Abstract
A recent computational analysis of the stabilizing intramolecular OH⋯O contact in 1,2-dialkyl-2,3-epoxycyclopentanol diastereomers has been extended to thiiriane, aziridine and phosphirane analogues. Density functional theory (DFT), second-order Møller-Plesset perturbation theory (MP2) and CCSD(T) coupled-cluster computations with simple methyl and ethyl substituents indicate that electronic energies of the isomers are lowered by roughly 3 to 4 kcal mol−1 when the OH group of these cyclopentanol systems forms an intramolecular contact with the O, S, N or P atom on the adjacent carbon. The results also suggest that S and P can participate in these stabilizing intramolecular interactions as effectively as O and N in constrained molecular environments. The stabilizing intramolecular OH⋯O, OH⋯S, OH⋯N and OH⋯P contacts also increase the covalent OH bond length and significantly decrease the OH stretching vibrational frequency in every system with shifts typically on the order of −41 cm−1.
1. Introduction
Previously known as the epoxy alcohol-aldol rearrangement [1], the Type III semipinacol rearrangement reaction [2,3] is the Lewis acid-mediated conversion of 2,3-epoxyalcohols to the corresponding -hydroxycarbonyl (Figure 1). This conversion, which can be accomplished with a wide range of Lewis acids [4,5,6,7,8,9], is often accompanied by 1,2-alkyl migration, a transformation that is historically regarded as a convenient route to accessing chiral quaternary centers. Apart from its presence in numerous synthetic methodologies, the reaction’s appeal is evident in industrial and commercial applications [10] as well as in the preparation of a variety of natural products [11,12,13,14]. Despite its utility, however, the mechanistic details of the semipinacol rearrangement are poorly understood. Chemists generally agree that an antiperiplanar arrangement between the migrating group (M in Figure 1) and the adjacent, epoxide CO bond is necessary to drive the reaction [10,15], an arrangement that explains why trans diastereomers fail to react [7,16]. However, additional, proposed mechanistic details for Type III semipinacol rearrangements [17,18,19,20] are not supported by experimental or theoretical evidence. This apparent void sparked an initial interest in investigating why 2,3-epoxyalcohols rearrange to the corresponding ketols.

Figure 1.
Lewis Acid-mediated Type III semipinacol rearrangement reaction converts 2,3-epoxyalcohols into the corresponding 1,3-ketol with concurrent 1,2-M group migration
To gain insight into these reactions, we recently examined the cis/trans energy differences in simple 1,2-dialkyl-2,3-epoxycyclopentanols [21]. Our quantum mechanical electronic structure computations from that study indicated that the trans diastereomers (right panel in Figure 2) had lower electronic energies than the corresponding cis structures in which the OH group pointed away from the epoxide (left panel in Figure 2). The corresponding relative energy is denoted and depicted schematically in the top half of Figure 3. However, when the OH group was rotated toward the epoxide O atom in the cis diastereomers (center panel in Figure 2), the substrate exhibited significant stabilization, yielding electronic energies lower than those of the trans structures. This relative energy is labeled and illustrated in the bottom half of Figure 3. To our knowledge, the OH⋯O interaction identified in that study was the first instance of a stabilizing intramolecular contact between epoxide and alcohol moieties to be reported. Furthermore, those findings offered compelling evidence indicating that intramolecular proton transfer may be involved in the mechanism responsible for converting the 1,2-dialkyl-2,3-epoxyalcohol to the corresponding 2,2-dialkyl-1,3-ketol.

Figure 2.
Three structural motifs of the 1,2-dimethyl-2,3-epoxycyclopentanols examined in Reference [21]: cis isomer without hydrogen bond (left), cis isomer with hydrogen bond (center), trans isomer (right).

Figure 3.
A schematic of the relative energies of the three different structural motifs: cis isomer without hydrogen bond, cis isomer with hydrogen bond, trans isomer.
The present study seeks to further characterize this intramolecular contact and any potential effects on the cis/trans energy differences of the aziridine, phosphirane and thiirane analogs of 1,2-dialkyl-2,3-epoxycyclopentanol. Specifically, our goal is to quantify any relative energy changes resulting from an intramolecular OH⋯N, OH⋯P or OH⋯S contact because N, P and S atoms can also potentially act as hydrogen bond acceptors. An extensive literature search revealed some stabilization in molecules containing alcohol and aziridine functional groups through an OH⋯N contact [22,23,24,25,26,27]. Because this interaction was similar to the OH⋯O contact reported in Reference [21], we anticipated that 1,2-dialkyl-2,3-aziridinylcyclopentanol would also adopt three comparable arrangements as shown in Figure 4. No instances of corresponding intramolecular OH⋯P or OH⋯S stabilizing contacts for phosphirane- and thiirane-containing systems have been reported, but the capacity of P and S atoms to accept intramolecular hydrogen bonds is well established both experimentally and theoretically [25,28,29,30,31,32,33].
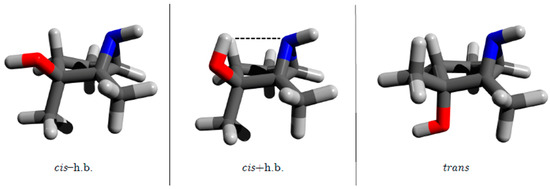
Figure 4.
