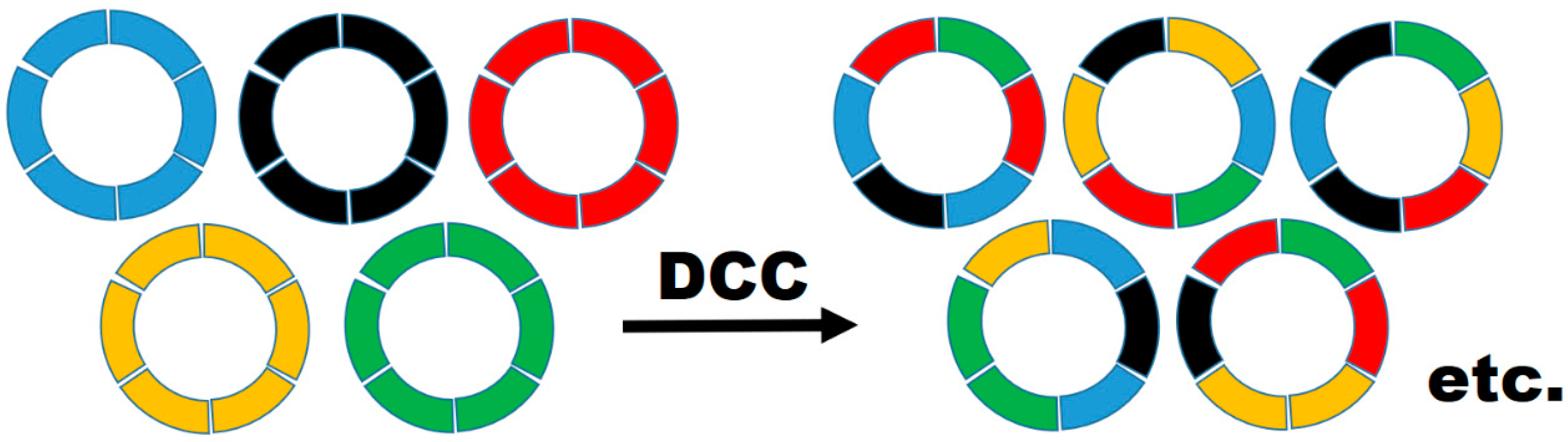
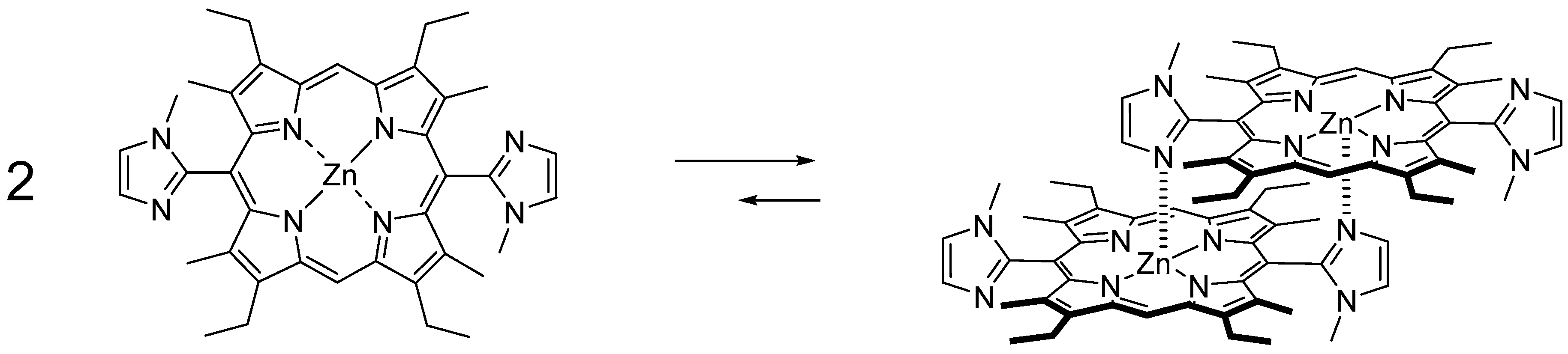
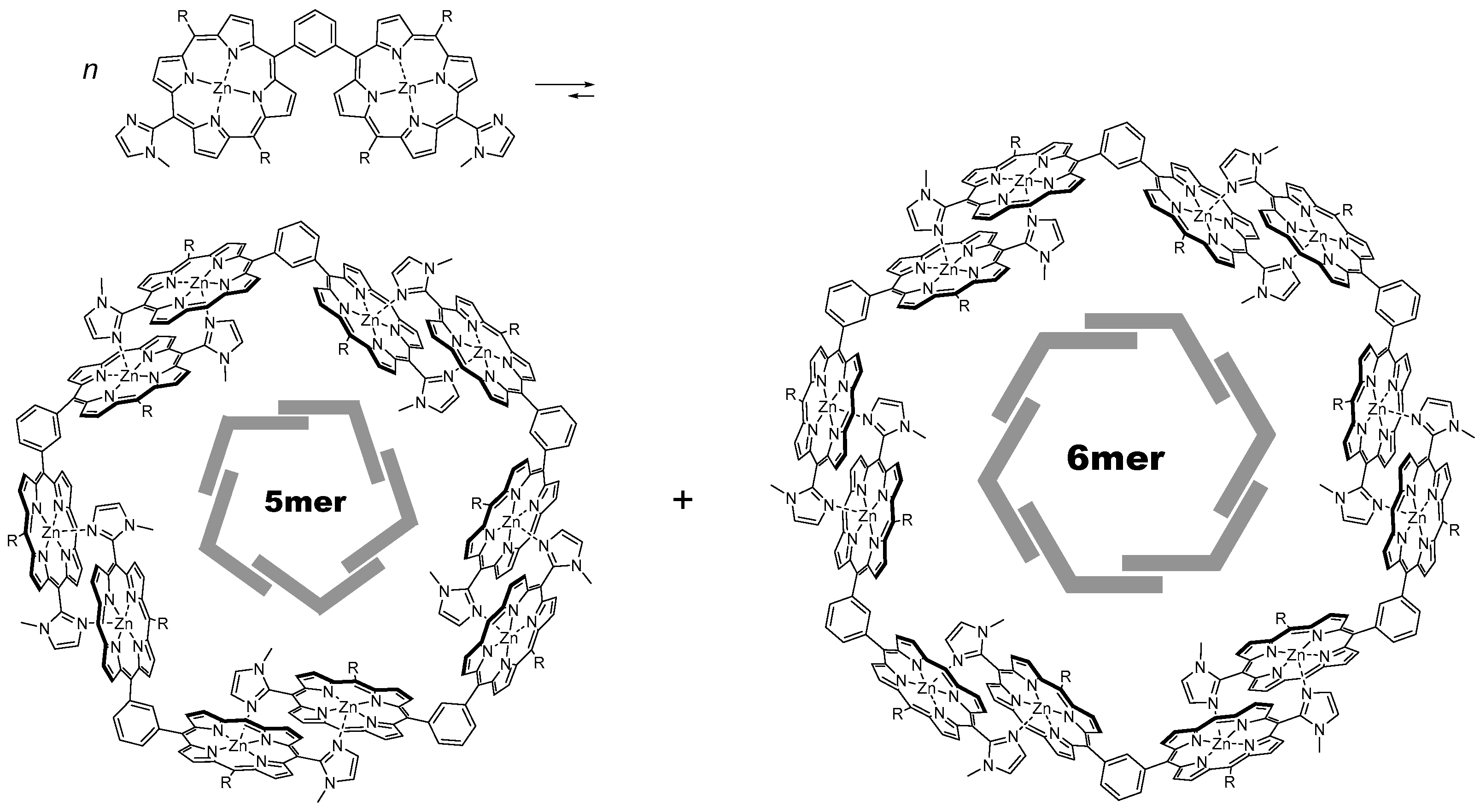
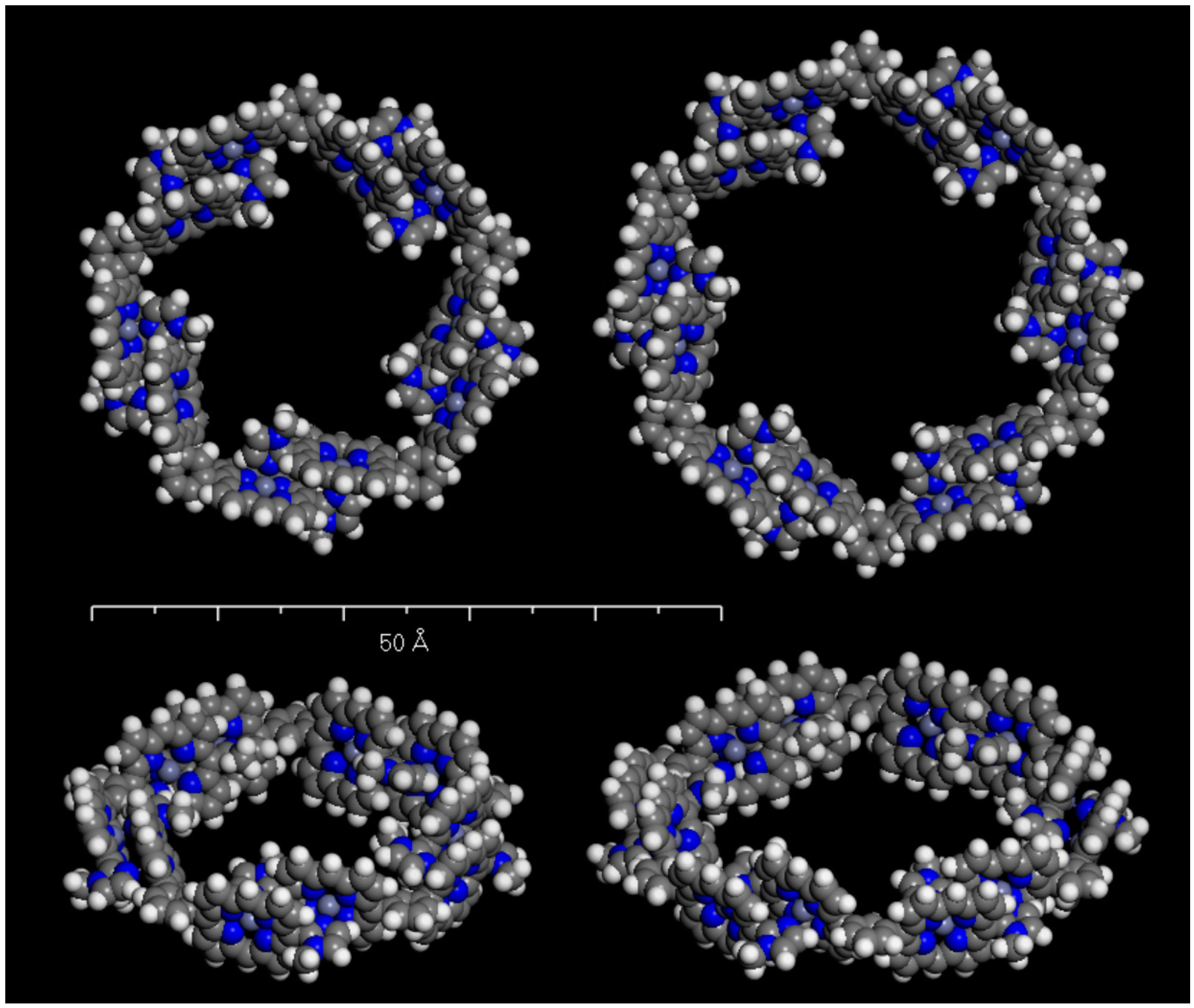
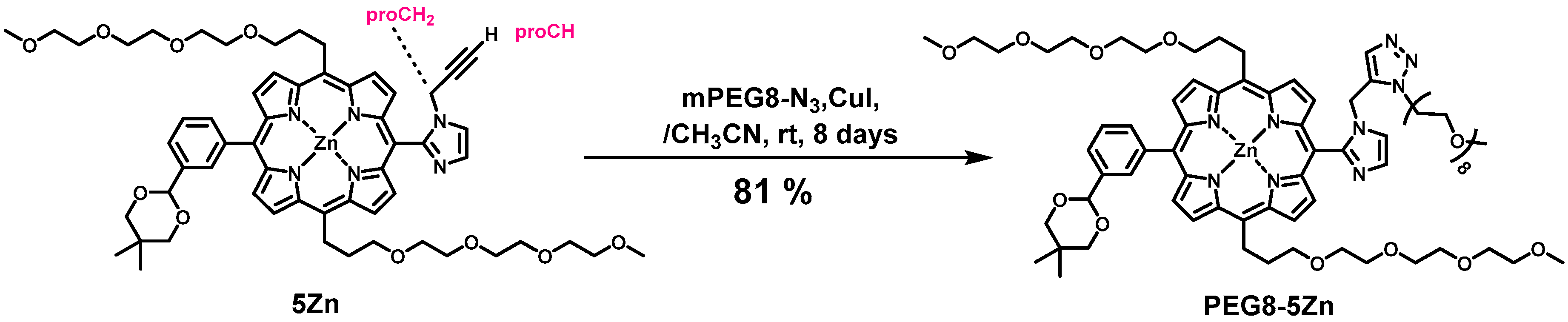3. Materials and Methods
General Procedure. All chemicals and solvents were of commercial reagent quality and used without further purification unless otherwise stated. 3-(4,4-Dimethyl-2,6-dioxan-1-yl)benzaldehyde
3 [
29] and 2,2′-(2,5,8,11-tetraoxapentadecane-15,15-diyl)bis(1
H-pyrrole)
4 [
31] were prepared according to the literature. PEG8N
3 (25-Azido-2,5,8,11,14,17,20,23-octaoxapentacosane) was purchased from TCI (Tokyo, Japan). CHCl
3 (Kanto, extra pure) stabilized with 0.5–1% ethanol was used. Reactions were monitored on silica gel 60F
254 TLC plates (Merck, Tokyo, Japan). Silica-gels utilized for column chromatography were purchased from Kanto Chemical Co. Inc. (Tokyo, Japan): Silica-Gel 60N (Spherical, Neutral) 63–210 μm and 40–50 μm (Flash).
