Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration
Abstract
1. Introduction
2. Results and Discussion
2.1. Overt Toxicity Associated with CRCE/APAP Administration
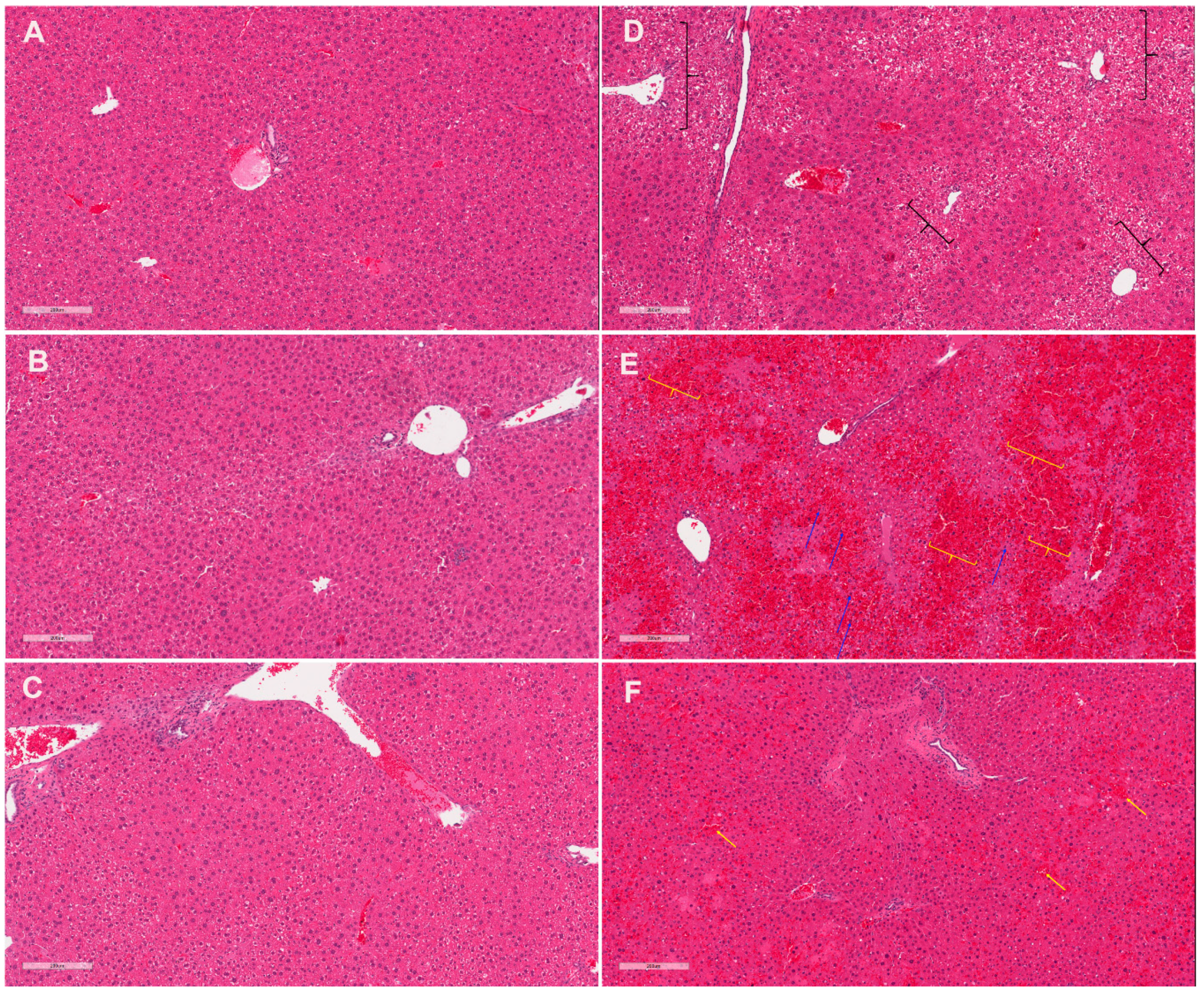
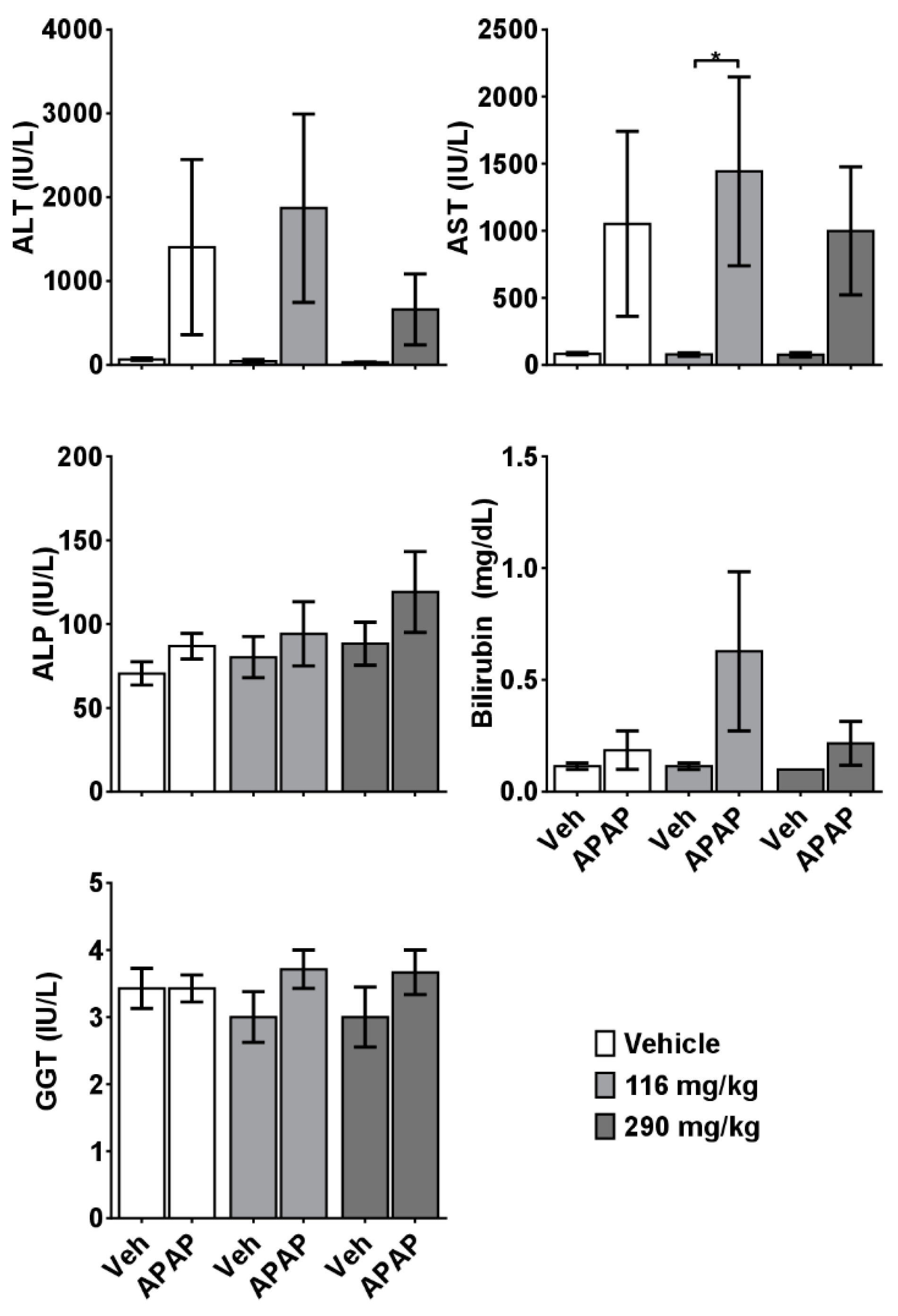
2.2. CRCE/APAP Cause Acute Liver Injury
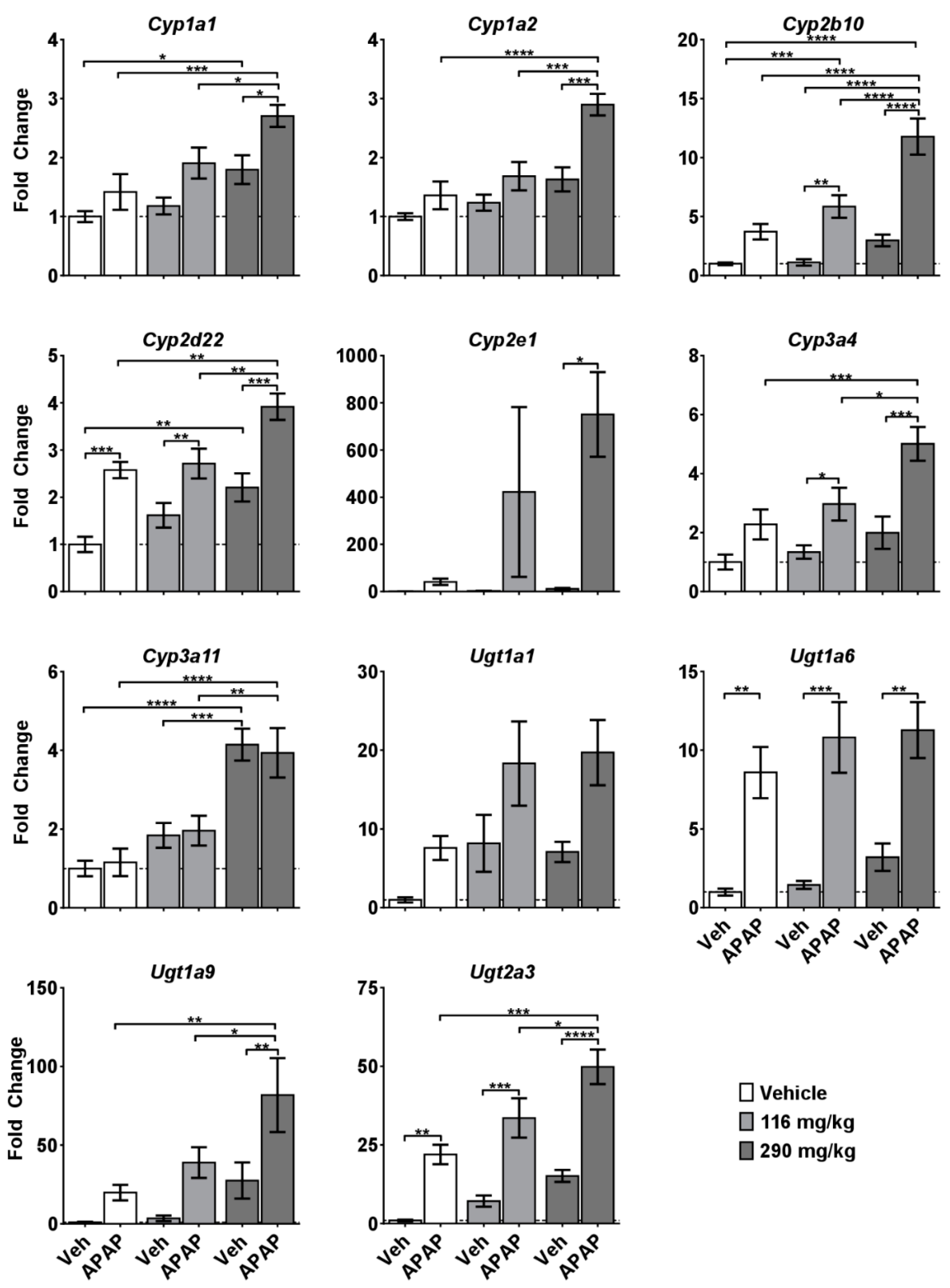
2.3. Molecular Alterations Associated with CRCE/APAP
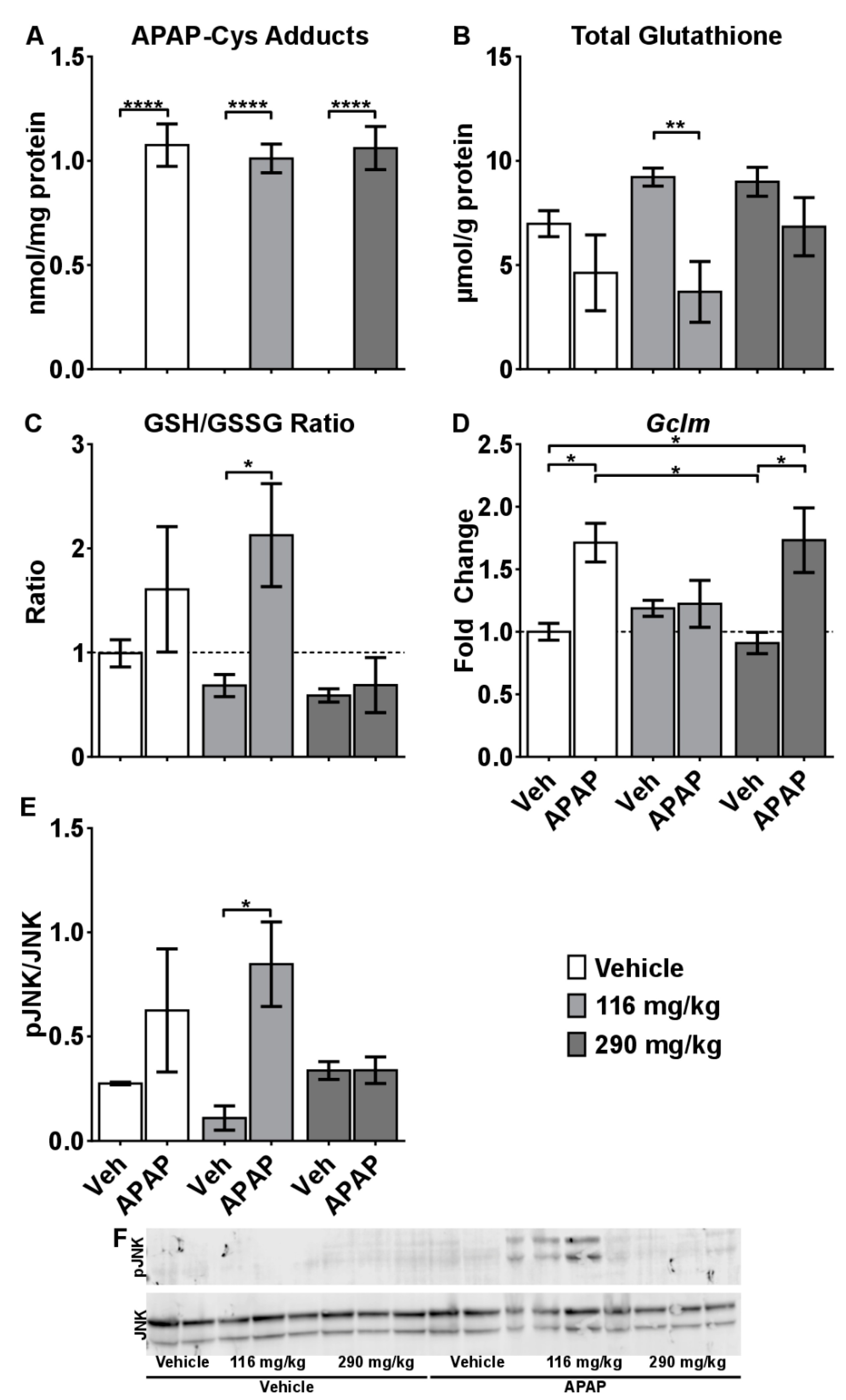
2.4. Mechanisms of CRCE/APAP-Induced Liver Injury
3. Conclusions
4. Materials and Methods
4.1. CBD Extract Characterization, Dosing Solution and Dose Calculations
4.2. Animals
4.3. Blood Sampling and Clinical Biochemistry
4.4. Histopathological Assessment
4.5. Analysis of mRNA Levels of Major Cytochromes and Transporter Genes
4.6. Analysis of the APAP Protein Adducts in the Liver Tissue
4.7. Glutathione Analysis
4.8. Western Blot
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devinsky, O.; Cross, J.H.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 377, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Pina, S.D.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, H.; Fleischman, R.W.; Grant, R.J. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol. Appl. Pharmacol. 