Avocado Oil: Characteristics, Properties, and Applications
Abstract
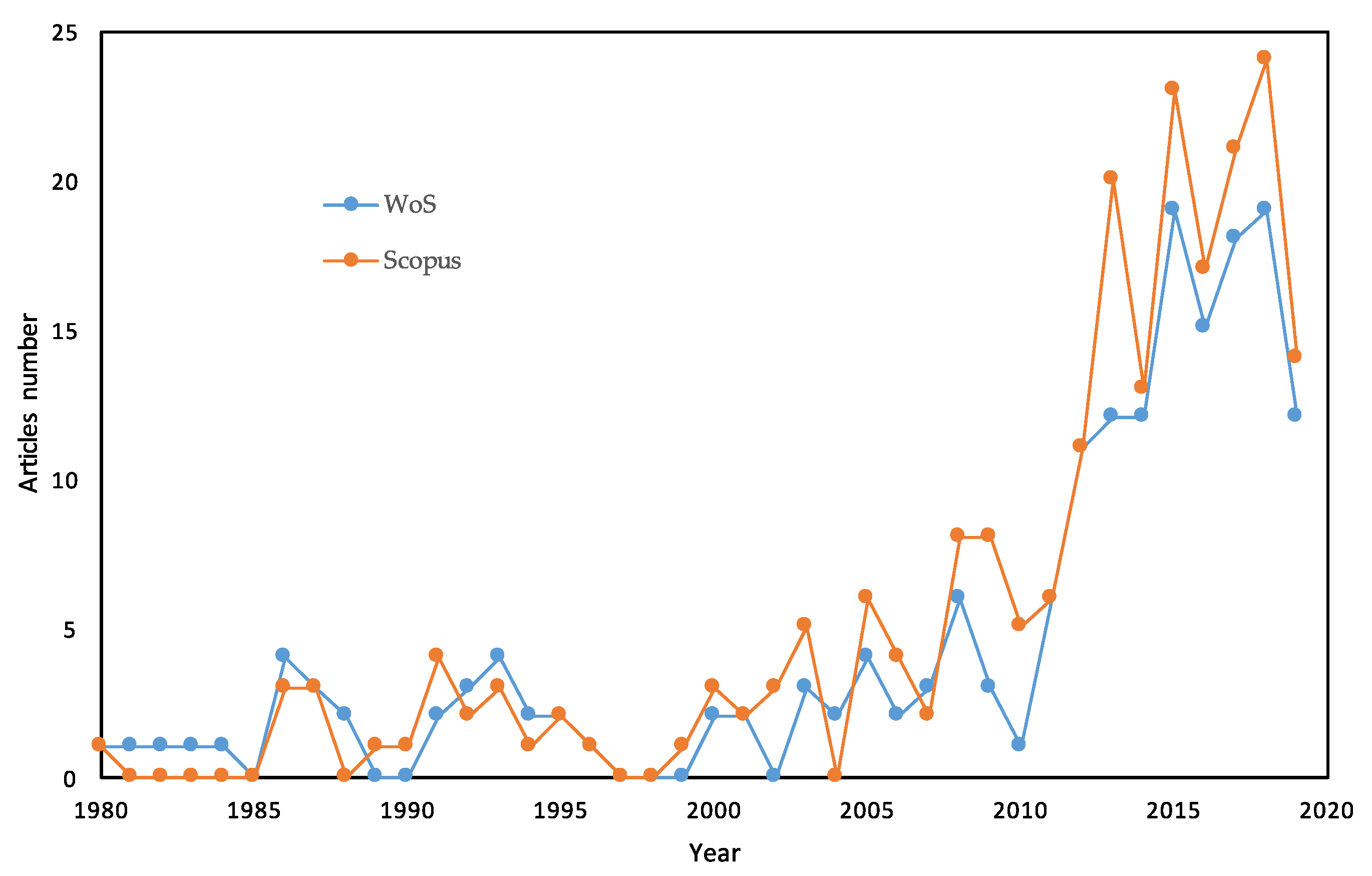
1. Introduction
2. Extraction Methods for Avocado Oil
2.1. Cold Pressed Method
2.2. Ultrasound-Assisted Aqueous Extraction Method (UAAE)
2.3. Supercritical CO2 Method
2.4. CO2 Subcritical Method
2.5. Enzymatic Extraction
2.6. Solvent Extraction
3. Procedures for the Conservation of Avocado Oil
4. Use of Analytical Techniques in the Quantification, Adulteration, and Contamination of Avocado Oil
5. Technological Applications of Avocado Oil
6. Composition of Avocado Oil
6.1. Characteristics According to the Variety and Origin of the Fruit
6.2. Physicochemical Characterization
6.3. Avocado Seed Oil
6.4. Comparison with Other Oils
7. Biological Effects
7.1. Human Health Effects
7.2. Experimental Studies in Animals
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UAAE | Ultrasound-assisted aqueous extraction |
| sCO2 | Subcritical CO2 |
| scCO2 | Supercritical CO2 |
| LPG | Liquefied gas of compressed oil |
| FFA | Free fatty acid |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
| SFA | Saturated fatty acid |
| TAG | Triacylglycerol |
| O | Oleic acid |
| P | Palmitic acid |
| L | Linoleic acid |
| COX | Cyclooxygenase enzyme |
| PCR | C reactive protein |
| FTIR | Infrared spectroscopy with Fourier transform |
| IOC | International olive council |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| APMAE | Atmospheric pressure microwave-assisted liquid–liquid extraction |
| PHAs | Polyhydroxyalkanoates |
| ECN48 | 48 equivalents of carbon atoms |
| VLDL | Very low-density lipoprotein |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
References
- Tan, C.; Tan, S.; Tan, S. Influence of Geographical Origins on the Physicochemical Properties of Hass Avocado Oil. J. Am. Oil Chem. Soc. 2017, 94, 1431–1437. [Google Scholar] [CrossRef]
- Wong, M.; Requejo-Jackman, C.; Woolf, A. What is unrefined, extra virgin cold-pressed avocado oil? Inform 2010, 21, 189–260. [Google Scholar]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chem. 2012, 1, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Zarrabal, O.; Nolasco-Hipolito, C.; Aguilar-Uscanga, M.; Melo-Santiesteban, G.; Hayward-Jones, P.; Barradas-Dermitz, D. Avocado oil supplementation modifies cardiovascular risk profile markers in a rat model of sucrose-induced metabolic changes. Dis. Markers 2014, 2014, 386–425. