Abstract
Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi are species endemic to Côte d’Ivoire. In this study, we determined, for the first time, the composition and biological activities of essential oils obtained from each of these plants. Essential oils were obtained by hydrodistillation from different organs of each plant with a Clevenger-type apparatus and analyzed by gas chromatography–mass spectrometry (GC-MS). Thirty-four components, accounting for more than 99.9% of the overall composition, were identified in the oils. The Z. psammophilum leaf and trunk bark oils exhibited two unusual methylketones, undecan-2-one and tridecan-2-one, whereas the root oil was rich in thymol and sesquiterpenoids. The Z. mezoneurispinosum leaf and trunk bark oils were rich in monoterpenoids, whereas sesquiterpenoids were predominant in the root oil. These samples produced, for the first time, some new chemical profiles of essential oils. The oils’ antioxidant activities were determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity and ferric reducing antioxidant power (FRAP) assays. The results showed that the essential oil isolated from roots of Z. mezoneurispinosum had the highest antioxidant activity, which is in accordance with the high thymol content of that oil. We also determined the lipoxygenase inhibitory activities of the essential oils. The results showed that all of the tested oils displayed high and close lipoxygenase inhibitory activities.
1. Introduction
The genus Zanthoxylum, family of Rutaceae, contains approximately 250 species that are distributed in subtropical and tropical climates in America, Africa, Asia, and Australia [1]. This genus is well-known for its interesting biological properties, including antioxidant, antimicrobial, antifungal, and anticancer properties [2,3,4,5,6,7]. These properties help to explain the extensive use of these plants in traditional medicine to treat a range of diseases, including anemia, cancer, sickle cell disease, infertility, rheumatism, osteoarthritis, and dysentery [8]. Furthermore, the fruits that species of the genus Zanthoxylum produce are consumed as food condiments in China [9,10].
Zanthoxylum psammophilum and Zanthoxylum mezoneurispinosum are two species of the genus Zanthoxylum that are endemic to Côte d’Ivoire [11]. Z. psammophilum is a thin, hairless lianascent plant that grows up to 15 m tall and 2 cm in diameter with solitary stem spines. Z. psammophilum presents a thick trunk bark that is easy to separate from the bark (the separation occurs at the vascular cambium level). Its leaves are composed of five to seven pairs of alternate leaflets with a moderately thorny petiole (Figure 1A). Z. mezoneurispinosum is a sarmentary shrub that grows to 10–15 m tall with twin stem spines. The trunk bark is not very thick and is quite difficult to collect. The spines of the relatively large, leafy shoots are black and measure 0.5–1 cm. They are located on either side of the petiole, with the tip bent downwards (Figure 1B). The nonvolatile compounds that the roots of these two species produce have already been studied. Two new benzophenanthridines, including 8-methoxy-7,8-dihydrofagaridine, were isolated from Z. psammophilum roots. Benzophenanthridines are known to have a wide range of interesting properties, including antibacterial, antifungal, and anticancer activities [12]. A cycloheptapeptide, akeassimezorine, was also found in Z. mezoneurispinosum roots [1]. To date, the volatile molecules of these two species have not been studied, either for their chemical composition or for their biological activities. The aim of this work is to isolate essential oils from the leaves, trunk bark, and roots of these two species, characterize the oils by GC-MS, and evaluate their antioxidant and lipoxygenase inhibitory activities.
Figure 1.
Leaves of Zanthoxylum psammophilum (A) and Zanthoxylum mezoneurispinosum (B).
2. Results
2.1. Yields
Different hydrodistillation yields were obtained depending on the species and on which fresh organ was hydrodistilled. Average yields of 0.5% and 0.05% for leaf oil, 0.2% and 3.2% for trunk bark oil, and 0.04% and 0.02% for root oil were obtained for Z. mezoneurispinosum and Z. psammophilum, respectively (Figure 2). Moreover, the obtained essential oils differed by their smells. For both species, leaves had a pronounced herbal scent, and roots had a spice-like scent. Nevertheless, the scent of the trunk bark differed between species. The Z. mezoneurispinosum trunk bark oil had a wood-like scent, whereas the Z. psammophilum trunk bark oil had a buttery note.
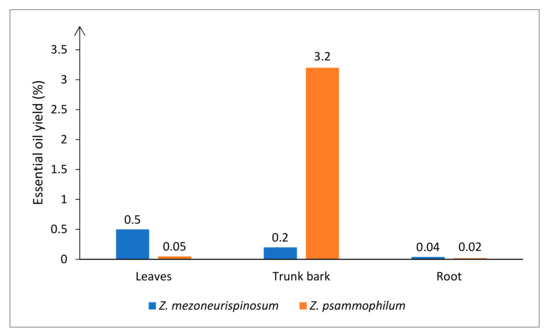
Figure 2.
Essential oil hydrodistillation yields (%) from Z. psammophilum and Z. mezoneurispinosum organs. Yields are expressed as g/100 g of fresh material.
2.2. Chemical Composition of Essential Oils
2.2.1. Zanthoxylum psammophilum
Several compounds were identified in the essential oils isolated from organs of Z. psammophilum. Sixteen molecules were found in the leaf oil, four in the trunk bark oil, and 24 in the root oil. These compounds represent more than 99.9% of each essential oil’s overall composition (Table 1). The leaf oil and the trunk bark oil consisted mainly of nonterpenic acyclic compounds (82.2% and >99.9%, respectively). The root oil was characterized by a high content of sesquiterpenes (75.3%).