Three structural motifs of the 1,2-dimethyl-2,3-aziridine cyclopentanols: cis isomer without hydrogen bond (left), cis isomer with hydrogen bond (center), trans isomer (right).
This investigation also provides some important theoretical extensions to the analysis presented in Reference [21]. In this work, the electronic structure computations are expanded to probe the effects of adding diffuse functions to the atomic orbital Gaussian basis sets. More importantly, quantification of these intramolecular contacts is extended beyond a simple relative energy scheme, which can overestimate the strength of the interaction [34], in contrast to intermolecular hydrogen bonds where direct computation of the hydrogen bond strength is relatively straightforward [35,36,37,38]. Thus, this investigation also reports physical characteristics, such as changes in OH stretching frequencies and other metrics widely considered among chemists to be distinctive features of hydrogen bonding.
In this paper, the term “hydrogen bond” is utilized to describe all reported OH⋯A contacts, where A = O, S, N and P. This semantic decision is predicated on the well-established role that intramolecular hydrogen bonding plays in conformer stabilization [39,40,41,42,43,44,45,46,47]. Although the “qualifying features of a hydrogen bond are contentious” [48], particularly in constrained intramolecular environments, such as between functional groups located on adjacent carbon atoms, there is strong experimental and theoretical evidence supporting intramolecular hydrogen bonding in instances that do not fit the formal definition [34,48,49,50]. Ultimately, this designation has no impact on the results presented in this manuscript. It merely simplifies the discussion of the relative energetics of the various cis and trans structures.
2. Computational Methods
To probe the ability of the thiirane, aziridine and phosphirane groups to accept an intramolecular hydrogen bond in the same manner as the epoxide group in the 1,2-dialkyl-2,3-epoxycyclopentanol systems, this work employs methyl (Me) and ethyl (Et) alky substituents in three different substitution patterns at the M/R positions (Figure 1): Me/Me, Me/Et and Et/Me. For these twelve different systems, the following three distinct configurations are examined: trans configuration; cis configuration with the intramolecular hydrogen bond (cis+h.b.); cis configuration without the intramolecular hydrogen bond (cis−h.b.). These variations give a total of 36 unique structures examined in this study, 6 of which are shown in Figure 2 and Figure 4.
Full geometry optimizations were performed on all 36 structures with the M06-2X [51] global hybrid density functional theory (DFT) method and two sets of correlation consistent triple zeta basis sets, one without and one with diffuse functions on all atoms (cc-pVTZ [52] and aug-cc-pVTZ [53,54] simply denoted TZ and aTZ, respectively, hereafter). M06-2X harmonic vibrational frequencies were also computed with the TZ and aTZ basis sets for every optimized structure to ensure they are minima with no imaginary frequencies (). For systems containing S and P, M06-2X computations were also performed using the cc-pV(T+d)Z basis set [55] for those centers and the TZ basis set for all other atoms, but those results have been relegated to the Supplementary Material because they are virtually identical to the M06-2X/TZ data discussed in detail in the next section. The nuclear magnetic resonance (NMR) chemical shielding constants were also calculated [56] at the M06-2X/TZ level of theory using the gauge-independent atomic orbital (GIAO) method [57].
Although prior studies [58,59] have shown the M06-2X functional can provide reliable conformational energetics for systems exhibiting intramolecular hydrogen bonding, MP2 [60] geometry optimizations and harmonic vibrational frequency computations were also carried out in this work with the same TZ basis set to provide an additional estimate of the energetics associated with these inter and intramolecular interactions. Using the MP2/TZ geometries, a subsequent set of single point energy computations were carried out with the CCSD(T) coupled-cluster method that includes all single and double substitutions along with a perturbative estimate of connected triple excitations.
To examine the intrinsic energetics of these intramolecular contacts, all computations were carried out on the isolated molecular species. The M06-2X and MP2 computations were performed with the Gaussian 09 software package [61]. All structures were optimized without constraints, and the M06-2X computations employed the default numerical integration grid in Gaussian 09. The residual Cartesian forces of the optimized structures did not exceed a.u.−1. The CCSD(T) energies were computed with Molpro 2015 [62,63]. The 1s-like core orbitals of C, N and O and the 1s, 2s and 2p-like core orbitals of P and S were frozen during all MP2 and CCSD(T) computations.
It should be noted that NH⋯O and PH⋯O contacts were also examined in the cis configurations of the aziridine and phosphirane systems. Preliminary M06-2X computations, however, indicate that the structures in which the hydroxyl group accepts what could be described as an intramolecular hydrogen bond from NH or PH are not stabilized to the extent of the corresponding conformations in which the OH group acts as the hydrogen bond donor. As such, only results associated with the OH⋯A interactions are reported and discussed here.
3. Results and Discussion
3.1. Energetics
In this study, the proposed stabilizing effects of the intramolecular OH⋯O, OH⋯S, OH⋯N and OH⋯P interactions were investigated in a series of 1,2-dialkyl-2,3-epoxy, thiiranyl, aziridinyl and phosphiranyl cyclopentanols. Because the cis/trans energetics appear to play such an important role in the underlying chemistry of Type III semipinacol rearrangement reactions, the same relative energies utilized in Reference [21] have been adopted for the current study (, and ). These terms are explicitly defined in Equations (1)–(3) and depicted schematically in Figure 3.