1H-NMR spectra were recorded by using JEOL ECA-500 (500 MHz, JEOL, Tokyo, Japan), JEOL ECA-300 (300 MHz, JEOL, Tokyo, Japan ), or JEOL ECZ-400 (400 MHz, JEOL, Tokyo, Japan ) and chemical shifts were recorded in parts per million (ppm) relative to tetramethylsilane. High-resolution MALDI–TOF mass spectra were collected on JEOL JMS-S3000 SpiralTOF with dithranol or
trans-2-[3-(4-
tert-butylphenyl)-2-methyl-2-propenylidene]-malononitrile (DCTB) as a matrix containing sodium iodide (NaI) and polyethylene glycol as an internal or an outer standard. The data analyses were carried out on mMass ver. 5.5 (
http://www.mmass.org/). UV–vis absorption spectra were collected on JASCO V-650 or V-660 spectrometer (JASCO Co. Tokyo, Japan). Steady-state fluorescence spectra were collected on Hitachi F-4500 spectrometer (Hitachi High-Tech Science Co., Tokyo, Japan) and corrected for the response of the detector system. The UV-vis absorption and fluorescence spectra were measured using a square cell (optical path = 10 mm).
Analytical high-performance liquid chromatography (HPLCs, JASCO Co. Tokyo, Japan) was carried out by using the following three systems:
[System 1] JASCO PU-2080plus and MD-2018plus system equipped with two TSK G2500HHR (Tosoh, 7.8 mm × 30 cm, exclusion limit: 20,000 Da) and one TSK G2000HHR (Tosoh, 7.8 mm × 30 cm, exclusion limit: 10,000 Da) columns using pyridine as an eluent.
[System 2] JASCO PU-2089 and MD-44010 system equipped with two TSK G4000HHR (Tosoh, 7.8 mm × 30 cm, exclusion limit: 40,000 Da) columns using CHCl3/THF (95:5 v/v) as an eluent.
[System 3] JASCO PU-2089 and MD-44010 system equipped with a TSK gel α-M (Tosoh, 7.8 mm × 30 cm, exclusion limit: 10,000,000 Da) column using a mixture of water/acetonitrile (4:6 v/v) as an eluent.
Preparative gel permeation chromatography (GPC) were carried out on LC-908 (Japan Analytical Industry, Tokyo, Japan) attached to one TSK G2500HHR (Tosoh, 21.5 mm × 30 cm, exclusion limit: 20,000 Da) and one G2000HHR (Tosoh, 21.5 mm × 30 cm, exclusion limit: 10,000 Da) columns eluted with pyridine. Infrared (IR) spectra were measured with JASCO FT/IR-4600 (JASCO Co. Tokyo, Japan) and ATR PRO ONE (JASCO Co. Tokyo, Japan) using ATR method.
1-(Prop-2-yn-1-yl)-1H-imidazole-2-carbaldehyde (
2). In a 20 mL flask were placed 1
H-Imidazole-2-carbaldehyde [
32] (50mg, 0.52 mol), LiBr∙H
2O (300 mg, 2.86 mmol), dry DMF (0.5 mL), and propargyl bromide (1.5 equiv, 58.7 μL, 0.78 mmol). The reaction mixture was heated to 60 °C. The reaction progress was monitored with TLC and additional propargyl bromide (1.5 equive, 58.7 μL, 0.78 mmol) was added to the reaction mixture (total ten equiv). The reaction progress almost stopped after 10.5 h, and then the mixture was quenched and neutralized with sat. NaHCO
3 aqueous solution. The product was extracted with ethyl acetate and washed with sat. NaHCO
3 aqueous solution and brine. The organic layer was dried over anhydrous MgSO
4 and the solvent was evaporated. Remaining DMF was removed by adding toluene using azeotropy. The residue was dried in vacuo to afford brown liquid as the crude product (35 mg). Further purification with a silica gel column (φ 3 cm × 7 cm, hexane → hexane:ethyl acetate = 1:1) gave the pure titled product as pale yellow liquid (20 mg, 29%). TLC
Rf = 0.6 (silica gel, chloroform:methanol = 11:1).
1H-NMR (300 MHz, DMSO-
d6)
δ (ppm) 9.71 (s, 1H), 7.73 (s, 1H), 7.31 (s, 1H), 5.25 (d,
J=3.0 Hz, 2H), 3.51 (t,
J=3.0 Hz, 1H).