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Zarrabal, O.; Nolasco-Hipolito, C.; Aguilar-Uscanga, M.; Melo-Santiesteban, G.; Hayward-Jones, P.; Barradas-Dermitz, D. Effect of dietary intake of avocado oil and olive oil on biochemical markers of liver function in sucrose-fed rats. Biomed Res. Int. 2014, 2014, 595479. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Indelicato, S.; Massenti, R.; Lo Bianco, R. Quantitative evaluation of the phenolic profile in fruits of six avocado (Persea americana) cultivars by ultra-high-performance liquid chromatography-heated electrospray-mass spectrometry. Int. J. Food Prop. 2017, 20, 1302–1312. [Google Scholar] [CrossRef]
- Woolf, A.; Wong, M.; Eyres, L.; McGhie, T.; Lund, C.; Olsson, S.; Wang, Y.; Bulley, C.; Wang, M.; Friel, E.; et al. Avocado oil. From cosmetic to culinary oil. In Gourmet and Health-Promoting Specialty Oils; Moreau, R., Kamal-Eldin, A., Eds.; AOCS Press: Urbana, IL, USA, 2009; pp. 73–125. [Google Scholar]
- NM. 2008. Norma Mexicana NMX-F-052- SCFI-2008. Aceites y grasas-aceite de aguacate - especificaciones. Available online: http://aniame.com/mx/wp-content/uploads/Normatividad/CTNNIAGS/NMX-F-052-SCFI-2008.pdf (accessed on 7 June 2019).
- Costagli, G.; Betti, M. Avocado oil extraction processes: Method for cold-pressed high-quality edible oil production versus traditional production. J. Agric. Eng. 2015, 46, 115–122. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonça, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef]
- Santana, I.; dos Reis, L.; Torres, A.; Cabral, L.; Freitas, S. Avocado (Persea americana Mill.) oil produced by microwave drying and expeller pressing exhibits low acidity and high oxidative stability. Eur. J. Lipid Sci. Technol. 2015, 117, 999–1007. [Google Scholar] [CrossRef]
- Chimsook, T.; Assawarachan, R. Effect of Drying Methods on Yield and Quality of the Avocado Oil. Adv. Mater. Res. Key Eng. Mater. 2017, 735, 127–131. [Google Scholar] [CrossRef]
- Dos Santos, M.; Alicieo, T.V.; Pereira, C.M.; Ramis-Ramos, G.; Mendonça, C.R. Profile of bioactive compounds in avocado pulp oil: Influence of the drying processes and extraction methods. J. Am. Oil Chem. Soc. 2014, 91, 19–27. [Google Scholar] [CrossRef]
- Norma para grasas y aceites comestibles no regulados por normas individuales. Available online: http://URL www.fao.org/input/download/standards/74/CXS_019s_2015.pdf (accessed on 16 April 2019).