1981, 58, 118–131. [Google Scholar] [CrossRef]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef] [PubMed]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Lichti, C.F.; Prather, P.L.; Zielinska, A.K.; Bratton, S.M.; Gallus-Zawada, A.; Finel, M.; Miller, G.P.; Radominska-Pandya, A.; Moran, J.H. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab. Dispos. 2009, 37, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Stout, S.M.; Cimino, N.M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef]
- Zendulka, O.; Dovrtelova, G.; Noskova, K.; Turjap, M.; Sulcova, A.; Hanus, L.; Jurica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2009, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.W.; Kelly, J.P.; Rosenberg, L.; Anderson, T.E.; Mitchell, A.A. Recent patterns of medication use in the ambulatory adult population of the United States: The Slone survey. JAMA 2002, 287, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Skinner, C.M.; Lin, H.; Ewing, L.E.; Kosanke, S.D.; Williams, D.K.; Avula, B.; Khan, I.A.; ElSohly, M.A.; Gurley, B.J.; et al. Safety assessment of the dietary supplement OxyELITE Pro (New Formula) in inbred and outbred mouse strains. Food Chem. Toxicol. 2017, 109, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.M.; Miousse, I.R.; Ewing, L.E.; Sridharan, V.; Cao, M.; Lin, H.; Williams, D.K.; Avula, B.; Haider, S.; Chittiboyina, A.G.; et al. Impact of obesity on the toxicity of a multi-ingredient dietary supplement, OxyELITE Pro (New Formula), using the novel NZO/HILtJ obese mouse model: Physiological and mechanistic assessments. Food Chem. Toxicol. 