Antioxidant and Lipoxygenase Inhibitory Activities of Essential Oils from Endemic Plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi
Abstract
1. Introduction
2. Results
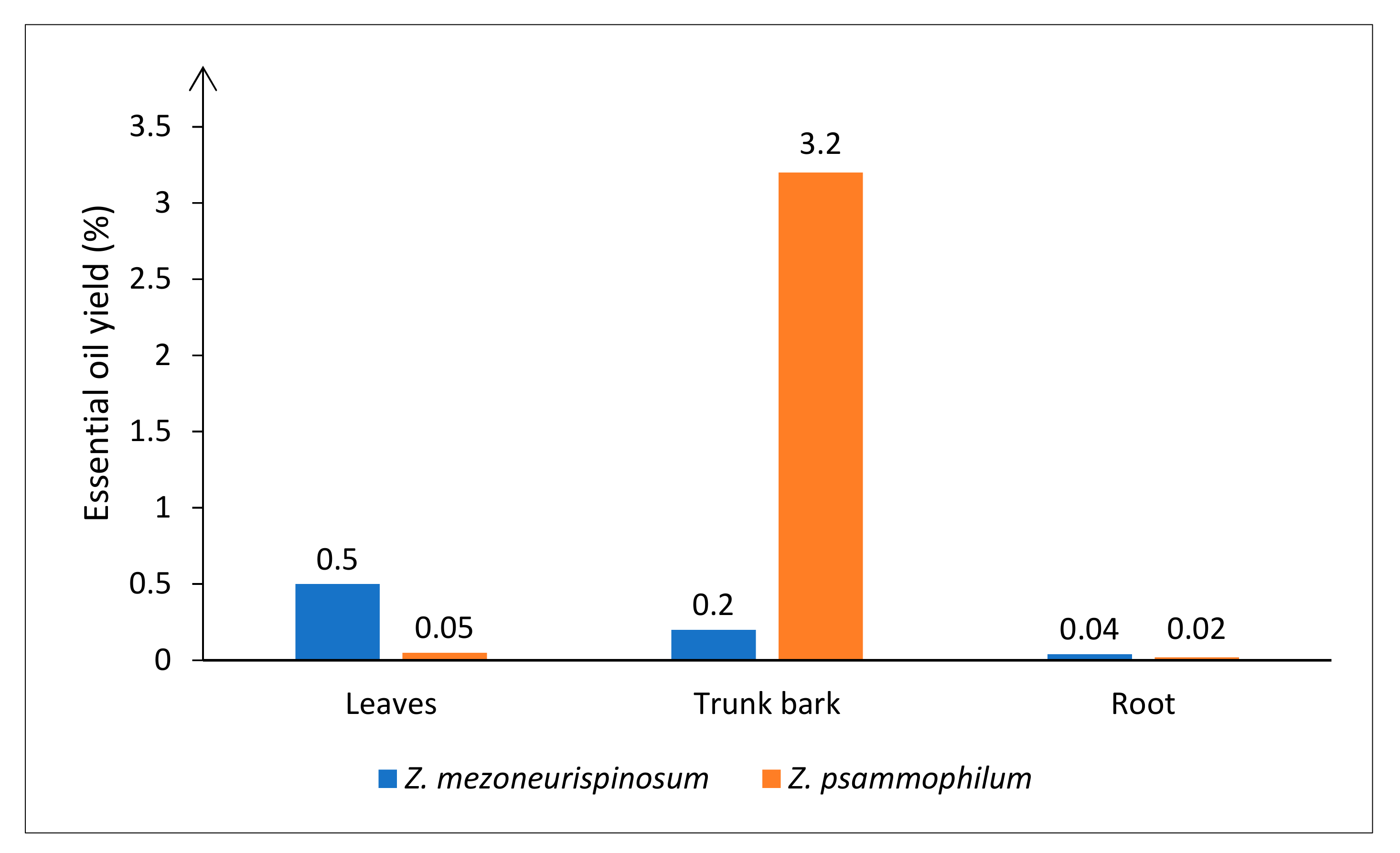
2.1. Yields
2.2. Chemical Composition of Essential Oils
2.2.1. Zanthoxylum psammophilum
2.2.2. Zanthoxylum mezoneurispinosum
2.3. Biological Activities of the Essential Oils
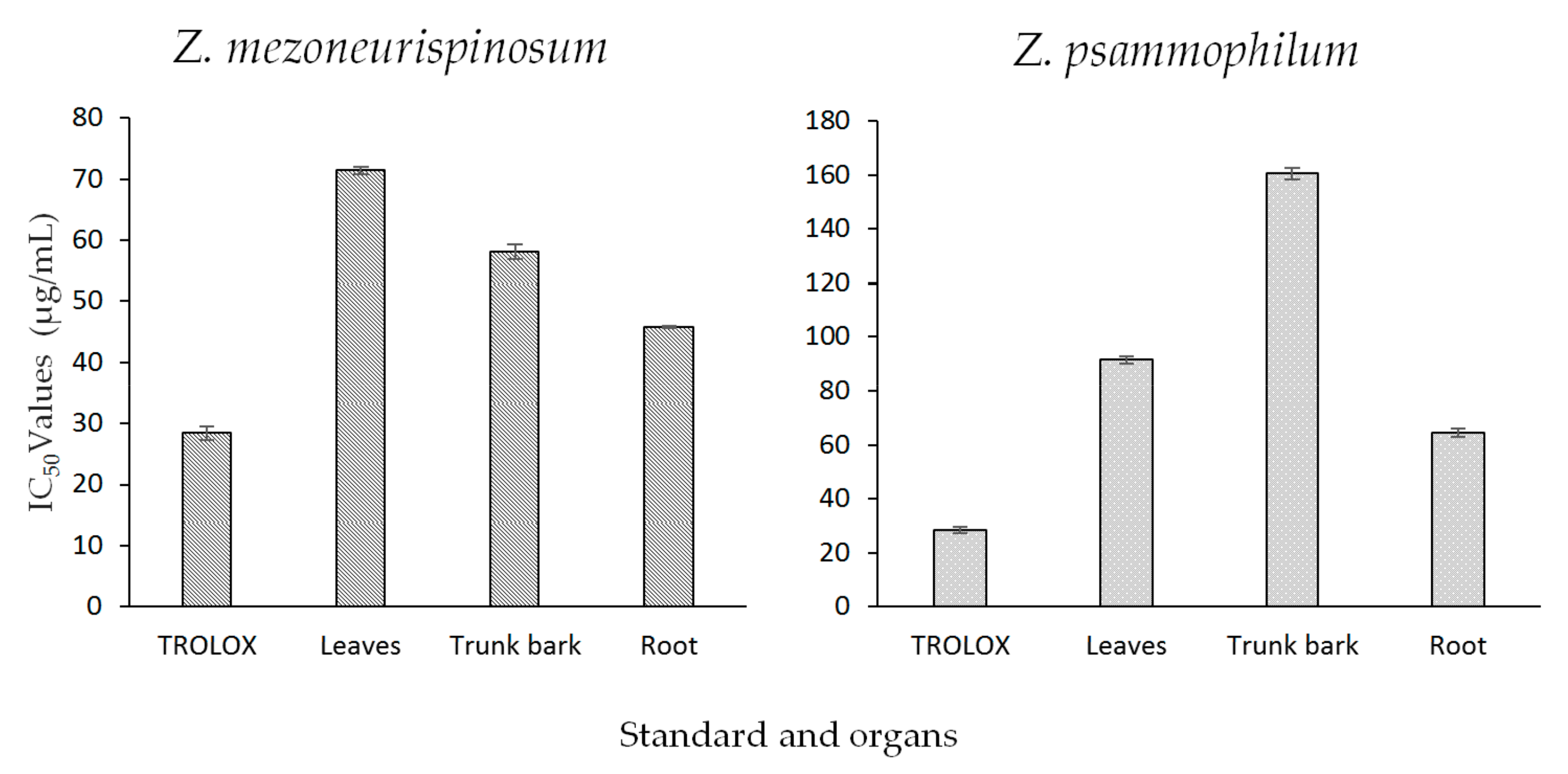
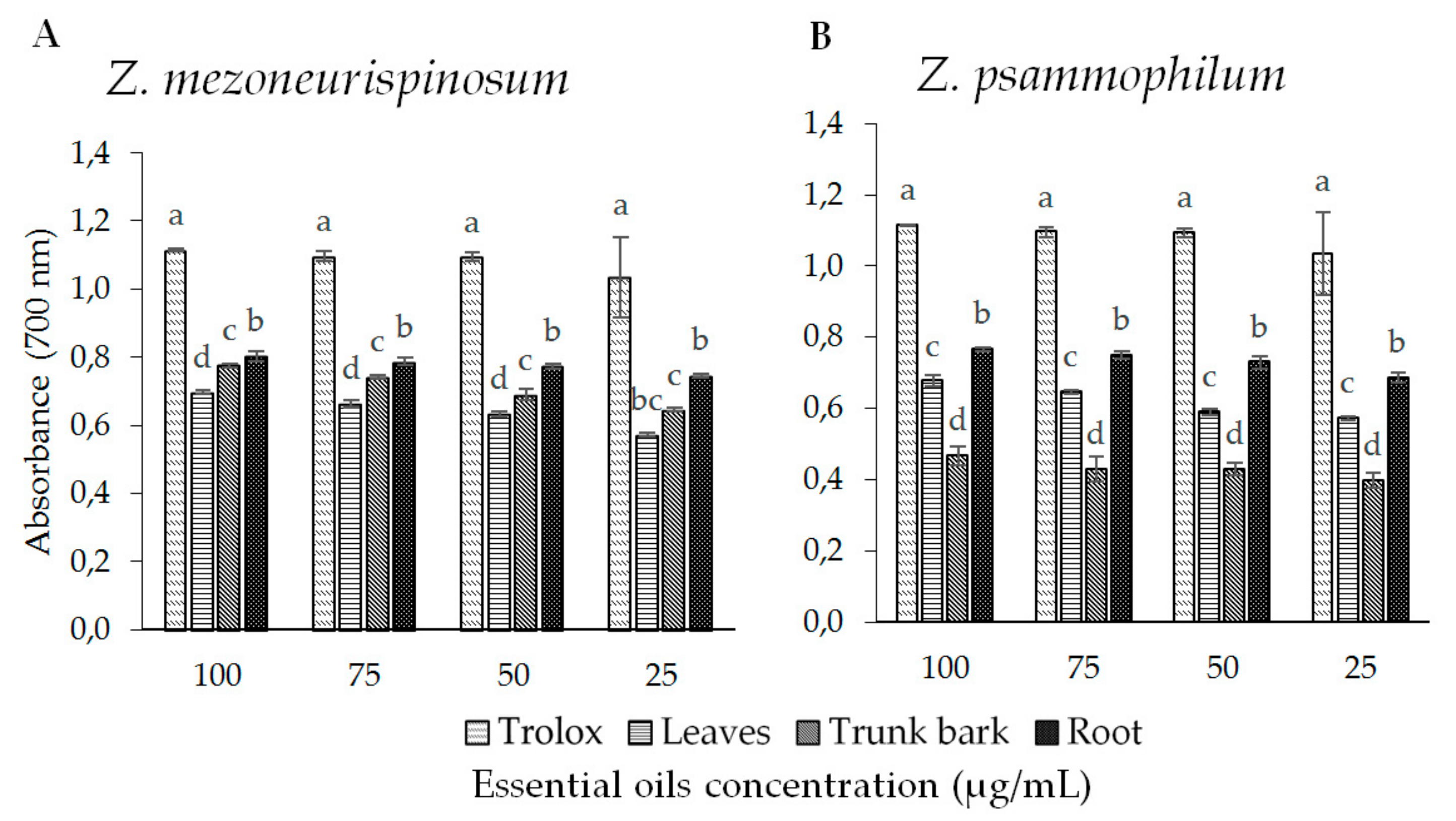
2.3.1. Antioxidant Activity
The DPPH Free Radical Scavenging Method
Ferric Reducing Antioxidant Power (FRAP) Assay
2.3.2. Lipoxygenase Inhibitory Activity
3. Discussion
3.1. Essential Oil Yields
3.2. Chemical Composition of the Essential Oils
3.3. Biological Properties of the Essential Oils
4. Material and Methods
4.1. Plant Materials and Hydrodistillation Procedure
4.2. Essential Oil Analyses
4.3. Biological Activities
4.3.1. Antioxidant Activity
2,2-diphenyl-1-picrylhydrazyl Radical Scavenging Capacity
Ferric-Reducing Power Determination
4.3.2. Lipoxygenase Inhibitory Activity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao-Kouassi, P.A.; Aké-Assi, E.; Martinez, A.; Le Magrex-Debar, E.; Gangloff, S.C.; Zèches-Hanrot, M. A New Cycloheptapeptide from Zanthoxylum mezoneurispinosum Aké Assi (Rutaceae). Mediterr. J. Chem. 2014, 3, 1013–1020. [Google Scholar] [CrossRef]
- Misra, L.N.; Wouatsa, N.A.V.; Kumar, S.; Venkatesh Kumar, R.; Tchoumbougnang, F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J. Ethnopharmacol. 2013, 148, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Tatsadjieu, L.N.; Essia Ngang, J.J.; Ngassoum, M.B.; Etoa, F.X. Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloïdes and Zanthoxylum leprieurii from Cameroon. Fitoterapia 2003, 74, 469–472. [Google Scholar] [CrossRef]
- Kpomah, E.D.; Uwakwe, A.A.; Abbey, B.W. Aphrodisiac studies of diherbal mixture of Zanthoxylum Leprieurii Guill. & Perr. and Piper guineense Schumach. & Thonn. on male wistar rats. Glob. J. Res. Med. Plants Iindigen. Med. 2012, 1, 381–390. [Google Scholar]
- Agyare, C.; Kisseih, E.; Yaa, I.; Poku, P.; Ossei, S. Medicinal plants used in wound care: Assessment of wound healing and antimicrobial properties of Zanthoxylum leprieurii. Issu. Biol. Sci. Pharm. Res. 2014, 2, 81–89. [Google Scholar]
- Tchabong, F.; Sameza, S.R.; Tchameni, M.L.; Mounbain, N.S.; Mouelle, F.; Jazet, S.A.; Menut, D.P.M.; Tchoumbougnang, C. Chemical composition, free radical scavenging and antifungal activity of Zanthoxylum leprieurii essential oils against Epidermophyton floccosum and Microsporum gypseum, two most prevalent cutaneous Mycosis. J. Pharm. 2018, 8, 13–19. [Google Scholar]