- Yang, S.; Hallett, I.; Rebstock, R.; Oh, H.; Kam, R.; Woolf, A.; Wong, M. Cellular Changes in “Hass” Avocado Mesocarp During Cold-Pressed Oil Extraction. J. Am. Oil Chem. Soc. 2018, 95, 229–238. [Google Scholar] [CrossRef]
- Xuan, T.; Hean, C.; Hamzah, H.; Ghazali, H. Optimization of ultrasound-assisted aqueous extraction to produce virgin avocado oil with low free fatty acids. J. Food Process Eng. 2017, 12656, 1–9. [Google Scholar]
- Martínez-Padilla, L.P.; Franke, L.; Xu, X.Q.; Juliano, P. Improved extraction of avocado oil by application of sono-physical processes. Ultrason. Sonochemistry 2018, 40, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Unda, K.; Saavedra, A. Numerical Simulation on Supercritical CO2 Fluid Dynamics in a Hollow Fiber Membrane Contactor. Computation 2019, 7, 8. [Google Scholar] [CrossRef]
- Corzzini, S.; Barros, H.; Grimaldi, R.; Cabral, F. Extraction of edible avocado oil using supercritical CO2 and a CO2/ethanol mixture as solvents. J. Food Eng. 2017, 194, 40–45. [Google Scholar] [CrossRef]
- Barros, H.D.; Coutinho, J.P.; Grimaldi, R.; Godoy, H.T.; Cabral, F.A. Simultaneous extraction of edible oil from avocado and capsanthin from red bell pepper using supercritical carbon dioxide as solvent. J. Supercrit. Fluids 2016, 107, 315–320. [Google Scholar] [CrossRef]
- Barros, H.; Grimaldi, R.; Cabral, F. Lycopene-rich avocado oil obtained by simultaneous supercritical extraction from avocado pulp and tomato pomace. J. Supercrit. Fluids 2017, 120, 1–6. [Google Scholar] [CrossRef]
- Restrepo, A.M.; Londoño, J.; González, D.; Benavides, Y.; Cardona, B.L. Comparación del aceite de aguacate variedad Hass cultivado en Colombia, obtenido por fluidos supercríticos y métodos convencionales: Una perspectiva desde la calidad. Rev. Lasallista Investig. 2012, 9, 151–161. [Google Scholar]
- Abaide, E.; Zabot, G.; Tres, M.; Martins, R.; Fagundez, J.; Nunes, L.; Druzian, S.; Soares, J.; Dal Prá, V.; Silva, J.; et al. Yield composition, and antioxidant activity of avocado pulp oil extracted by pressurized fluids. Food Bioprod. Process. 2017, 102, 289–298. [Google Scholar] [CrossRef]
- Xuan, T.; Hean, C.; Hamzah, H.; Ghazali, H. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. J. Supercrit. Fluids 2018, 135, 45–51. [Google Scholar]
- AOAC. Official methods of analysis of AOAC international, 18th ed.; Horwitz, W., Latimer, G., Eds.; AOAC International: Maryland, MD, USA, 2007. [Google Scholar]
- Buenrostro, M.; López-Munguia, A.C. Enzymatic extraction of avocado oil. Biotechnol. Lett. 1986, 8, 505–506. [Google Scholar] [CrossRef]
- Reddy, M.; Moodley, R.; Jonnalagadda, S.B. Fatty acid profile and elemental content of avocado (Persea americana Mill.) oil-effect of extraction methods. J. Environ. Sci. Health B 2012, 47, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Terry, L.A. Development of a rapid method for the sequential extraction and subsequent quantification of fatty acids and sugars from avocado mesocarp tissue. J. Agric. Food Chem. 2008, 56, 7439–7445. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Morris, R.; O’Brien, K. The oil content of avocado mesocarp. J. Sci. Food Agric. 1978, 29, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Moreno, A.; Dorantes, L.; Galíndez, J.; Guzmán, R.I. Effect of different extraction methods on fatty acids, volatile compounds, and physical and chemical properties of avocado (Persea americana Mill.) oil. J. Agric. Food Chem. 2003, 51, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Mostert, M.E.; Botha, B.M.; Du Plessis, L.M.; Duodu, K.G. Effect of fruit ripeness and method of fruit drying on the extractability of avocado oil with hexane and supercritical carbon dioxide. J. Sci. Food Agric. 2007, 87, 2880–2885. [Google Scholar] [CrossRef]
- Bizimana, V.; Breene, W.M.; Csallany, A.S. Avocado oil extraction with appropriate technology for developing countries. J. Am. Oil Chem. Soc. 1993, 70, 821–822. [Google Scholar] [CrossRef]