2018, 122, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Seo, J.E.; Wang, S.; Ashby, K.; Ballard, R.; Yu, D.; Ning, B.; Agarwal, R.; Borlak, J.; Tong, W.; et al. The Development of a Database for Herbal and Dietary Supplement Induced Liver Toxicity. Int. J. Mol. Sci. 2018, 19, 2955. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar] [PubMed]
- James, L.P.; McCullough, S.S.; Lamps, L.W.; Hinson, J.A. Effect of N-acetylcysteine on acetaminophen toxicity in mice: Relationship to reactive nitrogen and cytokine formation. Toxicol. Sci. 2003, 75, 458–467. [Google Scholar] [CrossRef]
- Fan, C.Q.; Crawford, J.M. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J. Clin. Exp. Hepatol. 2014, 4, 332–346. [Google Scholar] [CrossRef]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Wojcikowski, K.; Gobe, G. Animal studies on medicinal herbs: Predictability, dose conversion and potential value. Phytother. Res. 2014, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Muldrew, K.L.; James, L.P.; Coop, L.; McCullough, S.S.; Hendrickson, H.P.; Hinson, J.A.; Mayeux, P.R. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab. Dispos. 2002, 30, 446–451. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Lebofsky, M.; Norris, H.R.; Slawson, M.H.; Bajt, M.L.; Xie, Y.; Williams, C.D.; Wilkins, D.G.; Rollins, D.E.; Jaeschke, H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: Dose-response, mechanisms, and clinical implications. Toxicol. Appl. Pharmacol. 2013, 269, 240–249. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol. Mech. Methods 2015, 25, 589–595. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Du, K.; Xie, Y.; Bajt, M.L.; Ding, W.X.; Jaeschke, H. The role of the c-Jun N-terminal kinases 1/2 and receptor-interacting protein kinase 3 in furosemide-induced liver injury. Xenobiotica 2015, 45, 442–449. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available. |
| Vehicle (n = 7) | CBD, 116 mg/kg (n = 7) | CBD, 290 mg/kg (n = 8) | APAP (400 mg/kg) (n = 7) | CBD, 116 mg/kg + APAP (n = 8) | CBD, 290mg/kg + APAP (n = 8) | |
|---|---|---|---|---|---|---|
| Sinusoidal dilation | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.9 ± 0.4 | 2.0 ± 0.5 * | 0.8 ± 0.5 |
| Venous obstruction | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Atrophy | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 | 0.6 ± 0.2 | 0.3 ± 0.2 |
| Apoptosis | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Necrosis | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.6 | 2.0 ± 0.6 * | 0.8 ± 0.4 |
| Microvesicular steatosis | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Small droplet steatosis | 1.0 ± 0.3 | 0.7 ± 0.4 | 0.6 ± 0.3 | 0.3 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3 |