- Barkatullah, I.M.; Muhammad, N.; Rehman, I.; Rehman, M.; Khan, A. Chemical composition and biological screening of essential oils of Zanthoxylum armatum DC leaves. J. Clin. Toxicol. 2014, 3. [Google Scholar] [CrossRef]
- Agyare, C.; Asase, A.; Lechtenberg, M.; Niehues, M.; Deters, A.; Hensel, A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J. Ethnopharmacol. 2009, 125, 393–403. [Google Scholar] [CrossRef]
- Yang, X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, Y.; Zhou, L.; Shi, X.; Guo, Z.; Wang MJiang, W. Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum maxim. (Rutaceae) from China. J. Essent. Oil Res. 2009, 21, 174–178. [Google Scholar] [CrossRef]
- Ake Assi, L. Lectotypification de Fagara mezoneurispinosa (Rutaceae), basionyme de Zanthoxylum mezoneurispinosum, espèce endémique de Côte d’Ivoire. Adansonia 2009, 31, 169–174. [Google Scholar] [CrossRef]
- Yao-kouassi, P.A.; Martimez, A.; Malan, F.D.; Debar, E.L.M.; Gangloff, S.C.; Caron, C.; Coffy, A.A.; Hanrot, M.Z. Benzophénanthridines isolées de Zanthoxylum psammophilum. Int. J. Biol. Chem. Sci. 2014, 8, 377–385. [Google Scholar] [CrossRef]
- Tanoh, E.A.; Nea, F.; Yapi, T.A.; Boué, G.B.; Jean-Brice, B.; Tomi, F.; Tonzibo, Z.F. Essential oil of Zanthoxylum lepreurii Guill. & Perr. rich in undecan-2-one and tridecan-2-one. J. Essent. Oil Bear. Plants 2018, 21, 1397–1402. [Google Scholar]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I.; Hutton, I. Composition of the leaf oils of the Australian and lord howe Island species of Zanthoxylum (Rutaceae). J. Essent. Oil Res. 2000, 12, 285–291. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Lawal, O.A.; Adeniyi, B.A.; Alaka, S.A.; Tetede, E. Essential oil composition of three Zanthoxylum species. J. Essent. Oil Res. 2008, 20, 69–71. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Singh, P.; Sundriyal, R.C. Chemical constituents and biological activities of the genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem. 2011, 5, 412–416. [Google Scholar]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Bisht, R.; Negi, S.K. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC. Essent. Oil Iran. J. Pharmacol. Ther. 2012, 11, 68–72. [Google Scholar]
- Park, J.; Rodríguez-Moyá, M.; Li, M.; Pichersky, E.; San, K.Y.; Gonzalez, R. Synthesis of methyl ketones by metabolically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2012, 39, 1703–1712. [Google Scholar] [CrossRef]