- Ariza-Ortega, J.A.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Ramos-Cassellis, M.E.; Castañeda-Antonio, D.; Betanzos-Cabrera, G. Effect of electric field on the characteristics of crude avocado oil and virgin olive. J. Food Sci. Technol. 2017, 54, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Ortega, J.; Mendez-Ramos, M.; Diaz-Reyes, J.; Delgado-Macuil, R.; De-la-Torre, R. Study by Fourier Transform Infrared Spectroscopy of the Avocado Oils of the Varieties Hass, Criollo and Fuerte. J. Mater. Sci. Eng. 2010, 4, 61–64. [Google Scholar]
- Cerecedo-Cruz, L.; Azuara-Nieto, E.; Hernández-Álvarez, A.J.; González-González, C.R.; Melgar-Lalanne, G. Evaluation of the oxidative stability of Chipotle chili (Capsicum annuum L.) oleoresins in avocado oil. Grasas Aceites 2018, 69, 240. [Google Scholar] [CrossRef]
- Cicero, N.; Albergamo, A.; Salvo, A.; Bua, G.; Bartolomeo, G.; Mangano, V.; Rotondo, A.; Di Stefano, V.; Di Bella, G.; Dugo, G. Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market. Food Res. Int. 2018, 109, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Retief, L.; McKenzieb, JM.; Koch, K. A novel approach to the rapid assignment of 13C NMR spectra of major components of vegetable oils such as avocado, mango kernel and macadamia nut oils. Magn. Reson. Chem. 2009, 47, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Fernándes, G.D.; Gómez-Coca, R.B.; Pérez-Camino, M.C.; Moreda, W.; Barrera-Arellano, D. Chemical characterization of commercial and single-variety avocado oils. Grasas Aceites 2018, 69, 256. [Google Scholar] [CrossRef]
- Castejón, D.; Mateos-Aparicio, I.; Molero, M.D.; Cambero, MI.; Herrera, A. Evaluation and Optimization of the Analysis of Fatty Acid Types in Edible Oils by 1 H-NMR. Food Anal. Methods 2014, 7, 1285–1297. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A.; Riyanto, S.; Sudjadi, S.A.S.A.; Rosman, A.S.; Yusoff, F.M. Fourier transform infrared spectroscopy combined with multivariate calibrations for the authentication of avocado oil. Int. J. Food Prop. 2016, 19, 680–687. [Google Scholar] [CrossRef]
- Rohman, A.; Lumakso, F.A.; Riyanto, S. Use of partial least square-discriminant analysis combined with mid infrared spectroscopy for avocado oil authentication. J. Med. Plants Res. 2016, 10, 175–180. [Google Scholar] [CrossRef]
- Fuentes, E.; Báez, M.E.; Díaz, J. Microwave-assisted extraction at atmospheric pressure coupled to different clean-up methods for the determination of organophosphorus pesticides in olive and avocado oil. J. Chromatogr. A 2009, 1216, 8859–8866. [Google Scholar] [CrossRef]
- Arancibia, C.; Riquelme, N.; Zúñiga, R.; Matiacevicha, S. Comparing the effectiveness of natural and synthetic emulsifiers on oxidative and physical stability of avocado oil-based nanoemulsions. Innov. Food Sci. Emerg. Technol. 2017, 44, 159–166. [Google Scholar] [CrossRef]
- Caballero, E.; Soto, C.; Olivares, A.; Altamirano, C. Potential use of avocado oil on structured lipids MLM-type production catalysed by commercial immobilised lipases. PloS ONE 2014, 9, E107749. [Google Scholar] [CrossRef]
- Flores-Sánchez, A.; López-Cuellar, M.; Pérez-Guevara, F.; Figueroa López, U.; Martín-Bufájer, J.M.; Vergara-Porras, B. Synthesis of Poly-(R-hydroxyalkanoates) by Cupriavidus necator ATCC 17699 Using Mexican Avocado (Persea americana) Oil as a Carbon Source. Int. J. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Flores, M.; Vergara, C.E.; Guzman, L. Thermal analysis and antioxidant activity of oil extracted from pulp of ripe avocados. J. Therm. Anal. Calorim. 2017, 130, 959–966. [Google Scholar] [CrossRef]
- Galvão, M.D.S.; Narain, N.; Nigam, N. Influence of different cultivars on oil quality and chemical characteristics of avocado fruit. Food Sci. Technol. 2014, 34, 539–546. [Google Scholar] [CrossRef]
- Espinosa-Alonso, L.G.; Paredes-López, O.; Valdez-Morales, M.; Oomah, B.D. Avocado oil characteristics of Mexican creole genotypes. Eur. J. Lipid Sci. Technol. 2017, 119, 1600406. [Google Scholar] [CrossRef]
- Yanty, N.A.M.; Marikkar, J.M.N.; Long, K. Effect of varietal differences on composition and thermal characteristics of avocado oil. J. Am. Oil Chem. Soc. 2011, 88, 1997–2003. [Google Scholar] [CrossRef]
- Dreher, M.; Davenport, A. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Martínez-Nieto, L.; Barranco-Barranco, R.; Moreno-Romero, M.V. Extracción de aceite de aguacate: Un experimento industrial. Grasas Aceites 1992, 43, 11–15. [Google Scholar] [CrossRef]