| Large droplet steatosis | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Clear cell changes | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.8 ± 0.3 | 1.4 ± 0.4 | 0.6 ± 0.4 | 1.3 ± 0.5 |
| Portal inflammation | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ±0.0 | 0.5 ± 0.4 | 0.0 ± 0.0 |
| Lobular inflammation | 0.9 ± 0.2 | 1.2 ± 0.3 | 0.8 ± 0.2 | 0.6 ± 0.2 | 1.4 ± 0.6 | 0.8 ± 0.2 |
| Interface activity | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.4 | 0.0 ± 0.0 |
| Row | Column | Correlation | p-Value |
|---|---|---|---|
| Liver-to-body weight ratio | Bilirubin | 0.37 | 0.0210 |
| Liver-to-body weight ratio | Total GSH | −0.17 | 0.3897 |
| Liver-to-body weight ratio | GSSG/GSH | 0.28 | 0.1465 |
| Liver-to-body weight ratio | Sinusoidal dilation | 0.42 | 0.0065 |
| Liver-to-body weight ratio | Necrosis | 0.56 | 0.0002 |
| Bilirubin | Total GSH | −0.49 | 0.0043 |
| Bilirubin | GSSG/GSH | 0.48 | 0.0054 |
| Bilirubin | Sinusoidal dilation | 0.61 | <0.0001 |
| Bilirubin | Necrosis | 0.67 | <0.0001 |
| Total GSH | GSSG/GSH | −0.69 | <0.0001 |
| Total GSH | Sinusoidal dilation | −0.75 | <0.0001 |
| Total GSH | Necrosis | −0.70 | <0.0001 |
| GSSG/GSH | Sinusoidal dilation | 0.74 | <0.0001 |
| GSSG/GSH | Necrosis | 0.71 | <0.0001 |