Table 1.
The chemical composition of essential oils isolated from different organs of two Zanthoxylum species endemic to Côte d’Ivoire. Data are expressed as the mean of triplicates.
Leaves: A highly diverse set of molecules was found in the essential oil isolated from Z. psammophilum leaves (Table 1). Methylketones (79.4%) were the main compounds found in this oil; however, sesquiterpenes (14.9%) and diterpenes (2.9%) were also present. The leaf oil’s major components were found to be tridecan-2-one (54.4%), undecan-2-one (20.6%), pentadecan-2-one (4.4%), and tridecan-2-ol (3.8%).
Trunk bark: The essential oil isolated from Z. psammophilum trunk bark was mainly composed of methylketones, such as undecan-2-one (61.0%) and tridecan-2-one (37.1%). Other compounds, such as undecan-2-ol and tridecan-2-ol, were also found in this oil but in much lower quantities (1.5% and 0.4%, respectively) (Table 1).
Roots: As shown in Table 1, sesquiterpenes (75.3%), monoterpenes (18.2%), and methyl ketones (6.6%) were dominant in the essential oil isolated from Z. psammophilum roots. The main components were found to be β-caryophyllene (21.8%), thymol (15.7%), α-humulene (13.6%), elemol (5.2%), α-copaene (5.7%), β-selinene (4.9%), caryophyllene oxide (4.7%), δ-cadinene (4.5%), and cyclosativene (4.2%).
2.2.2. Zanthoxylum mezoneurispinosum
The analysis of the chemical composition of essential oils isolated from Z. mezoneurispinosum organs revealed 15 compounds for the leaves, 19 compounds for the trunk bark, and 27 compounds for the roots. These compounds represent more than 99.9% of each essential oil’s overall composition (Table 1). The essential oils isolated from the trunk bark and from the leaves were predominantly composed of monoterpenes (83.3% and 92.1%, respectively). Sesquiterpenes represented 16.7% and 7.9% of the composition of the trunk bark oil and the leaf oil, respectively. The essential oil isolated from the roots was mainly composed of sesquiterpenes (81.5%) and monoterpenes (18.6%).
Leaves: The essential oil isolated from Z. mezoneurispinosum leaves was rich in monoterpenes, including α-pinene (55.4%), sabinene (10.1%), (E)-β-ocimene (8.9%), β-myrcene (4.5%), linalool (3.8%), and terpineol (3.2%). β-caryophyllene (3.0%) was the main sesquiterpene found in this essential oil.
Trunk bark: The essential oil isolated from Z. mezoneurispinosum trunk bark was rich in monoterpenes, including α-pinene (50.9%), sabinene (9.7%), linalool (5.1%), terpineol (4.2%), and β-myrcene (3.2%). β-caryophyllene (2.5%) and γ-eudesmol (5.2%) were the main sesquiterpenes found in this essential oil.
Roots: The main compounds that were present in the essential oil isolated from Z. mezoneurispinosum roots included γ-elemene (24.8%), cyclosativene (11.9%), viridiflorol (7.1%), α-copaene (5.4%), and elemol (5.0%). β-selinene (4.5%) and γ-eudesmol (4.3%) were the main sesquiterpenes and α-pinene (11.0%) was the main monoterpene.
2.3. Biological Activities of the Essential Oils
2.3.1. Antioxidant Activity
The in vitro antioxidant activities of the essential oils isolated from the two Zanthoxylum species were evaluated in two different ways. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical test was used to evaluate the H-donating or radical scavenging ability of the oils. The ferric reducing antioxidant power (FRAP) assay was used to estimate the ferric-reducing capacity of the oils. For each tested essential oil and each assay, the concentration ranging from 100 to 25 µg/mL that caused a 50% inhibition of the oxidant activity (IC50) was determined and compared to a standard. A low IC50 value indicates a high antioxidant activity.
The DPPH Free Radical Scavenging Method
The antioxidant activities of essential oils isolated from different organs of both Zanthoxylum species were evaluated with the DPPH assay. Trolox, a molecule well-known for its antioxidant properties, was used as a standard. Figure 3 shows the results. The IC50 values of the essential oils varied by species and organ. For both plants, an increase in essential oil concentration induced a higher antioxidant activity (p-value <0.05). Moreover, at the same concentration and for the same organ, all essential oils isolated from Z. psammophilum had higher antioxidant activities than essential oils isolated from Z. mezoneurispinosum (p-value <0.001). For the Z. mezoneurispinosum specie, the essential oil isolated from the roots showed the highest antioxidant activity, with an IC50 value of 45.8 ± 0.1 µg/mL. The Trolox standard showed an IC50 value of 28.4 ± 1.1 µg/mL. The essential oils isolated from the trunk bark and leaves had IC50 values of 58.1 ± 1.2 µg/mL and 71.4 ± 0.5 µg/mL, respectively. The essential oils isolated from Z. psammophilum exhibited higher IC50 values: 64.5 ± 1.5 µg/mL for the roots, 91.5 ± 1.4 µg/mL for the leaves, and 160.5 ± 2.1 µg/mL for the trunk bark.
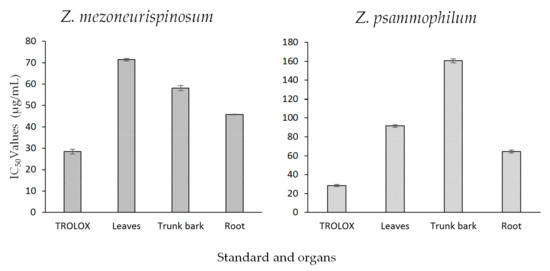
Figure 3.