- Saini, M.; Wang, Z.W.; Chiang, C.; Chao, Y. Metabolic engineering of Escherichia coli for production of butyric acid. J. Agric. Food Chem. 2014, 62, 4342–4348. [Google Scholar] [CrossRef]
- Pareja, M.; Qvarfordt, E.; Webster, B.; Mayon, P.; Pickett, J.; Birkett MGlinwood, R. Herbivory by a phloem-feeding insect inhibits floral volatile production. PLoS ONE 2012, 7, e31971. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.N.; Thang, T.D. Chemical composition of the essential oil of Zanthoxylum avicennae (Lam.) DC Leaves (Rutaceae) from Vietnam. J. Essent. Oil-Bear. Plants JEOP 2012, 15, 7–11. [Google Scholar] [CrossRef]
- Setzer, W.N.; Noletto, J.A.; Lawton, R.O.; Haber, W.A. Leaf essential oil composition of five Zanthoxylum species from Monteverde, Costa Rica. Mol. Divers. 2005, 9, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Fourough, M.; Mohaddese, M.; Ebrahim, R.; Maryam, M.S. Chemical composition, antimicrobial and antioxidant activities of Echinophora platyloba essential oil. Infectio 2017, 21, 176–181. [Google Scholar]
- Rafaela, K.L.; Maria, G.C.; Milene, A.A.; Paula, L.G.; Luı´s, R.B.; David, L.N. Bactericidal and antioxidant activity of essential oils from Myristica fragrans Houtt and Salvia microphylla H.B.K. J. Am. Oil Chem. Soc. 2011, 89, 523–528. [Google Scholar]
- Pino, J.A.; Bello, A.; Urquiola, A.; García, S. Leaf oil of Xylopia aromatica (Lam.) Mart. from Cuba. J. Essent. Oil Res. 2011, 2905, 9–11. [Google Scholar]
- Lee, G.W.; Chung, M.S.; Kang, M.B.Y.; Chung, B.Y.; Lee, S. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma 2016, 253, 683–690. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Di Stefano, A.; Taspınar, N.; Sozio, P. Protective effects of cyclosativene on H2O 2-induced injury in cultured rat primary cerebral cortex cells. Cytotechnology 2015, 67, 299–309. [Google Scholar] [CrossRef]
- Kang, M.-S.; Lee, H.-S. Acaricidal and insecticidal responses of Cinnamomum cassia oils and main constituents. Appl. Biol. Chem. 2018, 61, 653–659. [Google Scholar] [CrossRef]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef]
- Wungsintaweekul, J.; Sitthithaworn, W.; Putalun, W.; Pfeifhoffer, H.W. Antimicrobial, antioxidant activities and chemical composition of selected Thai spices. J. Sci. Technol. 2010, 32, 589–598. [Google Scholar]