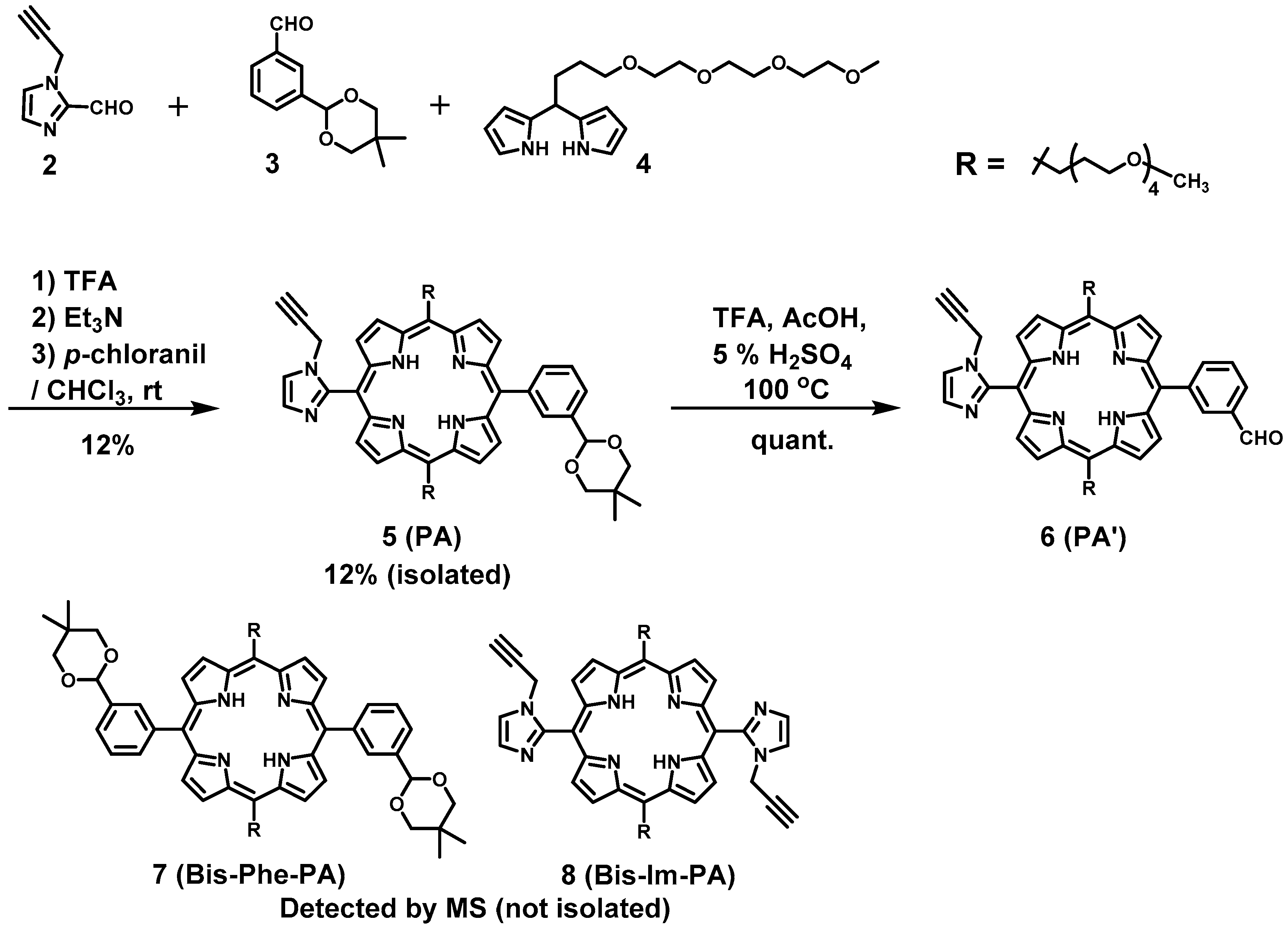
PA(PorphyrinA) (5). A 500 mL three-necked flask was charged with 1-(prop-2-yn-1-yl)-1H-imidazole-2-carbaldehyde (2) (206 mg, 1.53 mmol), 3-(4,4-dimethyl-2,6-dioxan-1-yl)benzaldehyde (3) (365 mg, 1.53 mmol), 2,2′-(2,5,8,11-tetraoxapentadecane-15,15-diyl)bis(1H-pyrrole) (4) (1.07 g, 3.04 mmol), and chloroform (284 mL). After bubbling with N2 gas for 30 min, trifluoroacetic acid (TFA) (234 μL, 3.06 mmol) in chloroform was added. After stirred for 4 h under dark, the color of the reaction mixture was changed from yellow to red and spots showing red emission by irradiation with 365 nm appeared on TLC. Triethylamine (TEA) (469 μL, 3.37 mmol) was added to neutralize the solution, and the mixture was stirred until the color became orange. To the mixture p-chloranil (1.28 g, 5.20 mmol) was added, and the reaction mixture was stirred for 14 h. The solvent was evaporated to dryness to give a black solid (4.12 g). The crude product was purified by an alumina column (φ 8 cm × 12 cm, chloroform → chloroform:acetone = 10:1). The second band eluted with chloroform and acetone was collected. The fractions were concentrated, and the residue was further purified with a flush silica gel column (φ 3 cm × 20 cm, chloroform:methanol = 50:1). The red band was collected, and concentrated under reduced preessure to afford 5 as a purple solid (22.7 mg, 12%). TLC Rf = 0.48 (silica gel, ethyl acetate); MALDI-TOF MS (matrix: dithranol) found m/z ([M + Na]+) 1035.5180, calcd for [C58H72N6O10 + Na]+ 1035.5208; 1H-NMR (300 MHz, CDCl3) δ (ppm) 9.55 (d, J = 3.9 Hz, 2H, β), 9.47 (d, J = 4.8 Hz, 2H, β), 8.86(d, J = 3.9 Hz, 2H, β), 8.78 (d, J = 4.8 Hz, 2H, β), 8.35, 8.26 (each s, 0.5H × 2, Ph2), 8.21 (d, J = 7.8 Hz, 0.5H, Ph6), 8.12 (d, J = 7.5 Hz, 0.5H, Ph6), 8.00 (d, J = 7.8 Hz, 1H, Ph4), 7.79–7.75 (m, 1H, Ph5), 7.73 (s, 2H, Im5), 5.67 (d, J = 5.4 Hz, 1H, acetal-CH), 5.07(t, J = 7.2 Hz, 4H, TEG), 4.38(dd, J = 2.7 Hz, 2H, propargyl CH2), 3.86–3.40(m, 28H, TEG, acetal-CH2), 3.31(s, 6H, TEG-CH3), 2.81–2.72(m, 4H, TEG), 2.25 (d, J = 2.7 Hz, 1H, propargyl CH), 1.36, 1.34(each s, 3H, acetal-CH3), 0.80, 0.79(each s, 3H, acetal-CH3), −2.67(s, 2H, Hinner); 13C-NMR (75 MHz, CDCl3) δ (ppm) 148.17, 149–144, 142.46, 137.14, 137.08, 134.98, 132.56, 132.43, 132.38, 130.54, 129.48, 127.98, 126.84, 126.78, 125.82, 120.80, 119.75, 119.69, 103.19, 102.15, 77.96, 77.41, 77.20, 74.44, 72.04, 70.91, 70.88, 70.82, 70.65, 70.47, 70.10, 59.12, 37.79, 37.19, 31.32, 30.47, 23.29, 22.03.