The relative cis/trans electronic energies of the isolated (in vacuo) species are reported in Table 1 for the TZ basis set. (The data for other basis sets is available in the Supplementary Material). All 3 methods give remarkably consistent results, and they indicate that the electronic energy of the trans configuration are significantly lower than those of the non-hydrogen bonded cis diastereomers. The corresponding M06-2X, MP2 and CCSD(T) values (Equation (1)) range from to kcal mol−1 for these systems. The identity of the bridging heteroatom (A) only has modest effect on these cis/trans energy differences, increasing by approximately 0.8 kcal mol−1 from the smallest values (for A = P and O) to the largest (for A = N). Replacing the Me group with Et at either the M or R position has a similar effect and can increase by roughly same amount: from to kcal mol−1 in the epoxide substrates (A = O), from to kcal mol−1 in the thiirane systems (A = S), from to kcal mol−1 in the aziridine compounds (A = N) and from to kcal mol−1 in the phosphirane analogs (A = P).

Table 1.
Relative electronic energies (, , all in kcal mol−1) computed with the TZ basis set.
The M06-2X, MP2 and CCSD(T) data provided in Table 1 and defined in Equation (2) show that all but one of the cis isomers become lower in energy than their trans counterparts when the OH group is oriented in the cis configuration to donate a hydrogen bond to the O, S, N or P atom. For the Me and Et substituted 1,2-dialkyl-2,3-epoxycyclopentanols, the h.b. structures have lower electronic energies with the TZ basis set than the isomers by to kcal mol−1 when A = O. The values are quite similar for A = S ( to kcal mol−1) and for A = N ( to kcal mol−1). Although the trend holds in the phosphirane systems, the and h.b. systems become isoenergetic when the Et substituent is at position 1 (i.e., M = Et). Diffuse functions have a negligible impact on the M06-2X energetics reported in Table 1. The corresponding results obtained with the aTZ basis set can be found in the Supplementary Material.
The aforementioned relative energies provide insight into the magnitude of stabilization imparted by the intramolecular OH⋯A contacts in these systems as defined by in Equation (3). The M06-2X, MP2 and CCSD(T) values for the Me and Et substituted 1,2-dialkyl-2,3-epoxycyclopentanols range from to kcal mol−1 for the OH⋯O interactions. The values reported in Table 1 reveal that both the OH⋯S and OH⋯N intramolecular contacts in the systems examined here are potentially stronger, ranging from to kcal mol−1 for the former and to kcal mol−1 for the latter. Although slightly smaller in magnitude than the values for the analogous OH⋯O contacts, these estimates of the stabilization from the intramolecular OH⋯P interaction still approach kcal mol−1.
The values reported in Table 1 are entirely consistent with those published elsewhere for intramolecular vicinal hydrogen bonds with an OH donor. For example, an analogous computational analysis of 2-substituted ethanols (with fluoro, amino and nitro groups) yielded corresponding energy differences near 2 kcal mol−1 [49]. The cis/trans energy difference for 2-fluoro and 2-chlorophenol were found to be slightly larger and on the order of 3 or 4 kcal mol−1 from DFT and MP2 computations [50], which is perhaps not surprising given that phenols are significantly more acidic than cyclopentanols. Subsequent analyses of these constrained intramolecular contacts based on the electron density, orbitals and/or electrostatic potential [64,65,66,67,68,69,70,71,72,73] are avoided in the present study because they have been shown to yield rather inconsistent results [34,48].
3.2. Bond Lengths, Vibrational Frequencies and NMR Chemical Shielding Constants
When a hydroxyl group forms a typical intermolecular hydrogen bond, the vibrational frequency associated with the covalent OH bond stretch shifts to a lower energy (commonly referred to as a “red shift”). In the gas phase, for example, when one water molecule donates a hydrogen bond to another to form the water dimer, the donor OH stretching frequency shifts to 3602 cm−1 which is 55 cm−1 lower than the symmetric OH stretch of the water monomer and 154 cm−1 lower than the asymmetric stretch [74,75]. Similar changes in spectroscopic and geometrical parameters are known to accompany intramolecular hydrogen bond formation [70,71,72,73,76,77].
Table 2 reports the analogous gas phase OH harmonic stretching frequencies computed with the M06-2X functional and the TZ basis set for all 36 optimized structures. The first three columns list the OH stretching frequencies for the , cis−h.b. and cis+h.b. systems, respectively. Although the OH groups adopt significantly different orientations in the and cis−h.b. configurations, the corresponding harmonic vibrational frequencies never differ by more than 5 cm−1 with the h.b. frequency typically having a slightly larger value by +2 cm−1. These differences are tabulated in the penultimate column of data denoted and are defined just like the energy difference in Equation (1).

Table 2.
Absolute and relative M06-2X/TZ harmonic OH stretching frequencies ( and in cm−1).