The IC50 values (µg/mL), obtained with the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, of essential oils isolated from different organs of Z. mezoneurispinosum (A) and Z. psammophilum (B). Data are expressed as the mean and standard value, n = 3.
Ferric Reducing Antioxidant Power (FRAP) Assay
The antioxidant activity of the essential oils was also evaluated using the reducing power test. The presence of reducing agents in oils causes a reduction of Fe3+, which is a complex in ferrous form. Therefore, we evaluated Fe2+ by measuring and monitoring the increase in blue color density in reaction media of essential oils of Z. mezoneurispinosum and Z. psammophilum. Figure 4A shows the results for the Z. mezoneurispinosum essential oil; Figure 4B shows the results for the Z. psammophilum essential oil. For each essential oil and each species, a dose effect was observed as a significant increase in antioxidant activity, which was found to be correlated with an increase in concentration (p-value <0.001). Moreover, all of the essential oils isolated from Z. mezoneurispinosum had higher antioxidant activities (p-value <0.001) than those isolated from Z. psammophilum.
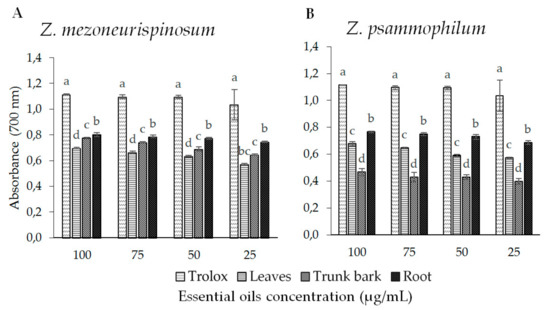
Figure 4.
The ferric-reducing power of essential oils isolated from different organs of Z. mezoneurispinosum (A) and Z. psammophilum (B). Data are expressed as the mean and standard value, n = 3. Within a concentration, mean values followed by the same letter are not significantly different according to Tukey’s test (p < 0.001).
2.3.2. Lipoxygenase Inhibitory Activity
The lipoxygenase inhibitory activities of essential oils isolated from different organs of Z. mezoneurispinosum and Z. psammophilum were evaluated by measuring the ability of each oil to inhibit the activity of lipoxygenase, which is an enzyme involved in the inflammation process. Quercetin was used as the reference standard. The IC50 values are presented in Table 2. The results showed that the essential oils isolated from different organs of Z. mezoneurispinosum had lower IC50 values than those isolated from different organs of Z. psammophilum (p-value <0.05). For all essential oils and both plant species, dose effects were observed as higher essential oil concentrations always resulted in greater lipoxygenase inhibitory activities (p-value <0.05).

Table 2.
Lipoxygenase inhibitory activity. Data are expressed as the mean of triplicates.
3. Discussion
3.1. Essential Oil Yields
In this study, essential oils were isolated from different organs of Z. psammophilum and Z. mezoneurispinosum, two plant species endemic to Côte d’Ivoire [13]. For several other Zanthoxylum species, the literature reports essential oil hydrodistillation yields that range from 0.05 to 1.90% (w/w) [14,15,16]. The yields of essential oils isolated from leaves and roots of Z. psammophilum (0.05% and 0.02%, w/w), and from leaves, trunk bark, and roots of Z. mezoneurispinosum (0.50%, 0.2% and 0.04%, w/w), that were obtained in the present study seem, therefore, to be consistent with those reported in the literature. The yield value of essential oil of 3.2% (w/w) that was obtained from the trunk bark of Z. psammophilum seems to be high; however, it is similar to the yield obtained from dried fruits of another Zanthoxylum species (Zanthoxylum leprieurii from Cameroon) [3] and can be explained by the morphology of the trunk bark of this specie presenting a thick bark.
3.2. Chemical Composition of the Essential Oils
This work represents the first report regarding the characterization of the chemical composition of the essential oils isolated from the different organs of Zanthoxylum species. Methylketones were found as main dominant chemical component of the essential oils isolated from aerial parts of Z. psammophilum. This is consistent with the chemical composition of essential oils isolated from the leaves and trunk bark of Z. leprieurii from Côte d’Ivoire [15]. Moreover, in Indian Z. armatum and Australian Z. pinnatum, methylketones have also been reported as major components of leaves essential oils (undecan-2-one (54.3%) and tridecan-2-one (31.7%)) for the former and undecan-2-one (46.0%) and tridecan-2-one (27.1%) for the latter [13,14,17]. However, when comparing the methylketones proportion in these two species with the ones in Z. psammophilum, it appeared that tridecan-2-one compound is the most dominant in Z. psammophilum leaves whereas in the trunk bark, it was the undecan-2-one which was the prevailing molecule. This could suggest that the leaves and trunk bark of Z. psammophilum could exhibit interesting properties as they are used for their flavor and fragrance in the food, pharmaceutical, and perfumery industries [18]. In combination with undecan-2-one, tridecan-2-one also have well-known antibacterial and insecticidal activities [19,20]. The composition of the essential oil isolated from Z. psammophilum roots was different to those isolated from the aerial parts of the plant, as β-caryophyllene (21.8%), α-humulene (13.6%), and thymol (15.7%) were the main compounds in the root oil. The high proportion of β-caryophyllene in this oil is interesting because this molecule is known to have several biological activities, including good antioxidant properties [21].