- Kang, O.H.; Chae, H.S.; Choi, J.G.; Oh, Y.C.; Lee, Y.S.; Kim, J.H.; Seung, M.J.; Jang, H.J.; Bae, K.H.; Lee, J.H.; et al. Ent-pimara-8(14), 15-dien-19-oic acid isolated from the roots of Aralia cordata inhibits induction of inflammatory mediators by blocking NF-κB activation and mitogen-activated protein kinase pathways. Eur. J. Pharmacol. 2008, 601, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tangjitjaroenkun, J.; Supabphol, R.; Chavasiri, W. Antioxidant effect of Zanthoxylum limonella Alston. J. Med. Plants Res. 2012, 6, 1407–1414. [Google Scholar] [CrossRef]
- Supabphol, R.; Tangjitjareonkun, J. Chemical constituents and biological activities of Zanthoxylum limonella (Rutaceae): A. Review. Trop. J. Pharm. Res. 2014, 13, 2119–2130. [Google Scholar] [CrossRef]
- Sati, S.C.; Sati, M.D.; Raturi, R.; Badoni, P.; Singh, H. Anti-Inflammatory and Antioxidant Activities of Zanthoxylum Armatum Stem Bark. Glob. J. Res. Eng. 2011, 11, 1–5. [Google Scholar]
- Asli, C.; Zehra, I.Y.; Tamer, U. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar]
- Dang, N.H.; Zhang, X.; Zheng, M.; Son, K.H.; Chang, H.W.; Kim, H.P.; Bae, K.; Kang, S.S. Inhibitory Constituents against Cyclooxygenases from Aralia cordata Thunb. Arch. Pharm. Res. 2005, 28, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−) -trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Dae-Seung, K.; Hyun-Ja, L.; Yong-Deok, J.; Yo-Han, H.; Ji-Ye, K.; Hyun-Jeong, K.; Hyun-Ji, S.; JongWook, K.; Beom Su, L.; Sung-Hoon, K. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar]
- Jing, C.; Wenqing, W.; Chunyang, S.; Jianguo, F. A Comparative Study of Sodium Houttuyfonate and 2-Undecanone for Their in Vitro and in Vivo Anti-Inflammatory Activities and Stabilities. Int. J. Mol. Sci. 2014, 15, 22978–22994. [Google Scholar]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of Antioxidant Capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb Rebey, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni Selami, I.; Saidani Tounsi, M.; Marzouk, B.; Fauconnier, M.-L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst. 2018, 152, 971–978. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. 2011, 40. [Google Scholar] [CrossRef]
- Benini, C.; Ringuet, M.; Wathelet, J.P.; Lognay, G.; Du Jardin, P.; Fauconnier, M.-L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2012, 27, 356–366. [Google Scholar]
- Sidali, L.; Brada, M.; Fauconnier, M.-L.; Lognay, G. Chemical composition and antioxidant activity of Thymus fontanesii essential oil from Algeria. J. Nat. Prod. 2018, 1–10. [Google Scholar] [CrossRef]