- Flores, M.A.; Perez-Camino, M.C.; Troca, J. Preliminary Studies on Composition, Quality and Oxidative Stability of Commercial Avocado Oil Produced in Chile. J. Food Sci. Eng. 2014, 4, 21–26. [Google Scholar]
- Pedreschi, R.; Hollak, S.; Harkema, H.; Otma, E.; Robledo, P.; Westra, E.; Defilippi, B.G. Impact of postharvest ripening strategies on ‘Hass’ avocado fatty acid profiles. South Afr. J. Bot. 2016, 103, 32–35. [Google Scholar] [CrossRef]
- Oliveira, A.; De Souza, E.; Rodrigues, R.; Cordeiro, D.; Gonçalves, R.; Ferreira, C.; Rodrigues, A.; De Medeiros, P.; Gonçalves, T.; Da Silva, A.; et al. Effect of Semisolid Formulation of Persea Americana Mill (avocado) Oil on Wound Healing in Rats. Evid. -Based Complement. Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Bora, P.S.; Narain, N.; Rocha, R.V.; Paulo, M.Q. Characterization of the oils from the pulp and seeds of avocado (cultivar: Fuerte) fruits. Grasas Aceites 2001, 52, 171–174. [Google Scholar]
- Martinez-Nieto, L.; Moreno-Romero, M.V. Evolución del contenido de ácidos grasos de aceite de aguacate durante la maduración. Grasas Aceites 1995, 46, 92–95. [Google Scholar] [CrossRef]
- Martinez-Nieto, L.; Moreno-Romero, M.V. Sterolic composition of the unsaponifiable fraction of oil of avocado of several varieties. Grasas Aceites 1994, 45, 402–403. [Google Scholar]
- Indriyani, L.; Rohman, A.; Riyanto, S. Physico-chemical characterization of avocado (Persea americana Mill.) oil from three Indonesian avocado cultivars. Res. J. Med. Plants 2016, 10, 67–78. [Google Scholar]
- Barrera-López, R.E.; Arrubla-Vélez, J.P. Análisis de Fitoesteroles en la Semilla de Persea americana Miller (var. Lorena) por Cromatografía de Gases y Cromatografía Líquida de Alta Eficiencia. Rev. Fac. Cienc. Básicas 2017, 13, 35–41. [Google Scholar]
- Segovia, F.J.; Corral-Pérez, J.J.; Almajano, M.P. Avocado seed: Modeling extraction of bioactive compounds. Ind. Crop. Prod. 2016, 85, 213–220. [Google Scholar] [CrossRef]
- Rengifo, P.G.; Carhuapoma, M.; Artica, L.; Castro, A.J.; López, S. Caracterización y actividad antioxidante del aceite de semilla de palta Persea americana MILL. Cienc. E Investig. 2015, 18, 33–36. [Google Scholar]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Furlan, C.P.B.; Valle, S.C.; Östman, E.; Maróstica, M.R.; Tovar, J. Inclusion of Hass avocado-oil improves postprandial metabolic responses to a hypercaloric-hyperlipidic meal in overweight subjects. J. Funct. Foods 2017, 38, 349–354. [Google Scholar] [CrossRef]
- Stucker, M.; Memmel, U.; Hoffmann, M.; Hartung, J.; Altmeyer, P. Vitamin B12 cream containing avocado oil in the therapy of plaque psoriasis. Dermatology 2001, 203, 141–147. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Gallegos-Corona, M.; Sánchez-Briones, L.; Calderón-Cortés, E.; Montoya-Pérez, R.; Rodriguez-Orozco, A.; Campos-García, J.; Saavedra-Molina, A.; Mejía-Zepeda, R.; Cortés-Rojo, C. Protective effects of dietary avocado oil on impaired electron transport chain function and exacerbated oxidative stress in liver mitochondria from diabetic rats. J. Bioenerg. Biomembr. 2015, 47, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Avila, O.; Esquivel-Martínez, M.; Olmos-Orizaba, B.; Saavedra-Molina, A.; Rodriguez-Orozco, A.; Cortés-Rojo, C. Avocado Oil Improves Mitochondrial Function and Decreases Oxidative Stress in Brain of Diabetic Rats. J. Diabetes Res. 2015, 2015, 485759. [Google Scholar] [CrossRef]
- Ortiz-Ávila, O.; Sámano-García, C.; Calderón-Cortés, E.; Pérez-Hernández, I.; Mejía-Zepeda, R.; Rodríguez-Orozco, A.; Saavedra-Molina, A.; Cortés-Rojo, C. Dietary avocado oil supplementation attenuates the alterations induced by type I diabetes and oxidative stress in electron transfer at the complex II-complex III segment of the electron transport chain in rat kidney mitochondria. J. Bioenerg. Biomembr. 2013, 45, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Wermam, M.J.; Mokady, S.; Neeman, I. Effect of dietary avocado oils on hepatic collagen metabolism. Ann. Nutr. Metab. 1991, 35, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Werman, M.; Mokady, S.; Neeman, I.; Auslaender, L.; Zeidler, A. The effect of avocado oils on some liver characteristics in growing rats. Food Chem. Toxicol. 1989, 27, 279–282. [Google Scholar] [CrossRef]