In stark contrast, the OH stretching frequency is significantly perturbed in the cis+h.b. structures all of which exhibit the intramolecular OH⋯A contacts. The last column of data in Table 2 gives the OH stretching frequency difference between the and cis+h.b. structures (analogous to the energy difference in Equation (2)). The intramolecular OH⋯A contacts cause the frequency to decrease by at least 25 cm−1 and by as much as 51 cm−1 (with an average change of 41 cm−1). The same trends are observed in the M06-2X/aTZ and MP2/TZ harmonic frequency computations within the Supplementary Material. As with the conformational energy differences, the OH stretching frequency shifts reported here are consistent with the other computational studies of 2-substituted alcohols (e.g., cm−1 for 2-fluoroethanol and cm−1 for 2-nitroethanol) [49,78]. The shifts can be larger for less constrained intramolecular OH⋯A contacts. The experimental difference between the free and hydrogen bonded OH stretches of 1,3-propanediol is cm−1 [42], but that is still appreciably smaller than the corresponding cm−1 shift induced by the formation of the analogous intermolecular hydrogen bond between methanol and dimethylether [31].
Table 3 provides the related geometrical parameters for each gas phase optimized structure. As expected, the trends in the covalent OH bond lengths reported in the first 3 columns of data are congruent with those for the OH stretching frequencies. The OH bond lengths are nearly identical for the corresponding and cis−h.b. structures. They never differ by more than 0.0004 Å (), and the h.b. bond length is almost always slightly shorter by approximately 0.0002 Å. In contrast, the differences in the bond lengths are an order of magnitude larger when comparing values for the and cis+h.b. structures (). The OH bond lengths in Table 3 are typically 0.003 Å longer in the structures exhibiting the intramolecular OH⋯A contacts in accord with the appreciably lower OH stretching frequencies in Table 2. The same bond length changes can be seen for the M06-2X/aTZ and MP2/TZ data in the Supplementary Material.

Table 3.
Absolute and relative M06-2X/TZ covalent OH bond lengths (R and in Å).
The corresponding gas phase M06-2X/TZ isotropic NMR chemical shielding constants () for the H atom in the OH functional groups are reported in the first 3 columns of data in Table 4 for the , cis−h.b. and cis+h.b. systems, respectively. The isotropic shielding constants range from 30.40 to 31.96 ppm with the trans isomers consistently giving the largest values. Without exception, the isotropic shielding constants for the cis−h.b. are smaller by to ppm as indicated by the column of data. However, when the OH group rotates to form an OH⋯A contact, tends to decrease further, by roughly a factor of 2, giving values that grow to as much as ppm (last column of Table 4). In other words, is generally smaller for the cis+h.b. structures and larger for the cis−h.b. conformations. The two exceptions to this trend occur when M = Et and A = S or P. Overall, both the sign and magnitude of these changes are consistent with the formation of intramolecular hydrogen bonds in similar systems. [71,72] The same trends are observed in the M06-2X/aTZ and MP2/TZ NMR data reported in the Supplementary Material. However, it should be noted that changes NMR chemical shifts (often denoted ) have the opposite sign as those associated with isotropic shielding constants ().

Table 4.
Absolute and relative M06-2X/TZ isotropic NMR chemical shielding constants for the hydroxyl H atom ( and in ppm.)
The properties discussed in this section, and changes thereof, are qualitatively consistent with hydrogen bond formation, and the Supplementary Material includes graphs that explore these relationships by plotting the relative electronic energies of the cis+h.b. structures ( or ) versus various metrics from Table 2, Table 3 and Table 4. Only the and covalent OH bond length parameters in Table 3 have a clear correlation with or , for which the coefficient of determination () from a simple linear regression ranges from 0.83 to 0.89. This value does not exceed 0.66 for any of the other relationships examined in the Supplementary Material. Trends for certain subsets of data could emerge (e.g., for a given hydrogen bond acceptor) as additional systems are investigated, but no general relationships across all systems are apparent from the data plotted in the Supplementary Material apart from those involving the covalent OH bond length. This result is perhaps not too surpising given the highly constrained nature of these intramolecular OH⋯A contacts and the diversity of hydrogen bond accepts (A = O, S, N and P).
4. Conclusions
The DFT, MP2 and CCSD(T) computations performed in this study reveal that all of the systems examined exhibit stabilizing OH⋯A intramolecular interactions in vacuo, where A = O, S, N and P. A total of 36 unique structures were characterized to probe the relative energetics with and without the intramolecular OH⋯A interaction. With simple Me and Et substituents, the conformers electronic energies are stabilized by to 2.5 to 4.1 kcal mol−1 when the OH group rotates toward the adjacent O, S, N or P atoms. Consequently, the cis configurations exhibiting these intramolecular contacts have lower electronic energies than their trans counterparts. The intramolecular OH⋯A contacts in the systems studied here also induce OH covalent bond elongation along with a commensurate decrease in the OH stretching frequency and the isotropic NMR chemical shielding constant for the hydroxyl H atom. These findings represent the first theoretical evidence describing a stabilizing intramolecular interaction between hydroxyl and thiirane/phosphirane moieties exhibiting many of the characteristics commonly associated intramolecular hydrogen bonding. From a synthetic perspective, the results also suggest that the cis diastereomers can potentially serve as a fascinating starting point toward accessing interesting -keto alcohols, amines, thiols, and phosphines via Type III semipinacol rearrangement reaction. Future work will probe the distance and directional dependencies of these constrained intramolecular interactions in these cyclic and analogous acyclic systems.