Monoterpenoids dominated the chemical composition of the essential oils isolated from aerial parts of Z. mezoneurispinosum. This is in agreement with the chemical composition of several other Zanthoxylum species [14,22,23]. Moreover, two new compounds, (E)-β-ocimene and γ-eudesmol, which, to our knowledge, have not been described before in these species, were present in their essential oils. These two molecules have interesting antioxidant, antibacterial, and antimicrobial properties [24,25]. Additionally, the essential oil isolated from Z. mezoneurispinosum roots was found to be rich in sesquiterpenoids, as high quantities of cyclosativene (11.9%) were present in this oil. This is an interesting finding, as it appears that this molecule has only been found before in trace quantities in leaf essential oils obtained from Xylopia aromatica and Persea americanum, which are grown in Cuba [26]. To our knowledge, this is the first time that high quantities of this compound have been described in an essential oil. The high content of cyclosativene is of consequence for the valorization of this essential oil as this molecule has strong antimicrobial, antioxidant, and insecticidal properties [27,28,29,30]. Moreover, γ-elemene (24.8%), which is a molecule known for its insecticidal, antimicrobial, and antioxidant properties, was reported in high quantities in essential oil isolated from Z. mezoneurispinosum roots [31,32].
The complete chemical characterization of essential oils isolated from organs of Z. psammophilum and Z. mezoneurispinosum allows us to highlight the potential of these two plants as a source of therapeutic molecules for use in traditional medicine. We found compounds with antibacterial, antimicrobial, and antioxidant activities in the different essential oils that we isolated. Moreover, other applications might be considered for some of the newly described essential oils. Some could, in fact, be used as a new source of raw material for the flavour industry or as a new bioactive agent in pest management.
3.3. Biological Properties of the Essential Oils
A wide range of molecules with interesting biological properties were identified in the essential oils produced from the different organs of the two Zanthoxylum species. Reports on the interesting biological properties of solvant extracts and essential oils from a range of Zanthoxylum species can also be found in the literature [33,34]. For instance, Zanthoxylum limonella and Z. leprieurii were shown to have antioxidant activities, and Z. armatum was shown to possess anti-inflammatory properties [35]. It would seem then plausible to reveal antioxidant and anti-inflammatory properties of the essential oils that were isolated in this paper.
Several analytical methods have been developed to determine the antioxidant capacity of natural substances in vitro. Here, the DPPH radical scavenging and FRAP methods were used to evaluate the antioxidant activity of the essential oils. The two tests showed that the essential oils isolated from Z. mezoneurispinosum have a better antioxidant activity than those isolated from Z. psammophilum, probably due to their different chemical composition. The weak antioxidant activity of the Z. psammophilum essential oils may be due to their high non-terpenic compound content. The essential oils isolated from Z. mezoneurispinozum were found to be rich in monoterpenes, which are molecules known for their high antioxidant activity [17]. Moreover, the essential oil isolated from Z. mezoneurispinosum roots contained a high proportion of thymol, which is a molecule that is known to have antioxidant properties [36]. The presence of this compound could explain why the antioxidant activity of the essential oil isolated from Z. mezoneurispinosum roots was higher than that isolated from Z. psammophilum.
Regarding anti-inflammatory activity, we investigated the lipoxygenase inhibitory properties of the isolated essential oils. The results showed that all of the tested essential oils have strong lipoxygenase inhibitory activity due to the presence of β-caryophyllene, α-pinene and methylketones. These compounds are well-known for their anti-inflammatory activity [37,38,39,40]. Our results are congruent with [41] who highlighted that other Zanthoxylum species, Z. capense, has good anti-inflammatory properties.
However, in vitro antioxidant and anti-inflammatory interesting results obtained in this study should be confirmed by in vivo assays before considering the utilization of those essential oils in human care [42].
4. Material and Methods
4.1. Plant Materials and Hydrodistillation Procedure
Various organs of Z. psammophilum and Z. mezoneurispinosum, including leaves, trunk bark, and roots, were collected in southeastern Côte d’Ivoire, namely Agboville (5°55′40″ N, 4°12′47″ W) and Grand Lahou (5°15′2.4″ N, 5°0′12″ W), in October and November 2017. The plant material was authenticated by botanists at the National Flora Center (CNF) (Abidjan, Côte d’Ivoire) and the vegetation improvement laboratory of Nangui Abrogoua University (Côte d’Ivoire). A specimen of each species (AA21002 and AA21009 for Z. psammophilum and Z. mezoneurispinosum, respectively) was deposited at the Herbarium of the CNF. The scent of the essential oils was determined by five trained panelists. Fresh material (1.0–1.5 kg) was hydrodistillated for 3 h using a Clevenger-type apparatus. Essential oils were collected, dried over anhydrous Na2SO4, stored in sealed amber vials, and kept under refrigeration (4–6 °C) until analysis. The essential oil hydrodistillation yield was determined as the ratio of the mass of oil after distillation to the mass of fresh organs.
4.2. Essential Oil Analyses
Ten milligrams of essential oil was dissolved in 100 mL of hexane and analyzed by GC-MS (Agilent, Santa Clara, CA, USA). For each essential oil, this manipulation was repeated three times.