- Jamali, C.A.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Phenological changes to the chemical composition and biological activity of the essential oil from Moroccan endemic thyme (Thymus maroccanus Ball). Ind. Crops Prod. 2013, 49, 366–372. [Google Scholar] [CrossRef]
- Soilhi, Z.; Rhimi, A.; Heuskin, S.; Fauconnier, M.-L.; Mekki, M. Essential oil chemical diversity of Tunisian Mentha scollection. Ind. Crops Prod. 2019, 131, 330–340. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. IJRASET 2015, 3, 2321–9653. [Google Scholar]
- Liu, C.M.; Perng, M.H.; Chen, C.Y. Antioxidant activities of crude extracts from peel and seed of Cinnamomum camphora. Biomed. Res. 2018, 29, 2854–2858. [Google Scholar]
- Lee, J.H.; Kang, B.S.; Hwang, K.H.; Kim, G.H. Evaluation for anti-inflammatory effects of Siegesbeckia glabrescens extract in vitro. Food Agric. Immunol. 2011, 22, 145–160. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chang, W.H.; Chen, C.S.; Liao, J.W.; Huang, C.J.; Lu, F.J.; Chia, Y.C.; Hsu, H.K.; Wu, J.J.; Yang, H.L. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008, 46, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chang, C.T.; Chao, W.W.; Lin, C.F.; Chou, S.T. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Nikhila, G.S.; Sangeetha, G.; Swapna, T.S. Anti-inflammatory properties of the root tubers of Gloriosa superba and its conservation through micropropagation. J. Med. Plant Res. 2014, 9, 1–7. [Google Scholar]
- Lee, J.H.; Chang, K.M.; Kim, G.H. Composition and anti-inflammatory activities of Zanthoxylum schinifolium essential oil: Suppression of inducible nitric oxide synthase, cyclooxygenase-2, cytokines and cellular adhesion. J. Sci. Food Agric. 2009, 89, 1762–1769. [Google Scholar] [CrossRef]
- Odukoya, O.A.; Houghton, P.J.; Raman, A. Lipoxygenase Inhibitors in the seeds of Aframomum danielli K. Schum (Zingiberaceae). Phytomedicine 1999, 6, 251–256. [Google Scholar] [CrossRef]
- Aghraz, A.; Benameur, Q.; Gervasi, T.; Ait Dra, L.; Ben-Mahdi, M.H.; Larhsini, M.; Markouk, M.; Cicero, N. Antibacterial activity of Cladanthus arabicus and Bubonium imbricatum essential oils alone and in combination with conventional antibiotics against Enterobacteriaceae isolates. Lett. Appl. Microbiol. 2018, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Aghraz, A.; Albergamo, A.; Benameur, Q.; Salvo, A.; Larhsini, M.; Markouk, M.; Gervasi, T.; Cicero, N. Polyphenols contents, heavy metals analysis and in vitro antibacterial activity of extracts from Cladanthus arabicus and Bubonium imbricatum of Moroccan Origin. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of essential oils are available from the authors in very small amount. |