Supplementary Materials
The Supplementary Materials are available online.
Author Contributions
Conceptualization, J.M.C. and G.S.T.; Methodology, G.S.T.; Software, G.S.T.; Validation, B.E.S.; Formal Analysis, B.E.S., J.M.C. and G.S.T.; Investigation, B.E.S., J.M.C. and G.S.T.; Resources, G.S.T.; Data curation, G.S.T.; Writing—original draft, B.E.S. and J.M.C.; Writing—review & editing, G.S.T.; Visualization, B.E.S.; Supervision, G.S.T.; Project administration, G.S.T.; Funding acquisition, G.S.T.
Funding
This research was funded by the University of Mississippi and the National Science Foundation (NSF), grant number CHE-1664998. The work was performed using resources at the Mississippi Center for Supercomputing Research, some of which were obtained with support from NSF grant number CHE-1338056.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shimazaki, M.; Hara, H.; Keisuke, S.; Tsuchihashi, G.I. On the use of epoxy alcohol-aldol rearrangement for stereoselective construction of quarternary carbon centers. Tetrahedron Lett. 1987, 28, 5891–5894. [Google Scholar] [CrossRef]
- Song, Z.L.; Fan, C.A.; Tu, Y.Q. Semipinacol Rearrangement in Natural Product Synthesis. Chem. Rev. 2011, 111, 7523–7556. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.E.; Isaacs, N.S. Mechanisms Of Epoxide Reactions. Chem. Rev. 1959, 59, 737–799. [Google Scholar] [CrossRef]
- Maruoka, K.; Hasegawa, M.; Yamamoto, H.; Suzuki, K.; Shimazaki, M.; Tsuchihashi, G. Epoxy silyl ether rearrangements: A new, stereoselective approach to the synthesis of .beta.-hydroxy carbonyl compounds. J. Am. Chem. Soc. 1986, 108, 3827–3829. [Google Scholar] [CrossRef]
- Clarke, C.; Fleming, I.; Fortunak, J.M.; Gallagher, P.T.; Honan, M.C.; Mann, A.; Nubling, C.O.; Raithby, P.R.; Wolff, J. An approach to the synthesis of gelsemine: The intramolecular reaction of an allylsilane with an acyliminium ion for the synthesis of one of the quaternary centres. Tetrahedron 1988, 44, 3931–3944. [Google Scholar] [CrossRef]
- Jung, M.E.; Lee, W.S.; Sun, D. Synthesis of Four Diastereomeric 3,5-Dialkoxy-2,4-dimethylalkanals by a Simple Extension of the Non-Aldol Aldol Process to Bis(propionates). Org. Lett. 1999, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Walsh, P.J. Asymmetric Addition of Alkylzinc Reagents to Cyclic Unsaturated Ketones and a Tandem Enantioselective Addition/Diastereoselective Epoxidation with Dioxygen. J. Am. Chem. Soc. 2003, 125, 9544–9545. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Kikkawa, T.; Nakajima, S.i.; Takanami, T. Highly Regio- and Stereoselective Rearrangement of Epoxides to Aldehydes Catalyzed by High-Valent Metalloporphyrin Complex, Cr(TPP)OTf. J. Am. Chem. Soc. 2004, 126, 9554–9555. [Google Scholar] [CrossRef]
- Kita, Y.; Matsuda, S.; Inoguchi, R.; Ganesh, J.K.; Fujioka, H. Lewis Acid-Promoted Rearrangement of 2,3-Epoxy Alcohol Derivatives: Stereochemical Control and Selective Formation of Two Types of Chiral Quaternary Carbon Centers from the Single Carbon Skeleton. J. Org. Chem. 2006, 71, 5191–5197. [Google Scholar] [CrossRef]
- Tu, Y.Q.; Fan, C.A.; Ren, S.K.; Chan, A.S.C. Zinc bromide as catalyst for the stereoselective construction of quaternary carbon: Improved synthesis of diastereomerically enriched spirocyclic diols. J. Chem. Soc. Perkin Trans. 1 2000, 3791–3794. [Google Scholar] [CrossRef]
- Angeles, A.R.; Waters, S.P.; Danishefsky, S.J. Total Syntheses of (+)- and (−)-Peribysin E. J. Am. Chem. Soc. 2008, 130, 13765–13770. [Google Scholar] [CrossRef] [PubMed]
- Tanino, K.; Onuki, K.; Asano, K.; Miyashita, M.; Nakamura, T.; Takahashi, Y.; Kuwajima, I. Total Synthesis of Ingenol. J. Am. Chem. Soc. 2003, 125, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Gu, P.; Tu, Y.Q.; Fan, C.A.; Zhang, Q. An Efficient Total Synthesis of (±)-Stemonamine. Org. Lett. 2008, 10, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Fenster, M.D.B.; Dake, G.R. An Asymmetric Formal Synthesis of Fasicularin. Chem. Eur. J. 2005, 11, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Epstein, O.L.; Cha, J.K. Rapid Access to the in,out-Tetracyclic Core of Ingenol. Angew. Chem. 2005, 44, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.M.; Snowden, T.S. Comparative reductive desymmetrization of 2,2-disubstituted-cycloalkane-1,3-diones. Tetrahedron 2008, 64, 2897–2905. [Google Scholar] [CrossRef]