| Sinusoidal dilation | Necrosis | 0.86 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewing, L.E.; McGill, M.R.; Yee, E.U.; Quick, C.M.; Skinner, C.M.; Kennon-McGill, S.; Clemens, M.; Vazquez, J.H.; McCullough, S.S.; Williams, D.K.; et al. Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration. Molecules 2019, 24, 2256. https://doi.org/10.3390/molecules24122256
Ewing LE, McGill MR, Yee EU, Quick CM, Skinner CM, Kennon-McGill S, Clemens M, Vazquez JH, McCullough SS, Williams DK, et al. Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration. Molecules. 2019; 24(12):2256. https://doi.org/10.3390/molecules24122256
Chicago/Turabian StyleEwing, Laura E., Mitchell R. McGill, Eric U. Yee, Charles M. Quick, Charles M. Skinner, Stefanie Kennon-McGill, Melissa Clemens, Joel H. Vazquez, Sandra S. McCullough, D. Keith Williams, and et al. 2019. "Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration" Molecules 24, no. 12: 2256. https://doi.org/10.3390/molecules24122256
APA StyleEwing, L. E., McGill, M. R., Yee, E. U., Quick, C. M., Skinner, C. M., Kennon-McGill, S., Clemens, M., Vazquez, J. H., McCullough, S. S., Williams, D. K., Kutanzi, K. R., Walker, L. A., ElSohly, M. A., James, L. P., Gurley, B. J., & Koturbash, I. (2019). Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration. Molecules, 24(12), 2256. https://doi.org/10.3390/molecules24122256