| N° | Compounds | Cas Number | Identification | RIa | RIb | Zanthoxylum mezoneurispinosum | Zanthoxylum psammophilum | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Trunk Bark | Root | Leaves | Trunk Bark | Root | ||||||
| 1 | α-pinene | 80-56-8 | MS, RI, STD | 931 | 933 | 55.4 ± 0.9 | 50.9 ± 3.6 | 11.0 ± 0.5 | ̶ | ̶ | ̶ |
| 2 | sabinene | 3387-41-5 | MS, RI, STD | 969 | 971 | 10.1 ± 0.1 | 9.7 ± 0.4 | 0.4 ± 0.0 | ̶ | ̶ | ̶ |
| 3 | β-pinene | 18,172-67-3 | MS, RI, STD | 970 | 974 | ̶ | ̶ | 0.7 ± 0.0 | ̶ | ̶ | ̶ |
| 4 | β-myrcene | 123-35-3 | MS, RI, STD | 987 | 989 | 4.5 ± 0.1 | 3.2 ± 0.5 | 1.1 ± 0.0 | ̶ | ̶ | ̶ |
| 5 | α-phellandrene | 99-83-2 | MS, RI, STD | 1000 | 1002 | 1.6 ± 0.5 | tr | ̶ | ̶ | ̶ | ̶ |
| 6 | p-cymene | 25,155-15-1 | MS, RI, STD | 1022 | 1023 | 2.5 ± 0.1 | 2.6 ± 0.5 | tr | ̶ | ̶ | ̶ |
| 7 | limonene | 138-86-3 | MS, RI, STD | 1023 | 1027 | tr | tr | 1.4 ± 0.1 | ̶ | ̶ | ̶ |
| 8 | eucalyptol | 470-82-6 | MS, RI, STD | 1026 | 1029 | ̶ | ̶ | 1.6 ± 0.1 | ̶ | ̶ | ̶ |
| 9 | (E)-β-ocimene | 13,877-91-3 | MS, RI, STD | 1041 | 1046 | 8.9 ± 0.2 | 1.7 ± 0.1 | 0.7 ± 0.1 | ̶ | ̶ | ̶ |
| 10 | linalool | 78-70-6 | MS, RI, STD | 1094 | 1098 | 3.8 ± 0.2 | 5.1 ± 1.0 | 0.3 ± 0.0 | ̶ | ̶ | tr |
| 11 | undecane | 1120-21-4 | MS, RI, STD | 1100 | 1099 | ̶ | ̶ | ̶ | 1.1 ± 0.1 | tr | ̶ |
| 12 | alloocimene | 7216-56-0 | MS, RI | 1125 | 1129 | 2.3 ± 0.1 | 2.1 ± 0.2 | 0.8 ± 0.1 | ̶ | ̶ | ̶ |
| 13 | 4-terpineol | 562-74-3 | MS, RI, STD | 1177 | 1178 | tr | 0.7 ± 0.2 | tr | tr | tr | 0.4 ± 0.0 |
| 14 | 4-isopropylcyclohexen-2-one | 500-02-7 | MS, RI | 1184 | 1186 | ̶ | 3.2 ± 0.9 | ̶ | ̶ | ̶ | ̶ |
| 15 | terpineol | 98-55-5 | MS, RI, STD | 1190 | 1190 | 3.2 ± 0.1 | 4.2 ± 0.6 | tr | tr | tr | tr |
| 16 | citronellol | 106-22-9 | MS, RI, STD | 1225 | 1227 | ̶ | ̶ | ̶ | ̶ | tr | 1.0 ± 0.0 |
| 17 | geraniol | 106-24-1 | MS, RI, STD | 1250 | 1254 | ̶ | ̶ | tr | ̶ | tr | 1.0 ± 0.0 |
| 18 | thymol | 89-83-8 | MS, RI, STD | 1287 | 1291 | tr | tr | 0.7 ± 0.2 | tr | tr | 15.7 ± 0.2 |
| 19 | undecan-2-one | 112-12-9 | MS, RI, STD | 1289 | 1293 | ̶ | ̶ | ̶ | 20.6 ± 0.1 | 61.0 ± 0.4 | 3.3 ± 0.2 |
| 20 | undecan-2-ol | 1653-30-1 | MS, RI, STD | 1298 | 1300 | ̶ | ̶ | ̶ | 2.3 ± 0.1 | 1.5 ± 0.2 | tr |
| 21 | α-cubebene | 17,699-14-8 | MS, RI | 1350 | 1353 | ̶ | tr | 0.4 ± 0.0 | ̶ | ̶ | ̶ |
| 22 | cyclosativene | 22,469-52-9 | MS, RI | 1368 | 1373 | ̶ | ̶ | 11.9 ± 0.2 | ̶ | ̶ | 4.2 ± 0.1 |