GC-MS was carried out using an Agilent GC system 7890B (Agilent, Santa Clara, CA, USA) fitted with a split-splitless injector and coupled to an Agilent MSD 5977B detector. One microliter of 0.01% essential oil solution was injected, and the analytical conditions were fixed as follows: injection mode: splitless at 300 °C; HP-5MS capillary column (Agilent, Santa Clara, CA, USA) (30 m × 0.25 mm, df = 0.25 µm); temperature program: from 50 °C (1 min) to 300 °C (5 min) at a rate of 5 °C/min. The carrier gas was helium at a flow rate of 1.2 mL/min. The mass spectra were recorded in Electron Ionization mode at 70 eV (scanned mass range: 40–400 m/z). The source and quadrupole temperatures were fixed at 230 °C and 150 °C, respectively. The component identification was performed on the basis of chromatographic retention indices (RI) and by comparison of the recorded spectra with a computed data library (Pal 600K®) [43]. RI values were measured on an HP-5MS column (Agilent, Santa Clara, CA, USA). RI calculations were performed in temperature program mode according to [44,45,46,47,48]; a mixture of homologues n-alkanes (C7–C30) was used under the same chromatographic conditions. The main components were confirmed by comparison of their retention and MS spectrum data with co-injected pure references (Sigma, Darmstadt, Germany) when commercially available.
4.3. Biological Activities
4.3.1. Antioxidant Activity
The antioxidant activities of essential oils produced from Zanthoxylum species were evaluated using in vitro DPPH and FRAP assays.
2,2-diphenyl-1-picrylhydrazyl Radical Scavenging Capacity
The free radical scavenging activity [43] was determined in triplicate according to [48] with slight modifications in concentrations [49,50]. Briefly, 0.5 mL of the essential oils at four concentrations (25, 50, 75, and 100 µg/mL) in methanol was added to 1 mL of a stable solution of DPPH (0.06 mM, methanol) [50]. The mixture was vortexed for about 1 min and then incubated at room temperature in the dark for 30 min. Absorbance was measured at 517 nm in a spectrophotometer Ultrospec 7000 UV–visible, dual beam spectrophotometer (GE Healthcare, Cambridge, UK). Trolox (Sigma, Darmstadt, Germany) was used as a standard (a positive control) under the same conditions. All tests were performed in triplicate. The scavenging activity of essential oils on DPPH radicals was calculated as DPPH (%) inhibition using the following equation:
% Inhibition = ((Ab − Aa)/Ab) × 100
Ab = absorbance of the blank sample.
Aa = absorbance of the test sample.
Ferric-Reducing Power Determination
The reducing power of essential oils and the standard (Trolox) was evaluated as described by Singleton and Hseu [51,52]. In brief, a 1 mL sample at four concentrations (25, 50, 75, and 100 µg/mL) (in triplicate for each concentration) and prepared in methanol was mixed with phosphate buffer (1 mL, 0.2 M, pH = 6.6) and 1% potassium ferricyanide [K3Fe(CN)6] solution (1 mL) and incubated at 50 °C for 20 min. Afterwards, trichloroacetic acid (TCA) (1 mL, 10% v/v) was added to the solution, which was then centrifuged for 10 min at 3000 g. The recovered supernatant was mixed with distilled water (1.5 mL) and 0.1% v/v FeCl3 (150 µL). The absorbance of the resulting solution was then measured at 700 nm [53]. An increase in absorbance (when compared to the blank) indicates an increase in the reducing power. Absorbance due to the essential oils themselves (at the different concentrations) was systematically subtracted for each assay.
4.3.2. Lipoxygenase Inhibitory Activity
The in vitro lipoxygenase inhibitory activity of essential oils was determined by measuring the inhibition power of essential oils on lipoxygenase activity (EC 1.13.11.12). The spectrophotometric method described by Lyckander and Malterud (1992) was used with some minor modifications. Briefly, a reaction mixture containing essential oils in various concentrations (100, 75, 50, and 25 µg/mL of methanol) (in triplicate for each concentration), lipoxygenase (Sigma, Darmstadt, Germany), and 35 µL (0.1 mg/mL) of a 0.2 M borate buffer solution (pH = 9.0) was incubated for 15 min at 25 °C. The reaction was then initiated by addition of 35 µL of a substrate solution (linoleic acid 250 µM) and the absorbance was measured at 234 nm. Quercetin (Sigma) was used as a standard inhibitor at the same concentration as the essential oils [54,55,56]. The inhibition percentage of lipoxygenase activity was calculated as follows:
where ODblank is the Optical Density (OD) of the reaction media without the essential oil, and ODsample is the OD of the reaction media with the essential oil minus the OD value of the diluted essential oil (to compensate for absorbance due to the essential oils themselves).
Inhibition percentage (%) = 100 × (ODblank − ODsample)/ODblank
The amount of DPPH that was inhibited by the essential oils and the lipoxygenase inhibitory activity of these essential oils were expressed as the percent concentration corresponding to a 50% inhibition (IC50).
4.4. Statistical Analysis
The DPPH and FRAP methods were used to determine the antioxidant activity of the essential oils. The measurements to determine the antioxidant activity as well as the lipoxygenase inhibitory activity were performed in triplicate. Two factors were considered for the statistical analysis: the organ and the concentration. Experimental data are expressed as mean ± SD. The analysis of variance between the different averages was carried out by ANOVA using the Minitab 18 software (Pennsylvania, PA, USA). The significance level for the tests was set at 5%. The structuring of averages was done using Dunnett’s test. A multiple comparison analysis was performed using Tukey’s test on both DPPH and lipoxygenase inhibitory activities.