- Wermam, M.J.; Mokady, S.; Nimni, M.E.; Neeman, I. The effect of various avocado oils on skin collagen metabolism. Connect. Tissue Res. 1990, 26, 1–10. [Google Scholar] [CrossRef]
- Lamaud, E.; Huc, A.; Wepierre, J. Effects of avocado and soya bean lipidic non-saponifiables on the components of skin connective tissue after topical application in the hairless rat: Biophysical and biomechanical determination. Int. J. Cosmet. Sci. 1982, 4, 143–152. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.; Wright, S.; Czarnecki, S.; Wilson, T.; Nicolosi, R. Cholesterol vehicle in experimental atherosclerosis 24: Avocado oil. J. Am. Coll. Nutr. 2003, 22, 52–55. [Google Scholar] [CrossRef]
- Salazar, M.J.; El Hafidi, M.; Pastelin, G.; Ramírez-Ortega, M.C.; Sánchez-Mendoza, M.A. Effect of an avocado oil-rich diet over an angiotensin II-induced blood pressure response. J. Ethnopharmacol. 2005, 98, 335–338. [Google Scholar] [CrossRef]
- Márquez-Ramírez, C.; Hernández de la Paz, J.; Ortiz-Avila, O.; Raya-Farias, A.; González-Hernández, J.; Rodríguez-Orozco, A.; Salgado-Garciglia, R.; Saavedra-Molina, A.; Godínez-Hernández, D.; Cortés-Rojo, C. Comparative effects of avocado oil and losartan on blood pressure, renal vascular function, and mitochondrial oxidative stress in hypertensive rats. Nutrition 2018, 54, 60–67. [Google Scholar]
- Del Toro-Equihua, M.; Velasco-Rodríguez, R.; López-Ascencio, R.; Vásquez, C. Effect of an avocado oil-enhanced diet (Persea americana) on sucrose-induced insulin resistance in Wistar rats. J. Food Drug Anal. 2016, 24, 350–357. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Abboud, R.; Alves-Pereira, V.; Soares da Costa, C.A.; Teles-Boaventura, G.; Alves-Chagas, M. The action of avocado oil on the lipidogram of wistar rats submitted to prolonged androgenic stimulum. Nutr. Hosp. 2015, 32, 696–701. [Google Scholar]
- Nicolella, H.D.; Neto, F.R.; Corrêa, M.B.; Lopes, D.H.; Rondon, E.N.; Dos Santos, L.F.; De Oliveira, P.F.; Damasceno, J.L.; Acésio, N.O.; Turatti, I.C.; et al. Toxicogenetic study of Persea americana fruit pulp oil and its effect on genomic instability. Food Chem. Toxicol. 2017, 101, 114–120. [Google Scholar] [CrossRef] [PubMed]
| Varieties and/or Country of Origin | Palmitic 16:0 | Estearic 18:0 | Palmitoleic 16:1 Ω7 | Oleic 18:1 Ω9 | Linoleic 18:2 Ω6 | α linolenic 18:3 Ω3 | Ref. |
|---|---|---|---|---|---|---|---|
| HASS | 18,62 | 0,49 | 8,47 | 60,17 | 10,97 | 0,98 | [51] |
| 13.4 | 0.6 | 3.9 | 65.3 | 15.2 | 1.3 | [46] | |
| 17.37 ± 0.0015 | 0.63 ± 0.0002 | 7.52 ± 0.0002 | 62.89 ± 0.0019 | 10.64 ± 0.0004 | 0.72 ± 0.0001 | [37] | |
| 18.17 ± 0.02 | 0.37 ± 0.00 | 4.03 ± 0.01 | 51.76 ± 0.04 | 11.12 ± 0.01 | 0.59 ± 0.00 | [52] | |
| 13.7 ± 1.5 | - | 3.4 ± 0.4 | 67.4 ± 3.0 | 14.4 ± 1.8 | 1.1 ± 0.01 | [53] 3 | |
| Australia | 25.63 ± 0.11 | 0.45 ± 0.16 | 7.29 ± 0.05 | 42.59 ± 0.16 | 20.87 ± 0.10 | 3.19 ± 0.06 | [1] |
| México | 22.59 ± 0.23 | 0.24 ± 0.02 | 11.63 ± 0.13 | 49.19 ± 0.57 | 14.72 ± 0.06 | 1.63 ± 0.16 | |
| New Zealand | 20.61 ± 0.16 | 0.30 ± 0.01 | 10.31 ± 0.03 | 50.97 ± 0.30 | 16.10 ± 0.11 | 1.72 ± 0.02 | |
| United States | 22.24 ± 0.05 | 0.93 ± 0.08 | 13.14 ± 0.01 | 47.69 ± 0.03 | 14.47 ± 0.01 | 1.54 ± 0.00 | |
| FORTUNE | 10. 75 | 0.48 | 3.14 | 74.32 | 10.03 | 0.85 | [51] |
| 20.5 | 0.5 | 6.8 | 60.6 | 13.2 | - | [47] | |
| BACON | 12.16 ± 0.04 | 0.38 ± 0.01 | 6.57 ± 0.01 | 61.72 ± 0.30 | 8.30 ± 0.02 | 0.44 ± 0.00 | [52] |
| PINKERTON | 16.93 ± 0.03 | 0.43 ± 0.01 | 7.33 ± 0.05 | 57.39 ± 0.18 | 8.25 ± 0.02 | 0.56 ± 0.00 | [52] |
| MARGARIDA | 23.66 | 3.58 | 47.20 | 13.46 | 1.60 | [54] | |
| FUERTE | 21.312 ± 0.550 | 0.762 ± 0.021 | 2.391 ± 0.188 | 64.436 ± 0.666 | 9.147 ± 0.030 | 0.467 ± 0.016 | [55] |
| 12.37 ± 0.01 | 0.51 ± 0.01 | 7.58 ± 0.00 | 64.62 ± 0.20 | 8.46 ± 0.02 | 0.47 ± 0.00 | [52] | |
| BREDA | 19.9–21.3 | - | 2.7–7.0 | 57.1–64.5 | 10.6–11.0 | 0.4–0.6 | [10] 1 |
| CRIOLLA MEXICANA | 28.12–34.48 | 0.23–1.07 | 6.64–8.5 | 40.73–42.72 | 15.52–18.88 | 1.51–2.14 | [24] 2 |
| DE MALASIA | 30.37 ± 0.06 | 1.30 ± 0.01 | 5.22±0.02 | 43.65 ± 0.04 | 17.45 ± 0.04 | 2.03 ± 0.01 | [49] |