| 23 | copaene | 3856-25-5 | MS, RI, STD | 1376 | 1379 | ̶ | tr | 5.4 ± 0.1 | ̶ | tr | 5.7 ± 0.0 |
| 24 | α-bergamotene | 17,699-05-7 | MS, RI, STD | 1411 | 1417 | ̶ | ̶ | ̶ | 0.7 ± 0.0 | ̶ | ̶ |
| 25 | α-gurjunene | 489-40-7 | MS, RI | 1412 | 1419 | ̶ | ̶ | ̶ | 0.8 ± 0.0 | ̶ | ̶ |
| 26 | β-caryophyllene | 87-44-5 | MS, RI, STD | 1419 | 1423 | 3.0 ± 0.1 | 2.5 ± 0.1 | tr | 1.7 ± 0.0 | tr | 21.8 ± 0.1 |
| 27 | cadina-4(14),5-diene | 54,324-03-7 | MS, RI | 1430 | 1434 | 0.7 ± 0.0 | tr | 2.5 ± 0.1 | ̶ | ̶ | ̶ |
| 28 | γ-elemene | 3242-08-8 | MS, RI | 1440 | 1444 | tr | 1.6 ± 0.1 | 24.8 ± 0.3 | tr | tr | 0.8 ± 0.2 |
| 29 | α-humulene | 6753-98-6 | MS, RI, STD | 1456 | 1458 | 1.4 ± 0.1 | 1.2 ± 0.1 | tr | 1.1 ± 0.1 | tr | 13.6 ± 0.2 |
| 30 | alloaromadendrene | 24,246-27-9 | MS, RI | 1457 | 1466 | ̶ | ̶ | ̶ | ̶ | ̶ | 1.55 ± 0.1 |
| 31 | germacrene D | 23,986-74-5 | MS, RI, STD | 1482 | 1486 | 0.7 ± 0.0 | tr | 2.4 ± 0.2 | ̶ | ̶ | ̶ |
| 32 | β-selinene | 17,066-67-0 | MS, RI | 1488 | 1493 | tr | tr | 4.5 ± 0.2 | tr | tr | 4.9 ± 0.1 |
| 33 | tridecan-2-one | 593-08-8 | MS, RI, STD | 1490 | 1495 | tr | tr | tr | 54.4 ± 0.4 | 37.1 ± 0.3 | 3.3 ± 0.1 |
| 34 | selina-4(14),7(11)-diene | 515-17-3 | MS, RI | 1495 | 1498 | tr | 0.8 ± 0.3 | 0.9 ± 0.0 | tr | tr | 2.7 ± 0.1 |
| 35 | tridecan-2-ol | 1653-31-2 | MS, RI, STD | 1495 | 1501 | ̶ | ̶ | ̶ | 3.8 ± 0.0 | 0.4 ± 0.0 | tr |
| 36 | γ-cadinene | 39,029-41-9 | MS, RI | 1513 | 1517 | ̶ | ̶ | ̶ | tr | tr | 1.0 ± 0.1 |
| 37 | δ-cadinene | 483-76-1 | MS, RI | 1524 | 1527 | 1.5 ± 0.1 | 1.3 ± 0.1 | 3.7 ± 0.2 | 0.8 ± 0.0 | tr | 4.5 ± 0.1 |
| 38 | elemol | 639-99-6 | MS, RI, STD | 1547 | 1552 | tr | 1.7 ± 0.5 | 5.0 ± 0.3 | 1.4 ± 0.0 | tr | 5.2 ± 0.0 |
| 39 | nerolidol | 7212-44-4 | MS, RI, STD | 1557 | 1564 | ̶ | ̶ | ̶ | 1.68 ± 0.05 | tr | 0.4 ± 0.01 |
| 40 | sphathulenol | 6750-60-3 | MS, RI | 1578 | 1584 | 0.7 ± 0.03 | 1.0 ± 0.2 | 2.5 ± 0.0 | tr | ̶ | 0.5 ± 0.1 |
| 41 | caryophyllene oxide | 1139-30-6 | MS, RI, STD | 1583 | 1588 | ̶ | ̶ | ̶ | 1.1 ± 0.0 | tr | 4.7 ± 0.2 |
| 42 | guaiol | 489-86-1 | MS, RI | 1600 | 1604 | ̶ | ̶ | 1.6 ± 0.2 | ̶ | ̶ | ̶ |
| 43 | viridiflorol | 552-02-3 | MS, RI, STD | 1600 | 1612 | ̶ | ̶ | 7.1 ± 0.2 | ̶ | ̶ | ̶ |
| 44 | γ-eudesmol | 1209-71-8 | MS, RI, STD | 1627 | 1626 | ̶ | 5.2 ± 0.9 | 4.3 ± 0.1 | ̶ | ̶ | ̶ |
| 45 | agarospirol | 1460-73-7 | MS, RI | 1642 | 1641 | ̶ | 1.6 ± 1.1 | 0.7 ± 0.1 | ̶ | ̶ | ̶ |
| 46 | ζ-cadinol | 5937-11-1 | MS, RI | 1639 | 1645 | Tr | tr | 2.0 ± 0.1 | tr | tr | 1.7 ± 0.2 |