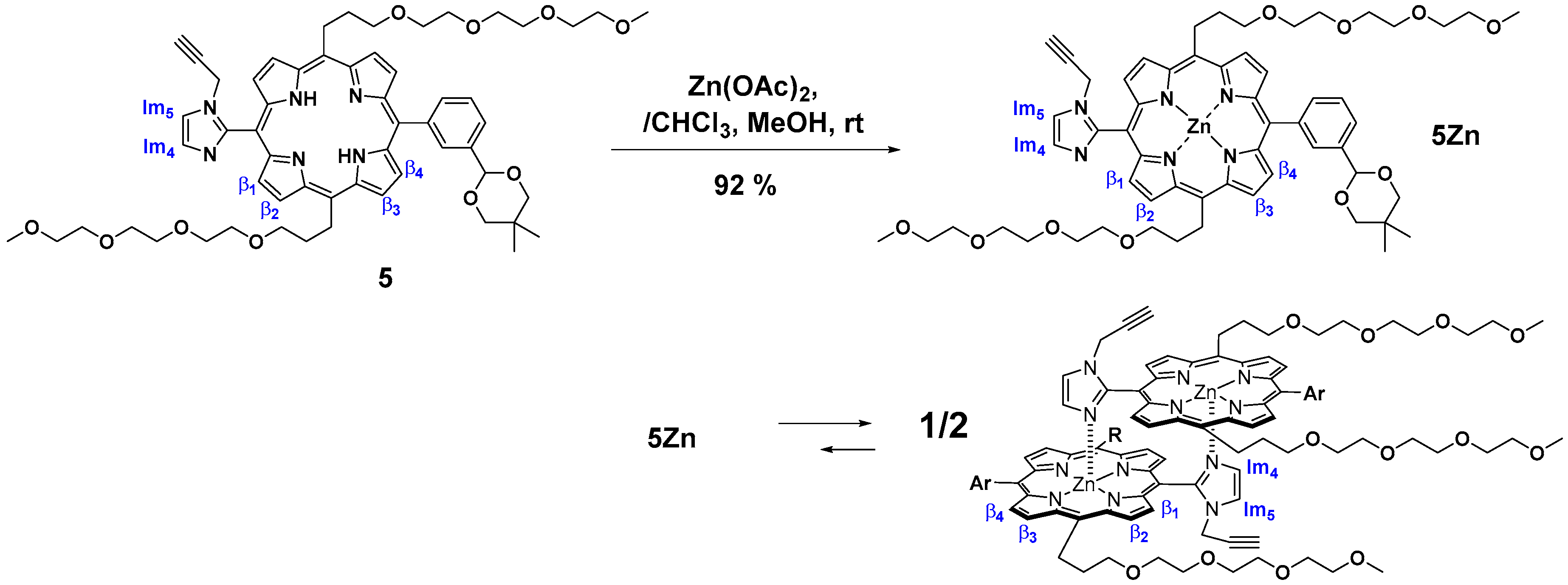
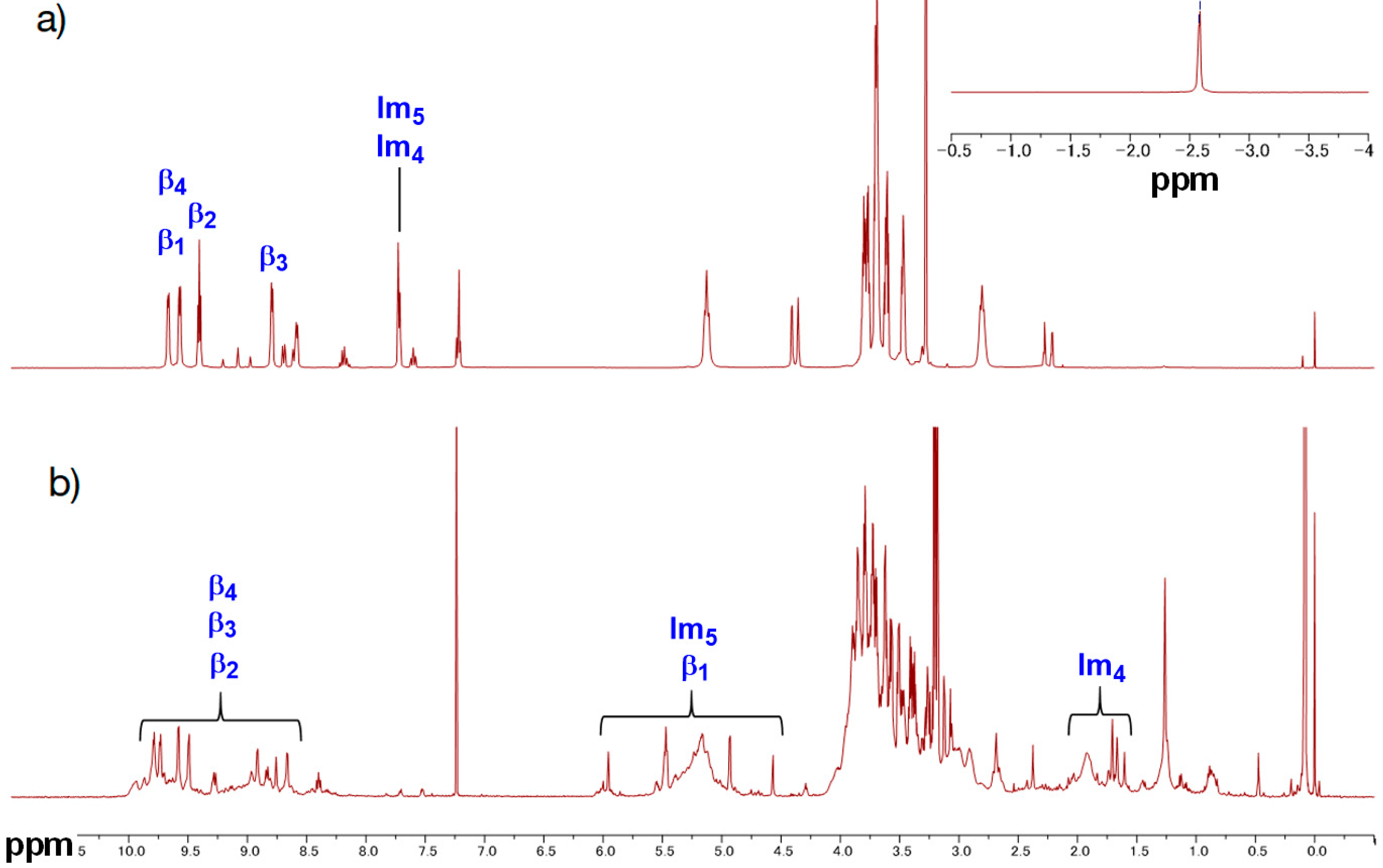
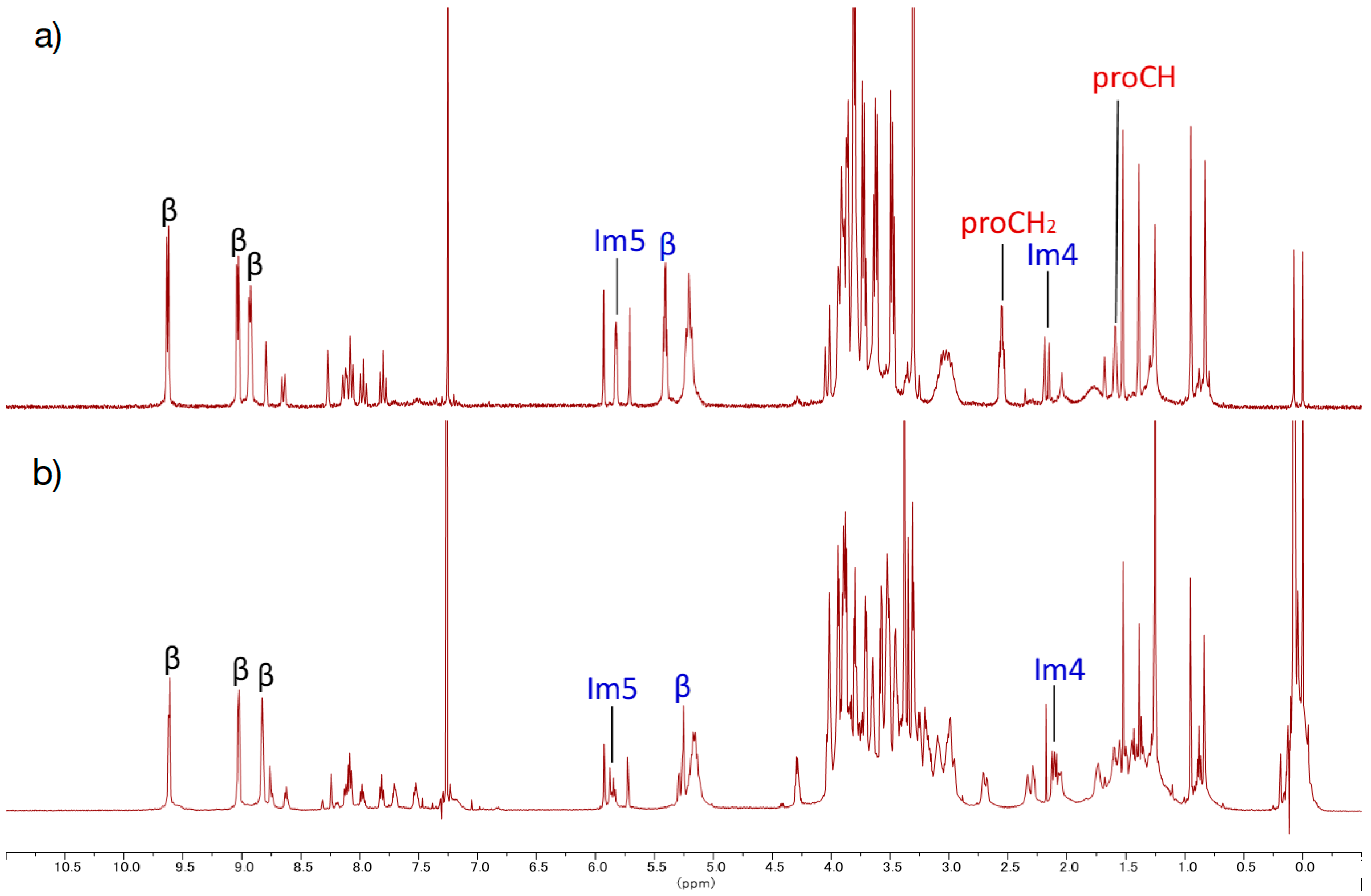
PA-Zn(PorphyrinA-Zn) (5Zn). 5 (24 mg, 2.34 × 10−5 mol) was dissolved in chloroform (5 mL) in a 35 mL flask. A saturated methanol solution of Zn(OAc)2 (17 mg, 9.4 × 10−5 mol) was added to the solution, and the mixture was stirred for 2 h at rt in the dark. The mixture was washed with water (×3), brine (×1) and dried over anhydrous Na2SO4. The solvent was evaporated to dryness, giving the titled compound as a purple solid (23.2 mg, 92%). MALDI-TOF-MS (matrix: DCTB) found m/z [M + Na]+ 1097.4863, calcd for [C58H70N6O10Zn + Na]+ 1097.4343; 1H-NMR (300 MHz, CDCl3, observed as atropisomers) δ (ppm) 9.63 (d, J = 4.5 Hz, 4H, β), 9.03 (d, J = 4.5 Hz, 4H, β), 8.93 (d, J = 4.5 Hz, 4H, β), 8.80(s, 1H, Ph2), 8.65 (d, J = 7.2 Hz, 1H, Ph6), 