5. Conclusions
The aim of this study was to evaluate the potential of Z. psammophilum and Z. mezoneurispinosum, two species endemic to Côte d’Ivoire, as a source of therapeutic molecules for use in traditional medicine or other applications. The results showed that the essential oils isolated from these plants contain interesting molecules and display some good and previously unknown biological properties. Although the essential oils exhibited only moderate antioxidant activities, all isolated oils displayed elevated lipoxygenase inhibitory activities. Essential oils isolated from these plants could, therefore, be used in traditional medicine, given this interesting property. However, subsequent studies on these oils should take into account the plant’s age and the harvesting season to better understand their impact on the oils’ composition and biological properties. Moreover, the chemical characterization of these oils led to the identification of molecules that potentially have other biological activities, such as antimicrobial properties. Hence, it could be interesting to test the antimicrobial activity of these essential oils as plant extracts are increasingly being used in therapeutics to control multi-resistant organisms [57,58]. The use of these newly described essential oils would, of course, require an extensive study of their cytotoxicity before application in human or animal care. The insecticidal activity of these essential oils could also represent another potential source of valorization, as the plant protection sector is always searching for new bio-based active compounds to replace conventional insecticides. Perfumers are also avid for new scents for their creations, which may constitute a high added-value valorization for these newly described essential oils.
However, it is important to mention that both plants are endemic and, currently, non-cultivated. If local populations begin to use them for their properties or in other applications, preservation initiatives (e.g., plant nurseries) will be crucial to prevent them from disappearing.
Author Contributions
Conceptualization, E.A.T., M.-L.F. and F.Z.T.; methodology, E.A.T.; software, E.A.T., T.K.K. and M.S.; validation, M.-L.F. and F.Z.T.; formal analysis, E.A.T.; investigation, E.A.T. and F.N.; resources, E.A.T. and M.-L.F.; data curation, E.A.T., M.G. and F.N.; writing—original draft preparation, E.A.T. and M.G.; writing—review and editing, M.G., M.-L.F. and F.Z.T.; visualization, E.A.T., T.K.K. and M.G.; supervision, M.-L.F. and F.Z.T.; project administration, M.-L.F. and F.Z.T.; funding acquisition, M.-L.F. and F.Z.T.
Funding
This research was funded by the Ministry of Higher Education of Côte d’Ivoire, grant number 100/AMBACI/AA. The APC was funded by the Education, Audiovisual and Culture Executive Agency (EACEA) through EOHUB project, grant number 600873-EPP-1-2018-1ES-EPPKA2-KA.
Acknowledgments
The authors thank all the staff of the Natural Molecules’ Chemistry Laboratory (Gembloux Agro-Bio Tech) for their welcome and availability. We are especially grateful to Ipou Joseph for his support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yao-Kouassi, P.A.; Aké-Assi, E.; Martinez, A.; Le Magrex-Debar, E.; Gangloff, S.C.; Zèches-Hanrot, M. A New Cycloheptapeptide from Zanthoxylum mezoneurispinosum Aké Assi (Rutaceae). Mediterr. J. Chem. 2014, 3, 1013–1020. [Google Scholar] [CrossRef]
- Misra, L.N.; Wouatsa, N.A.V.; Kumar, S.; Venkatesh Kumar, R.; Tchoumbougnang, F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J. Ethnopharmacol. 2013, 148, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Tatsadjieu, L.N.; Essia Ngang, J.J.; Ngassoum, M.B.; Etoa, F.X. Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloïdes and Zanthoxylum leprieurii from Cameroon. Fitoterapia 2003, 74, 469–472. [Google Scholar] [CrossRef]
- Kpomah, E.D.; Uwakwe, A.A.; Abbey, B.W. Aphrodisiac studies of diherbal mixture of Zanthoxylum Leprieurii Guill. & Perr. and Piper guineense Schumach. & Thonn. on male wistar rats. Glob. J. Res. Med. Plants Iindigen. Med. 2012, 1, 381–390. [Google Scholar]
- Agyare, C.; Kisseih, E.; Yaa, I.; Poku, P.; Ossei, S. Medicinal plants used in wound care: Assessment of wound healing and antimicrobial properties of Zanthoxylum leprieurii. Issu. Biol. Sci. Pharm. Res. 2014, 2, 81–89. [Google Scholar]
- Tchabong, F.; Sameza, S.R.; Tchameni, M.L.; Mounbain, N.S.; Mouelle, F.; Jazet, S.A.; Menut, D.P.M.; Tchoumbougnang, C. Chemical composition, free radical scavenging and antifungal activity of Zanthoxylum leprieurii essential oils against Epidermophyton floccosum and Microsporum gypseum, two most prevalent cutaneous Mycosis. J. Pharm. 2018, 8, 13–19. [Google Scholar]
- Barkatullah, I.M.; Muhammad, N.; Rehman, I.; Rehman, M.; Khan, A. Chemical composition and biological screening of essential oils of Zanthoxylum armatum DC leaves. J. Clin. Toxicol. 2014, 3. [Google Scholar] [CrossRef]
