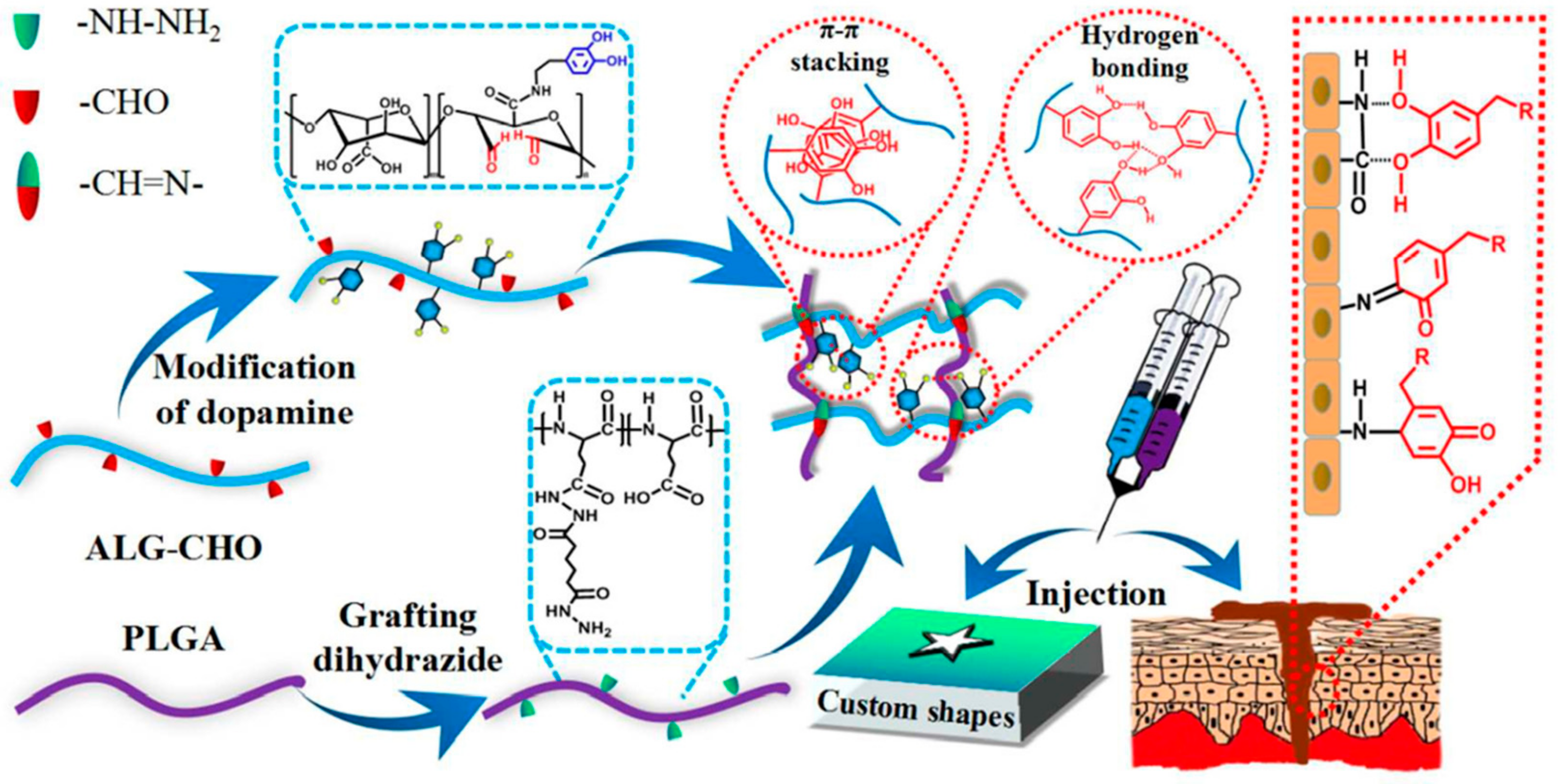
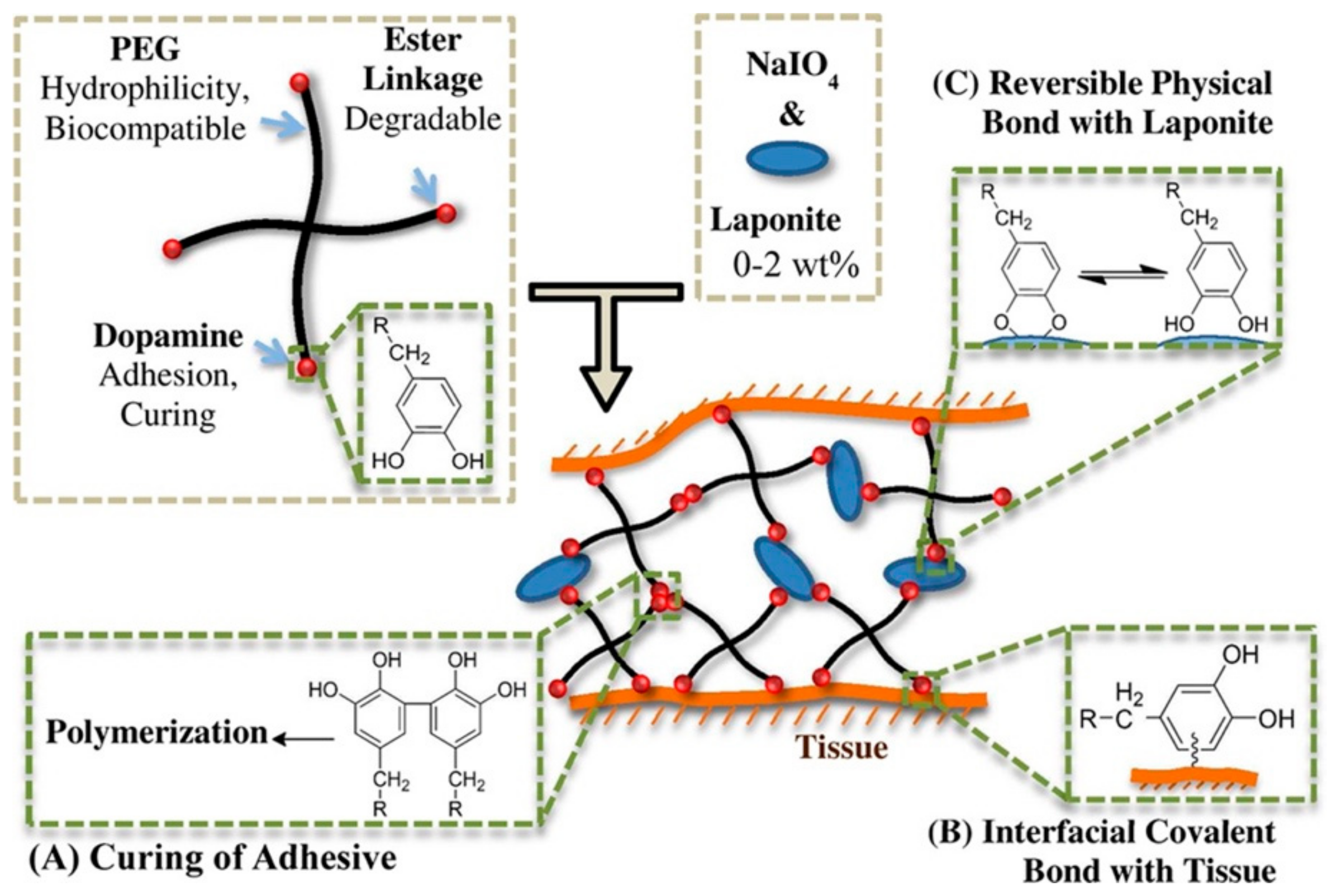
| Adhesion Pattern | Adhesion Strength (kPa) |
|---|---|
| Rapid-curing skin-fat gluing | 9.3 ± 4.9 |
| 2-h-curing skin-fat gluing | 24.7 ± 3.3 |
| 24-h-curing skin-fat gluing | 12.9 ± 0.5 |
| 2-h-curing skin-collagen gluing | 20.4 ± 4.0 |
| 2-h-curing cartilage gluing | 194.4 ± 20.7 |
| Substrate | Materials Used | Crosslinker | Reported Adhesive Strength | References |
|---|---|---|---|---|
| Mouse Subcutaneous Tissue | a CHI-C/Plu-SH | None | 15.0 ± 3.5 kPa | [67] |
| Mouse Subcutaneous Tissue | CHI/Pluronic F127 | None | 1.9 ± 1.8 kPa | [67] |
| Mouse Subcutaneous Tissue | CHI/Plu-SH | None | 5.3 ± 2.6 kPa | [67] |
| Mouse Subcutaneous Tissue | CHI-C/Pluronic F127 | None | 6.6 ± 1.0 kPa | [67] |
| Rabbit Small Intestinal Mucosa | Physical Mixture of Chitosan, Hydrocaffeic Acid | NaIO4 | 1.01 ± 0.09 kPa | [94] |
| Rabbit Small intestine | Chitosan | None | 0.35 ± 0.05 kPa | [94] |
| Rabbit Small intestine | Physical Mixture of Chitosan, Hydrocaffeic Acid | None | 0.76 ± 0.11 kPa | [94] |
| Porcine Skin | Recombinant MAP | NaIO4 and F3+ | ~200 kPa | [95] |
| Porcine Skin | b γ-PGA-DA | H2O2 and HRP | 58.2 kPa | [96] |
| Gelatin-coated Glass | c PLGA/ALG-CHO | Schiff Based Reaction | 8.5 ± 1.0 kPa | [72] |