8.27 (s, 1H, Ph2), 8.13 (d, J = 7.5 Hz, 1H, Ph6), 8.08 (t, J = 7.5 Hz, 2H, Ph4), 7.98 (t, J = 7.5 Hz, 1H, Ph5), 7.80 (t, J = 7.5 Hz, 1H, Ph5), 5.93, 5.71(each s, 2H, acetal-CH), 5.83-5.81 (m, 2H, Im5), 5.41(t, J = 4.5 Hz, 4H, β), 5.21 (t, J = 6.6 Hz, 8H, TEG), 4.05–3.46 (m, 56H, TEG, acetal-CH2), 3.30 (s, 12H, TEG-CH3), 3.08–2.95 (m, 8H, TEG), 2.57-2.53 (m, 4H, propargyl CH2), 2.19 (s, 1H, Im4), 2.15 (s, 1H, Im4), 1.60–1.58 (m, 2H, propargyl CH), 1.53, 1.39 (each s, 6H, acetal-CH3), 0.95, 0.83 (each s, 6H, acetal-CH3); 13C-NMR (125 MHz, CDCl3) δ(ppm) 151.14, 149.83, 149.08, 147.82, 145.49, 143.89, 136.66, 136.45, 135.39, 132.33, 129.31, 126.41, 122, 128.36, 126.90, 124.92, 121.27, 119.23, 116.10, 102.41, 94.76, 77.97, 77.79, 75.35, 73.69, 71.81, 70.85, 70.80, 70.71, 70.63, 70.44, 70.33, 38.29, 31.82, 58.92, 58.87, 35.20, 30.44, 30.28, 23.35, 21.91.