| 47 | α-cadinol | 481-34-5 | MS, RI | 1658 | 1659 | ̶ | ̶ | ̶ | 1.2 ± 0.2 | tr | 2.0 ± 0.2 |
| 48 | pentadecan-2-one | 2345-28-0 | MS, RI, STD | 1696 | 1697 | ̶ | ̶ | ̶ | 4.4 ± 0.0 | tr | tr |
| 49 | juniper camphor | 473-04-1 | MS, RI | 1700 | 1703 | ̶ | ̶ | 1.8 ± 0.2 | ̶ | ̶ | ̶ |
| 50 | phytol | 150-86-7 | MS, RI, STD | 2111 | 2117 | ̶ | ̶ | ̶ | 2.9 ± 0.2 | tr | tr |
| Monoterpene hydrocarbons (%) | 85.1 | 70.1 | 15.9 | 0.0 | 0.0 | 0.0 | |||||
| Oxygenated monoterpenes (%) | 7.0 | 13.2 | 2.6 | 0.0 | 0.0 | 18.2 | |||||
| Sesquiterpene hydrocarbons (%) | 7.2 | 7.4 | 56.5 | 5.1 | 0.0 | 60.8 | |||||
| Oxygenated sesquiterpenes (%) | 0.7 | 9.4 | 25.0 | 9.8 | 0.0 | 14.5 | |||||
| Diterpenes (%) | 0.0 | 0.0 | 0.0 | 2.9 | 0.0 | 0.0 | |||||
| Others (%) | 0.0 | 0.0 | 0.0 | 82.2 | >99.9 | 6.6 | |||||
| Total identified | >99.9 | >99.9 | >99.9 | >99.9 | >99.9 | >99.9 | |||||
| IC50 values (µg/mL) of essential oils isolated from different organs of Zanthoxylum species | ||
| Z. mezoneurispinosum | Z. psammophilum | |
| Leaves | 26.4 ± 0.2 | 28.4 ± 0.1 |
| Trunk bark | 26.2 ± 0.2 | 31.3 ± 0.0 |
| Root | 25.3 ± 0.2 | 27.6 ± 0.1 |
| Quercetin | 21.6 ± 0.1 | 21.6 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanoh, E.A.; Nea, F.; Kenne Kemene, T.; Genva, M.; Saive, M.; Tonzibo, F.Z.; Fauconnier, M.-L. Antioxidant and Lipoxygenase Inhibitory Activities of Essential Oils from Endemic Plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi. Molecules 2019, 24, 2445. https://doi.org/10.3390/molecules24132445
Tanoh EA, Nea F, Kenne Kemene T, Genva M, Saive M, Tonzibo FZ, Fauconnier M-L. Antioxidant and Lipoxygenase Inhibitory Activities of Essential Oils from Endemic Plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi. Molecules. 2019; 24(13):2445. https://doi.org/10.3390/molecules24132445
Chicago/Turabian StyleTanoh, Evelyne A., Fatimata Nea, Tierry Kenne Kemene, Manon Genva, Matthew Saive, Felix Z. Tonzibo, and Marie-Laure Fauconnier. 2019. "Antioxidant and Lipoxygenase Inhibitory Activities of Essential Oils from Endemic Plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi" Molecules 24, no. 13: 2445. https://doi.org/10.3390/molecules24132445
APA StyleTanoh, E. A., Nea, F., Kenne Kemene, T., Genva, M., Saive, M., Tonzibo, F. Z., & Fauconnier, M.-L. (2019). Antioxidant and Lipoxygenase Inhibitory Activities of Essential Oils from Endemic Plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi. Molecules, 24(13), 2445. https://doi.org/10.3390/molecules24132445