| REED | 18.18 | 0.40 | 6.56 | 60.25 | 13.03 | 1.40 | [51] |
| ANTILLANA | 18.87 | 0.59 | 4.16 | 63.07 | 11.83 | 1.32 | [51] |
| VARIETY NO INDICATED | 18.74 ± 0.06 | 0.51 ± 0.00 | 7.88 ± 0.01 | 54.40 ± 0.10 | 10.87 ± 0.01 | 0.61 ± 0.00 | [3] |
| 12.87 | 1.45 | 3.86 | 57.44 | 18.70 | 0.92 | [53] |
| Varieties | β-Sitosterol | α-Tocopherol | γ-Tocopherol | ∆5-avenasterol | Campesterol | Estigmasterol | Sitoestanol | Campestanol | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| HASS | 91.917 ± 0.027 | - | - | - | 6.091 ± 0.026 | 0.001 ± 0.001 | - | - | [57] |
| 95.2 | - | - | - | 4.7 | 0.13 ± 0.00 | - | - | [51] | |
| 82.95 ± 0.06 | 86.75 ± 0.62 | 9.02 ± 0.09 | 6.63 ± 0.07 | 5.88 ± 0.01 | - | 0.46 ± 0.01 | 0.04 ± 0.00 | [52] | |
| FUERTE | 94.767 ± 0.012 | - | - | - | 5.043 ± 0.012 | 0.001 ± 0.001 | 0.57 ± 0.04 | - | [57] |
| 92.9 | - | - | - | 6.4 | - | - | - | [51] | |
| 80.56 ± 0.08 | 103.11 ± 6.87 | 20.35 ± 1.22 | 8.81 ± 0.03 | 4.62 ± 0.02 | 0.15 ± 0.00 | - | 0.04 ± 0.00 | [52] | |
| BACON | 92.189 ± 0.012 | - | - | - | 6.096 ± 0.010 | 0.011 ± 0.001 | - | - | [57] |
| 82.6 ± 0.03 | 51.90 ± 0.04 | 71.61 ± 0.57 | 9.16 ± 0.03 | 3.71 ± 0.01 | 0.40 ± 0.01 | 0.58 ± 0.04 | 0.05 ± 0.00 | [52] | |
| REED | 94.605 ± 0.027 | - | - | - | 5.123 ± 0.021 | 0.001 ± 0.001 | - | - | [57] |
| ANTILLANA | 91.2 | - | - | - | 8.6 | - | - | - | [51] |
| 89.3 | - | - | - | 10.6 | - | - | - | [51] | |
| PINKERTON | 84.08 ± 0.08 | 45.62 ± 0.19 | 13.71 ± 0.56 | 5.86 ± 0.01 | 6.00 ± 0.01 | 0.11 ± 0.00 | 0.41 ± 0.03 | 0.04 ± 0.00 | [52] |
| VARIETY NO INDICATED | - | - | - | 9.42 ± 1.69 | 18.36 ± 1.44 | 1.11 ± 0.12 | 2.19 ± 0.22 | 0.43 ± 0.03 | [3] |
| Animal Model | Protocol Used | Conclusions | Ref. |
|---|---|---|---|
| Male Wistar rats | Daily administration of 1.0 mL/250 g of avocado oil by gavage or losartan at 40 mg/kg for 45 days. | (a) Avocado oil mimics the effects of losartan. (b) Effects of avocado oil could be mediated by decreased actions of Angiotensin-II in mitochondria. (c) The intake of avocado oil could mitigate the harmful effects of hypertension in kidneys. | [74] |
| Diabetic male rats Goto-Kakizaki; the control rats were Wistar males. | Daily administration of avocado oil was 1 mL/250 g of weight for a period of 3, 6 and 12 months to Winstar and Goto-Kakizaki rats. | In the brain: (a) Improvement of mitochondrial function. (b) Decreased levels of free radicals and lipid peroxidation. (c) Improvement of the reduced/oxidized glutathione ratio. (d) Prevention of mitochondrial dysfunction. | [66] |
| In vitro (Chinese hamster lung fibroblasts/V79 cells) and in vivo models (Swiss mice). | Swiss mice were given avocado oil in different concentrations of 250, 500, 1000 and 2000 mg/kg in an in vivo model. In vitro model was applied to different concentrations of avocado oil: 100, 200 and 400 μg/mL. | (a) Avocado pulp oil has no genotoxic effects. (b) The oil was effective in reducing the chromosomal damage induced by methyl methanesulfonate and doxorubicin. However, an increase in hepatic enzyme aspartate aminotransferase was found to be a marker of liver damage. | [77] |
| Wistar rats | Administration of a diet with different concentrations of avocado oil to insulin resistant rats. | (a) The dietary addition of 5–20% avocado oil can reduce glucose tolerance and insulin resistance, induced by the high sucrose diet, in Wistar rats. (b) The addition of 5–30% avocado oil reduced the body weight gain induced by the high sucrose diet in Wistar rats. | [75] |
| Male Wistar rats | Administration of avocado oil (1 g/250 g weight) to diabetic rats for 90 days. | In the liver: (a) Promoted an improvement in the functionality of the electron transport chain. (b) Decreased the generation of free radicals. (c) Diminished the harmful effects of oxidative stress in the liver. | [65] |
| Male Wistar rats | Diet supplemented with avocado oil to rats exposed to prolonged androgenic stimulation. | Avocado oil exerted a direct regulatory effect on the lipid profile. | [76] |