- Wang, B.M.; Song, Z.L.; Fan, C.A.; Tu, Y.Q.; Shi, Y. Lewis Acid Promoted Highly Stereoselective Rearrangement of 2,3-Aziridino Alcohols: A New Efficient Approach to Amino Carbonyl Compounds. Org. Lett. 2002, 4, 363–366. [Google Scholar] [CrossRef]
- Tu, Y.Q.; Sun, L.D.; Wang, P.Z. Stereoselective Reductive Rearrangement of Hydroxy Epoxides: A New Method for Synthesis of 1,3-Diols1. J. Org. Chem. 1999, 64, 629–633. [Google Scholar] [CrossRef]
- Jung, M.E.; D’Amico, D.C. Enantiospecific synthesis of all four diastereomers of 2-methyl-3- [(trialkylsilyl)oxy]alkanals: Facile preparation of aldols by non-aldol chemistry. J. Am. Chem. Soc. 1993, 115, 12208–12209. [Google Scholar] [CrossRef]
- Zhu, Y.; Shu, L.; Tu, Y.; Shi, Y. Enantioselective Synthesis and Stereoselective Rearrangements of Enol Ester Epoxides. J. Org. Chem. 2001, 66, 1818–1826. [Google Scholar] [CrossRef]
- Abdo, Y.A.; Weeks, J.W.; Layfield, W.; Tremlett, W.M.; Graham, J.W.; Tabor, M.E.; Causey, S.E.; Carr, J.M.; Tschumper, G.S. Intramolecular Hydrogen Bonding in α-Epoxy Alcohols: A Conformational Analysis of 1,2-Dialkyl-2,3-epoxycyclopentanol Diastereomers. Chem. Lett. 2018, 47, 156–159. [Google Scholar] [CrossRef]
- De Ceglie, M.C.; Degennaro, L.; Falcicchio, A.; Luisi, R. Restricted rotations and stereodynamics of aziridine-2-methanol derivatives. Tetrahedron 2011, 67, 9382–9388. [Google Scholar] [CrossRef]
- Wang, M.C.; Wang, D.K.; Zhu, Y.; Liu, L.T.; Guo, Y.F. Enantiopure N-ferrocenylmethylaziridin-2-ylmethanols from l-serine: Synthesis, crystal structure and applications. Tetrahedron Asymmetry 2004, 15, 1289–1294. [Google Scholar] [CrossRef]
- Wang, M.C.; Wang, Y.H.; Li, G.W.; Sun, P.P.; Tian, J.X.; Lu, H.J. Applications of conformational design: Rational design of chiral ligands derived from a common chiral source for highly enantioselective preparations of (R)- and (S)-enantiomers of secondary alcohols. Tetrahedron Asymmetry 2011, 22, 761–768. [Google Scholar] [CrossRef]
- Wojtulewski, S.; J Grabowski, S. Different donors and acceptors for intramolecular hydrogen bonds. Chem. Phys. Lett. 2003, 378, 388–394. [Google Scholar] [CrossRef]
- Grabowski, S. An estimation of strength of intramolecular hydrogen bonds—Ab initio and AIM studies. J. Mol. Struct. 2001, 562, 137–143. [Google Scholar] [CrossRef]
- Froimowicz, P.; Zhang, K.; Ishida, H. Intramolecular Hydrogen Bonding in Benzoxazines: When Structural Design Becomes Functional. Chem. Eur. J. 2016, 22, 2691–2707. [Google Scholar] [CrossRef]
- Grabowski, S.J. Ab Initio Calculations on Conventional and Unconventional Hydrogen BondsStudy of the Hydrogen Bond Strength. J. Phys. Chem. A 2001, 105, 10739–10746. [Google Scholar] [CrossRef]
- Howard, D.L.; Kjaergaard, H.G. Hydrogen bonding to divalent sulfur. Phys. Chem. Chem. Phys. 2008, 10, 4113–4118. [Google Scholar] [CrossRef]
- Hansen, A.S.; Du, L.; Kjaergaard, H.G. Positively Charged Phosphorus as a Hydrogen Bond Acceptor. J. Phys. Chem. Lett. 2014, 5, 4225–4231. [Google Scholar] [CrossRef]
- Lane, J.R.; Hansen, A.S.; Mackeprang, K.; Kjaergaard, H.G. Kinetic Energy Density as a Predictor of Hydrogen- Bonded OH-Stretching Frequencies. J. Phys. Chem. A 2017, 121, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Møller, K.H.; Hansen, A.S.; Kjaergaard, H.G. Gas Phase Detection of the NH-P Hydrogen Bond and Importance of Secondary Interactions. J. Phys. Chem. A 2015, 119, 10988–10998. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Tang, S.; Hansen, A.; Frandsen, B.; Maroun, Z.; Kjærgaard, H. Subtle differences in the hydrogen bonding of alcohol to divalent oxygen and sulfur. Chem. Phys. Lett. 2017, 667, 146–153. [Google Scholar] [CrossRef]