| Gelatin-coated Glass | d PLGA/ALG-CHO-Catechol | Hydrogen Bonding and Schiff Based reaction | 24.9 kPa | [72] |
| Porcine Skin | PLGA/ALG-CHO-Catechol | Hydrogen Bonding and Schiff Based reaction | 15.5 kPa | [72] |
| Wet Rat Skin | e HCA-g-HECTS | NaIO4 | 73.56 kPa | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, W.-Y.; Hu, Z.; Liu, H.-Z.; Ouyang, Q.-Q.; Zhang, D.-Y.; Li, S.-D.; Li, P.-W.; Yang, Z.-M. Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules 2019, 24, 2586. https://doi.org/10.3390/molecules24142586
Quan W-Y, Hu Z, Liu H-Z, Ouyang Q-Q, Zhang D-Y, Li S-D, Li P-W, Yang Z-M. Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules. 2019; 24(14):2586. https://doi.org/10.3390/molecules24142586
Chicago/Turabian StyleQuan, Wei-Yan, Zhang Hu, Hua-Zhong Liu, Qian-Qian Ouyang, Dong-Ying Zhang, Si-Dong Li, Pu-Wang Li, and Zi-Ming Yang. 2019. "Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications" Molecules 24, no. 14: 2586. https://doi.org/10.3390/molecules24142586
APA StyleQuan, W.-Y., Hu, Z., Liu, H.-Z., Ouyang, Q.-Q., Zhang, D.-Y., Li, S.-D., Li, P.-W., & Yang, Z.-M. (2019). Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules, 24(14), 2586. https://doi.org/10.3390/molecules24142586