- Agyare, C.; Asase, A.; Lechtenberg, M.; Niehues, M.; Deters, A.; Hensel, A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J. Ethnopharmacol. 2009, 125, 393–403. [Google Scholar] [CrossRef]
- Yang, X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, Y.; Zhou, L.; Shi, X.; Guo, Z.; Wang MJiang, W. Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum maxim. (Rutaceae) from China. J. Essent. Oil Res. 2009, 21, 174–178. [Google Scholar] [CrossRef]
- Ake Assi, L. Lectotypification de Fagara mezoneurispinosa (Rutaceae), basionyme de Zanthoxylum mezoneurispinosum, espèce endémique de Côte d’Ivoire. Adansonia 2009, 31, 169–174. [Google Scholar] [CrossRef]
- Yao-kouassi, P.A.; Martimez, A.; Malan, F.D.; Debar, E.L.M.; Gangloff, S.C.; Caron, C.; Coffy, A.A.; Hanrot, M.Z. Benzophénanthridines isolées de Zanthoxylum psammophilum. Int. J. Biol. Chem. Sci. 2014, 8, 377–385. [Google Scholar] [CrossRef]
- Tanoh, E.A.; Nea, F.; Yapi, T.A.; Boué, G.B.; Jean-Brice, B.; Tomi, F.; Tonzibo, Z.F. Essential oil of Zanthoxylum lepreurii Guill. & Perr. rich in undecan-2-one and tridecan-2-one. J. Essent. Oil Bear. Plants 2018, 21, 1397–1402. [Google Scholar]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I.; Hutton, I. Composition of the leaf oils of the Australian and lord howe Island species of Zanthoxylum (Rutaceae). J. Essent. Oil Res. 2000, 12, 285–291. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Lawal, O.A.; Adeniyi, B.A.; Alaka, S.A.; Tetede, E. Essential oil composition of three Zanthoxylum species. J. Essent. Oil Res. 2008, 20, 69–71. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Singh, P.; Sundriyal, R.C. Chemical constituents and biological activities of the genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem. 2011, 5, 412–416. [Google Scholar]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Bisht, R.; Negi, S.K. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC. Essent. Oil Iran. J. Pharmacol. Ther. 2012, 11, 68–72. [Google Scholar]
- Park, J.; Rodríguez-Moyá, M.; Li, M.; Pichersky, E.; San, K.Y.; Gonzalez, R. Synthesis of methyl ketones by metabolically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2012, 39, 1703–1712. [Google Scholar] [CrossRef]
- Saini, M.; Wang, Z.W.; Chiang, C.; Chao, Y. Metabolic engineering of Escherichia coli for production of butyric acid. J. Agric. Food Chem. 2014, 62, 4342–4348. [Google Scholar] [CrossRef]
- Pareja, M.; Qvarfordt, E.; Webster, B.; Mayon, P.; Pickett, J.; Birkett MGlinwood, R. Herbivory by a phloem-feeding insect inhibits floral volatile production. PLoS ONE 2012, 7, e31971. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.N.; Thang, T.D. Chemical composition of the essential oil of Zanthoxylum avicennae (Lam.) DC Leaves (Rutaceae) from Vietnam. J. Essent. Oil-Bear. Plants JEOP 2012, 15, 7–11. [Google Scholar] [CrossRef]
- Setzer, W.N.; Noletto, J.A.; Lawton, R.O.; Haber, W.A. Leaf essential oil composition of five Zanthoxylum species from Monteverde, Costa Rica. Mol. Divers. 2005, 9, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Fourough, M.; Mohaddese, M.; Ebrahim, R.; Maryam, M.S. Chemical composition, antimicrobial and antioxidant activities of Echinophora platyloba essential oil. Infectio 2017, 21, 176–181. [Google Scholar]
- Rafaela, K.L.; Maria, G.C.; Milene, A.A.; Paula, L.G.; Luı´s, R.B.; David, L.N. Bactericidal and antioxidant activity of essential oils from Myristica fragrans Houtt and Salvia microphylla H.B.K. J. Am. Oil Chem. Soc. 2011, 89, 523–528. [Google Scholar]
- Pino, J.A.; Bello, A.; Urquiola, A.; García, S. Leaf oil of Xylopia aromatica (Lam.) Mart. from Cuba. J. Essent. Oil Res. 2011, 2905, 9–11. [Google Scholar]
- Lee, G.W.; Chung, M.S.; Kang, M.B.Y.; Chung, B.Y.; Lee, S. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma 2016, 253, 683–690. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Di Stefano, A.; Taspınar, N.; Sozio, P. Protective effects of cyclosativene on H2O 2-induced injury in cultured rat primary cerebral cortex cells. Cytotechnology 2015, 67, 299–309. [Google Scholar] [CrossRef]
- Kang, M.-S.; Lee, H.-S. Acaricidal and insecticidal responses of Cinnamomum cassia oils and main constituents. Appl. Biol. Chem. 2018, 61, 653–659. [Google Scholar] [CrossRef]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef]
- Wungsintaweekul, J.; Sitthithaworn, W.; Putalun, W.; Pfeifhoffer, H.W. Antimicrobial, antioxidant activities and chemical composition of selected Thai spices. J. Sci. Technol. 2010, 32, 589–598. [Google Scholar]
- Kang, O.H.; Chae, H.S.; Choi, J.G.; Oh, Y.C.; Lee, Y.S.; Kim, J.H.; Seung, M.J.; Jang, H.J.; Bae, K.H.; Lee, J.H.; et al. Ent-pimara-8(14), 15-dien-19-oic acid isolated from the roots of Aralia cordata inhibits induction of inflammatory mediators by blocking NF-κB activation and mitogen-activated protein kinase pathways. Eur. J. Pharmacol. 2008, 601, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tangjitjaroenkun, J.; Supabphol, R.; Chavasiri, W. Antioxidant effect of Zanthoxylum limonella Alston. J. Med. Plants Res. 2012, 6, 1407–1414. [Google Scholar] [CrossRef]