- Rosenberg, R.E. The Strength of Hydrogen Bonds between Fluoro-Organics and Alcohols, a Theoretical Study. J. Phys. Chem. A 2018, 122, 4521–4529. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.C.; Gray, J.L.; Hardwick, A.J.; Nguyen, L.T.; Tschumper, G.S. Getting down to the fundamentals of hydrogen bonding: Anharmonic vibrational frequencies of the hetero and homogeneous dimers of HF and H2O from ab initio electronic structure computations. J. Chem. Theory Comput. 2014, 10, 5426–5435. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.N.; Tschumper, G.S. Hydrogen Bonding in the Mixed HF/HCl Dimer: Is It Better to Give or Receive? J. Comput. Chem. 2018, 39, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Sexton, T.M.; Howard, J.C.; Tschumper, G.S. Dissociation Energy of the H2O⋯HF Dimer. J. Phys. Chem. A 2018, 122, 4902–4908. [Google Scholar] [CrossRef]
- Dreux, K.M.; Tschumper, G.S. Examination of the structures, energetics, and vibrational frequencies of small sulfur-containing prototypical dimers, (H2S)2 and H2O/H2S. J. Comput. Chem. 2019, 40, 229–236. [Google Scholar] [CrossRef]
- De Oliveira, P.R.; Rittner, R. The relevant effect of an intramolecular hydrogen bond on the conformational equilibrium of cis-3-methoxycyclohexanol compared to trans-3-methoxycyclohexanol and cis-1,3-dimethoxycyclohexane. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 1737–1745. [Google Scholar] [CrossRef]
- Kuhn, B.; Mohr, P.; Stahl, M. Intramolecular Hydrogen Bonding in Medicinal Chemistry. J. Med. Chem. 2010, 53, 2601–2611. [Google Scholar] [CrossRef]
- Takeda, T.; Suzuki, Y.; Kawamata, J.; Noro, S.I.; Nakamura, T.; Akutagawa, T. The emergent intramolecular hydrogen bonding effect on the electronic structures of organic electron acceptors. Phys. Chem. Chem. Phys. 2017, 19, 23905–23909. [Google Scholar] [CrossRef] [PubMed]
- Karas, L.J.; Batista, P.R.; Viesser, R.V.; Tormena, C.F.; Rittner, R.; de Oliveira, P.R. Trends of intramolecular hydrogen bonding in substituted alcohols: A deeper investigation. Phys. Chem. Chem. Phys. 2017, 19, 16904–16913. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, M.; Lee, J.Y.C.; Cowley, R.E.; Kim, S.; Siegler, M.A.; Solomon, E.I.; Karlin, K.D. Intramolecular Hydrogen Bonding Enhances Stability and Reactivity of Mononuclear Cupric Superoxide Complexes. J. Am. Chem. Soc. 2018, 140, 9042–9045. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Zhao, Y.; Zeng, X.; Zou, J. Efficient Absorption of CO2 by Introduction of Intramolecular Hydrogen Bonding in Chiral Amino Acid Ionic Liquids. Energy Fuels 2018, 32, 6130–6135. [Google Scholar] [CrossRef]
- Lakshmipriya, A.; Chaudhary, M.; Mogurampelly, S.; Klein, M.L.; Suryaprakash, N. Intramolecular Hydrogen Bonding Appetency for Conformational Penchants in Oxalohydrazide Fluoro Derivatives: NMR, MD, QTAIM, and NCI Studies. J. Phys. Chem. A 2018, 122, 2703–2713. [Google Scholar] [CrossRef]
- Hubbard, T.A.; Brown, A.J.; Bell, I.A.W.; Cockroft, S.L. The Limit of Intramolecular H-Bonding. J. Am. Chem. Soc. 2016, 138, 15114–15117. [Google Scholar] [CrossRef] [PubMed]
- Giordanetto, F.; Tyrchan, C.; Ulander, J. Intramolecular Hydrogen Bond Expectations in Medicinal Chemistry. Med. Chem. Lett. 2017, 8, 139–142. [Google Scholar] [CrossRef]
- Lane, J.R.; Schroder, S.D.; Saunders, G.C.; Kjaergaard, H.G. Intramolecular Hydrogen Bonding in Substituted Aminoalcohols. J. Phys. Chem. A 2016, 120, 6371–6378. [Google Scholar] [CrossRef]
- Nagy, P.I. Theoretical Studies of the Solvent Effect on the Conformation of the HO-C-C-X (X = F, NH2, NO2) Moiety with Competing Intra- and Intermolecular Hydrogen Bonds. J. Phys. Chem. A 2012, 116, 7726–7741. [Google Scholar] [CrossRef]
- Abraham, M.H.; Abraham, R.J.; Aliev, A.E.; Tormena, C.F. Is there an intramolecular hydrogen bond in 2-halophenols? A theoretical and spectroscopic investigation. Phys. Chem. Chem. Phys. 2015, 17, 25151–25159. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Dunning, T.H.; Peterson, K.A.; Wilson, A.K. Gaussian basis sets for use in correlated molecular calculations. X. The atoms aluminum through argon revisited. J. Chem. Phys. 2001, 114, 9244–9253. [Google Scholar] [CrossRef]