| Wistar rats | Administration of a diet with olive oil and avocado oil to rats exposed to a diet rich in sucrose. | In the liver: (a) A diet high in sucrose causes hepatic impairment, partially reversible by the administration of avocado oil, which does not occur at the pancreatic level. (b) Avocado oil (independent of its extraction method) exhibits effects similar to those of olive oil in the fatty acid profile. | [5] |
| Wistar rats | Administration of a diet with olive oil and avocado oil to rats exposed to a diet rich in sucrose. | At the cardiovascular level: In rats fed with sucrose, it was observed that avocado oil reduces the levels of triglycerides, very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL), without affecting the levels of high-density lipoprotein (HDL). It also reduces the level of ultrasensitive CRP, indicating that the inflammatory processes associated with metabolic syndrome are partially reestablished. | [4] |
| Male Wistar rats | Avocado oil administration: 1 mL of avocado oil/250 g of weight daily for a period of 90 days. | At the renal level: (a) Protection against induced oxidative stress. (b) ROS generation decrease. (c) Improvement of the activities of complexes II and III. (d) Lower peroxidizability index in diabetic mitocondria. | [67] |
| Male Wistar rats | The control group received a laboratory pellet, while the treated group received a diet rich in 10% avocado oil (weight/weight) for a period of two weeks. | The administration of a diet rich in avocado oil to rats for two weeks modifies the content of fatty acids in cardiac and renal membranes. | [73] |
| Male rabbits of the New Zealand White strain. | Rabbits were fed a semi-purified diet containing 0.2% cholesterol and 14% oil for 90 days (corn, coconut, olive and avocado). | The atherogenic power of avocado oil is equivalent to that of olive oil and lower than that of corn and coconut oil. | [72] |
| Female rats and chicks. | Rats and chicks were fed 10% avocado oil (w/w). | Rats and chickens fed unrefined avocado oil showed a significant decrease in total collagen solubility in the liver. | [68] |
| Charles river female rats. | Growing rats were fed for eight weeks with 10% (w/w) refined or unrefined avocado oil or soybean oil, using different extraction media. | Rats fed with unrefined avocado oil extracted with hexane from intact fruit (unsaponifiables) or avocado seed oil showed significant increases in the content of soluble collagen in the skin, although the total collagen content was not affected. Rats fed refined or unrefined soybean oils showed no effects. | [70] |
| Charles river female rats. | The rats were fed diets containing 10% avocado oil (w/w) for four weeks. | The consumption of avocado oil extracted from intact fruits can cause changes in metabolism in the liver. Serum alkaline phosphatase activity increased in rats fed seed oil, oil extracted with unrefined solvent of intact fruit or unsaponifiables, and aspartate aminotransferase activity increased significantly in the group fed avocado seed oil. | [69] |
| Rats | Application of oil mixtures in the skin of the dorsal area of rats for 15 days, treatment with a mixture of 2/3 of soybean oil and 1/3 avocado in a 5% solution of sweet almond oil. | The mixture of soybean and avocado oils decreases the degree of collagen cross-linking, delaying wound healing. | [71] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, M.; Saravia, C.; Vergara, C.E.; Avila, F.; Valdés, H.; Ortiz-Viedma, J. Avocado Oil: Characteristics, Properties, and Applications. Molecules 2019, 24, 2172. https://doi.org/10.3390/molecules24112172
Flores M, Saravia C, Vergara CE, Avila F, Valdés H, Ortiz-Viedma J. Avocado Oil: Characteristics, Properties, and Applications. Molecules. 2019; 24(11):2172. https://doi.org/10.3390/molecules24112172
Chicago/Turabian StyleFlores, Marcos, Carolina Saravia, Claudia E. Vergara, Felipe Avila, Hugo Valdés, and Jaime Ortiz-Viedma. 2019. "Avocado Oil: Characteristics, Properties, and Applications" Molecules 24, no. 11: 2172. https://doi.org/10.3390/molecules24112172
APA StyleFlores, M., Saravia, C., Vergara, C. E., Avila, F., Valdés, H., & Ortiz-Viedma, J. (2019). Avocado Oil: Characteristics, Properties, and Applications. Molecules, 24(11), 2172. https://doi.org/10.3390/molecules24112172