- Supabphol, R.; Tangjitjareonkun, J. Chemical constituents and biological activities of Zanthoxylum limonella (Rutaceae): A. Review. Trop. J. Pharm. Res. 2014, 13, 2119–2130. [Google Scholar] [CrossRef]
- Sati, S.C.; Sati, M.D.; Raturi, R.; Badoni, P.; Singh, H. Anti-Inflammatory and Antioxidant Activities of Zanthoxylum Armatum Stem Bark. Glob. J. Res. Eng. 2011, 11, 1–5. [Google Scholar]
- Asli, C.; Zehra, I.Y.; Tamer, U. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar]
- Dang, N.H.; Zhang, X.; Zheng, M.; Son, K.H.; Chang, H.W.; Kim, H.P.; Bae, K.; Kang, S.S. Inhibitory Constituents against Cyclooxygenases from Aralia cordata Thunb. Arch. Pharm. Res. 2005, 28, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−) -trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Dae-Seung, K.; Hyun-Ja, L.; Yong-Deok, J.; Yo-Han, H.; Ji-Ye, K.; Hyun-Jeong, K.; Hyun-Ji, S.; JongWook, K.; Beom Su, L.; Sung-Hoon, K. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar]
- Jing, C.; Wenqing, W.; Chunyang, S.; Jianguo, F. A Comparative Study of Sodium Houttuyfonate and 2-Undecanone for Their in Vitro and in Vivo Anti-Inflammatory Activities and Stabilities. Int. J. Mol. Sci. 2014, 15, 22978–22994. [Google Scholar]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of Antioxidant Capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb Rebey, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni Selami, I.; Saidani Tounsi, M.; Marzouk, B.; Fauconnier, M.-L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst. 2018, 152, 971–978. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. 2011, 40. [Google Scholar] [CrossRef]
- Benini, C.; Ringuet, M.; Wathelet, J.P.; Lognay, G.; Du Jardin, P.; Fauconnier, M.-L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2012, 27, 356–366. [Google Scholar]
- Sidali, L.; Brada, M.; Fauconnier, M.-L.; Lognay, G. Chemical composition and antioxidant activity of Thymus fontanesii essential oil from Algeria. J. Nat. Prod. 2018, 1–10. [Google Scholar] [CrossRef]
- Jamali, C.A.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Phenological changes to the chemical composition and biological activity of the essential oil from Moroccan endemic thyme (Thymus maroccanus Ball). Ind. Crops Prod. 2013, 49, 366–372. [Google Scholar] [CrossRef]
- Soilhi, Z.; Rhimi, A.; Heuskin, S.; Fauconnier, M.-L.; Mekki, M. Essential oil chemical diversity of Tunisian Mentha scollection. Ind. Crops Prod. 2019, 131, 330–340. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. IJRASET 2015, 3, 2321–9653. [Google Scholar]
- Liu, C.M.; Perng, M.H.; Chen, C.Y. Antioxidant activities of crude extracts from peel and seed of Cinnamomum camphora. Biomed. Res. 2018, 29, 2854–2858. [Google Scholar]
- Lee, J.H.; Kang, B.S.; Hwang, K.H.; Kim, G.H. Evaluation for anti-inflammatory effects of Siegesbeckia glabrescens extract in vitro. Food Agric. Immunol. 2011, 22, 145–160. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chang, W.H.; Chen, C.S.; Liao, J.W.; Huang, C.J.; Lu, F.J.; Chia, Y.C.; Hsu, H.K.; Wu, J.J.; Yang, H.L. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008, 46, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chang, C.T.; Chao, W.W.; Lin, C.F.; Chou, S.T. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Nikhila, G.S.; Sangeetha, G.; Swapna, T.S. Anti-inflammatory properties of the root tubers of Gloriosa superba and its conservation through micropropagation. J. Med. Plant Res. 2014, 9, 1–7. [Google Scholar]
- Lee, J.H.; Chang, K.M.; Kim, G.H. Composition and anti-inflammatory activities of Zanthoxylum schinifolium essential oil: Suppression of inducible nitric oxide synthase, cyclooxygenase-2, cytokines and cellular adhesion. J. Sci. Food Agric. 2009, 89, 1762–1769. [Google Scholar] [CrossRef]
- Odukoya, O.A.; Houghton, P.J.; Raman, A. Lipoxygenase Inhibitors in the seeds of Aframomum danielli K. Schum (Zingiberaceae). Phytomedicine 1999, 6, 251–256. [Google Scholar] [CrossRef]
- Aghraz, A.; Benameur, Q.; Gervasi, T.; Ait Dra, L.; Ben-Mahdi, M.H.; Larhsini, M.; Markouk, M.; Cicero, N. Antibacterial activity of Cladanthus arabicus and Bubonium imbricatum essential oils alone and in combination with conventional antibiotics against Enterobacteriaceae isolates. Lett. Appl. Microbiol. 2018, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Aghraz, A.; Albergamo, A.; Benameur, Q.; Salvo, A.; Larhsini, M.; Markouk, M.; Gervasi, T.; Cicero, N. Polyphenols contents, heavy metals analysis and in vitro antibacterial activity of extracts from Cladanthus arabicus and Bubonium imbricatum of Moroccan Origin. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of essential oils are available from the authors in very small amount. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).