- Helgaker, T.; Jaszuński, M.; Ruud, K. Ab Initio Methods for the Calculation of NMR Shielding and Indirect Spin-Spin Coupling Constants. Chem. Rev. 1999, 99, 293–352. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. How Accurate Are the Minnesota Density Functionals for Noncovalent Interactions, Isomerization Energies, Thermochemistry, and Barrier Heights Involving Molecules Composed of Main-Group Elements? J. Chem. Theory Comput. 2016, 12, 4303–4325. [Google Scholar] [CrossRef]
- Kozuch, S.; Bachrach, S.M.; Martin, J.M. Conformational Equilibria in Butane-1,4-diol: A Benchmark of a Prototypical System with Strong Intramolecular H-bonds. J. Phys. Chem. A 2014, 118, 293–303. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M. Molpro: A general purpose quantum chemistry program package. Comput. Mol. Sci. 2012, 2, 242–253. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO, version 2015.1; A Package of ab Initio Programs; Ab Initio Software: Lexington, MA, USA, 2015. [Google Scholar]
- Alkorta, I.; Rozas, I.; Elguero, J. Non-conventional hydrogen bonds. Chem. Soc. Rev. 1998, 27, 163–170. [Google Scholar] [CrossRef]
- Tang, T.H.; Deretey, E.; Knak Jensen, S.J.; Csizmadia, I.G. Hydrogen bonds: Relation between lengths and electron densities at bond critical points. Eur. Phys. J. D 2006, 37, 217–222. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Molins, E.; Espinosa, E. Universal Features of the Electron Density Distribution in Hydrogen-Bonding Regions: A Comprehensive Study Involving HX (X = H, C, N, O, F, S, Cl) Interactions. Chem. Eur. J. 2010, 16, 2442–2452. [Google Scholar] [CrossRef] [PubMed]
- Nowroozi, A.; Raissi, H.; Hajiabadi, H.; Jahani, P.M. Reinvestigation of intramolecular hydrogen bond in malonaldehyde derivatives: An ab initio, AIM and NBO study. Int. J. Quantum Chem. 2011, 111, 3040–3047. [Google Scholar] [CrossRef]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theor. Comp. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Thomsen, D.L.; Axson, J.L.; Schroder, S.D.; Lane, J.R.; Vaida, V.; Kjaergaard, H.G. Intramolecular Interactions in 2-Aminoethanol and 3-Aminopropanol. J. Phys. Chem. A 2013, 117, 10260–10273. [Google Scholar] [CrossRef]
- Rusinska-Roszak, D.; Sowinski, G. Estimation of the Intramolecular OH...OC Hydrogen Bond Energy via the Molecular Tailoring Approach. Part I: Aliphatic Structures. J. Chem. Inf. Model. 2014, 54, 1963–1977. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V.; Sigalov, M.V. Estimating the energy of intramolecular hydrogen bonds from 1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199–11211. [Google Scholar] [CrossRef]
- Quiquempoix, L.; Bogdan, E.; Wells, N.J.; Le Questel, J.Y.; Graton, J.; Linclau, B. A Study of Intramolecular Hydrogen Bonding in Levoglucosan Derivatives. Molecules 2017, 22, 518. [Google Scholar] [CrossRef]
- Raissi, H.; Yoosean, M.; Mollania, F.; Farzad, F.; Nowroozi, A.R.; loghmaninejad, D. Ab initio and DFT computational studies on molecular conformations and strength of the intramolecular hydrogen bond in different conformers of 3-amino-2-iminomethyl acryl aldehyde. Comput. Theor. Chem. 2011, 966, 299–305. [Google Scholar] [CrossRef]
- Otto, K.E.; Xue, Z.; Zielke, P.; Suhm, M.A. The Raman spectrum of isolated water clusters. Phys. Chem. Chem. Phys. 2014, 16, 9849–9858. [Google Scholar] [CrossRef] [PubMed]
- Strickler, S.J. Molecular spectra and molecular structure. Volume 3, electronic spectra and electronic structure of polyatomic molecules (Herzberg, Gerhard). J. Chem. Educ. 1967, 44, A760. [Google Scholar] [CrossRef]
- Iogansen, A. Direct proportionality of the hydrogen bonding energy and the intensification of the stretching XH vibration in infrared spectra. Spectrochim. Acta A 1999, 55, 1585–1612. [Google Scholar] [CrossRef]
- Das, P.; Das, P.K.; Arunan, E. Conformational Stability and Intramolecular Hydrogen Bonding in 1,2-Ethanediol and 1,4-Butanediol. J. Phys. Chem. A 2015, 119, 3710–3720. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hedberg, K. Conformational analysis. 13. 2-Fluoroethanol. An investigation of the molecular structure and conformational composition at 20, 156, and 240 °C. Estimate of the anti-gauche energy difference. J. Am. Chem. Soc. 1989, 111, 6909–6913. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).