PA’(PorphyrinA’) (6). In a 50 mL flask, 5 (75.3 mg, 74.3 μmol) was dissolved in acetic acid (1.6 mL), and then TFA (0.8 mL) and 5% aqueous H2SO4 (0.4 mL) were added to the solution. The mixture was refluxed at 100 ○C for 1.5 h. Reaction mixture was transferred to a 500 mL beaker and neutralized with saturated NaHCO3 aqueous solution. The product was extracted with chloroform (×5). Organic layer was washed with water (×3) and brine (×1), and dried over anhydrous Na2SO4. The solvent was evaporated to dryness, giving 6 as a purple solid (77.6 mg, quantitative). 1H-NMR (300 MHz, CDCl3, observed as atropisomers) δ (ppm) 10.34, 10.33 (each s, 1H, -CHO), 9.57 (d, J = 4.8 Hz, 2H, β), 9.52 (d, J = 4.8 Hz, 2H, β), 8.78 (d, J = 4.8 Hz, 4H, β), 8.73, 8.64 (each s, 1H, Ph2), 8.49, 8.41 (each d, 1H, J = 7.5 Hz, Ph6), 8.37, 8.35 (each d, partially overlapped each other, Ph4), 7.99–7.45 (m, 1H, Ph5), 7.75 (s, 2H, Im5, Im4), 5.09 (t, J = 7.2 Hz, 4H, TEG), 4.41 (dd, J = 2.4 Hz, 2H, propargyl CH2), 3.88 (s, 4H, TEG), 3.82–3.49 (m, 28H, TEG), 3.33 (s, 6H, m, TEG), 2.81–2.73 (m, 4H, TEG), 2.25 (d, J = 2.4 Hz, 1H, propargyl CH), −2.69 (s, 2H, Hinner); 13C-NMR (100 MHz, CDCl3)δ (ppm) 192.76, 148.32, 143.68, 139.66, 134.98, 134.93, 135.09, 134.88, 131.85, 130.80, 129.64, 128.37, 128.96, 129.14, 128.89, 127.57, 127.54, 119.97, 119.86, 118.84, 103.70, 77.41, 74.50, 72.03, 70.90, 70.87, 70.83, 70.66, 70.46, 70.03, 59.13, 37.82, 37.19, 31.31.
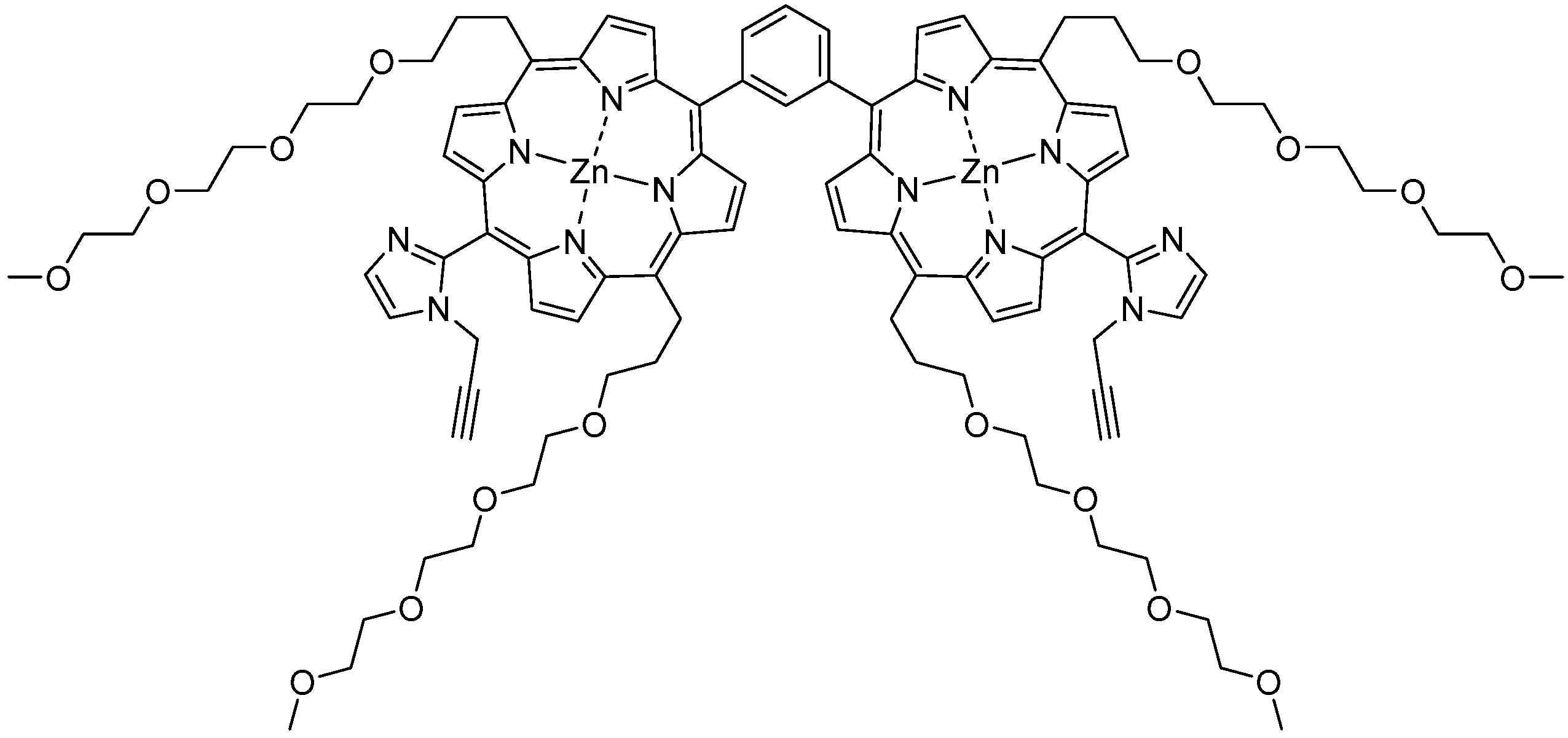
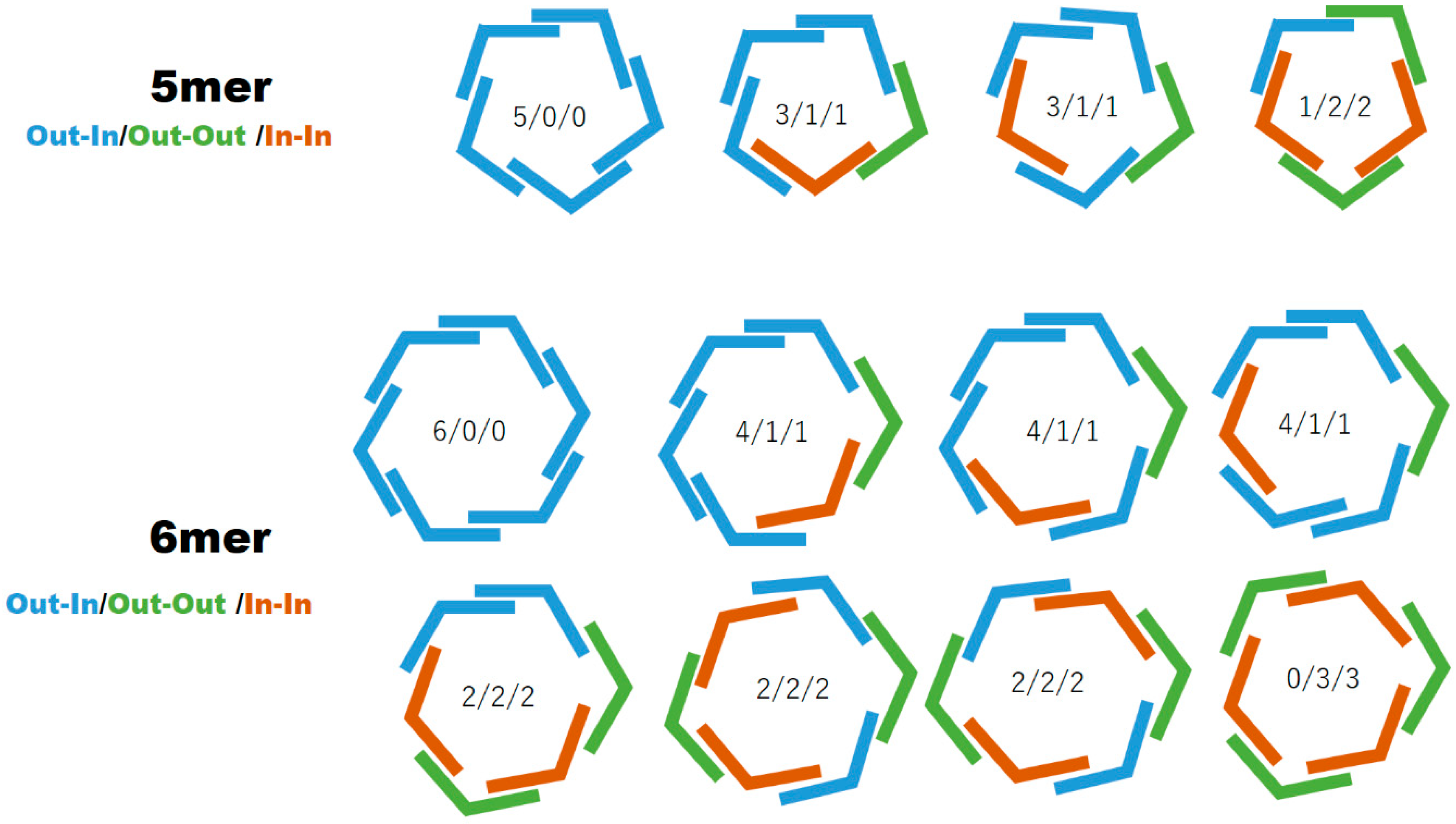
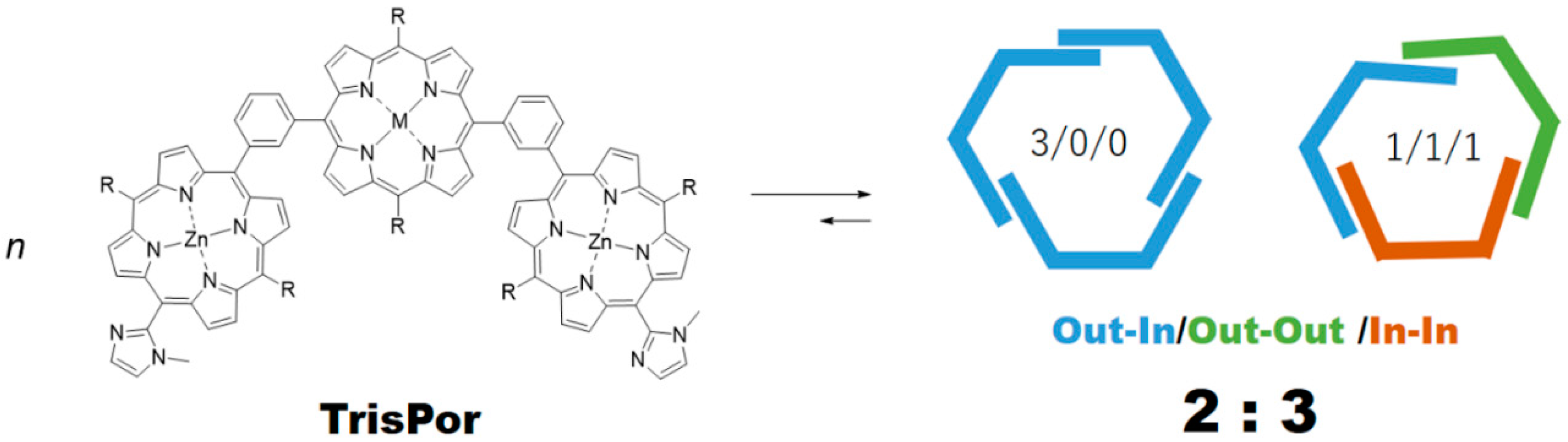
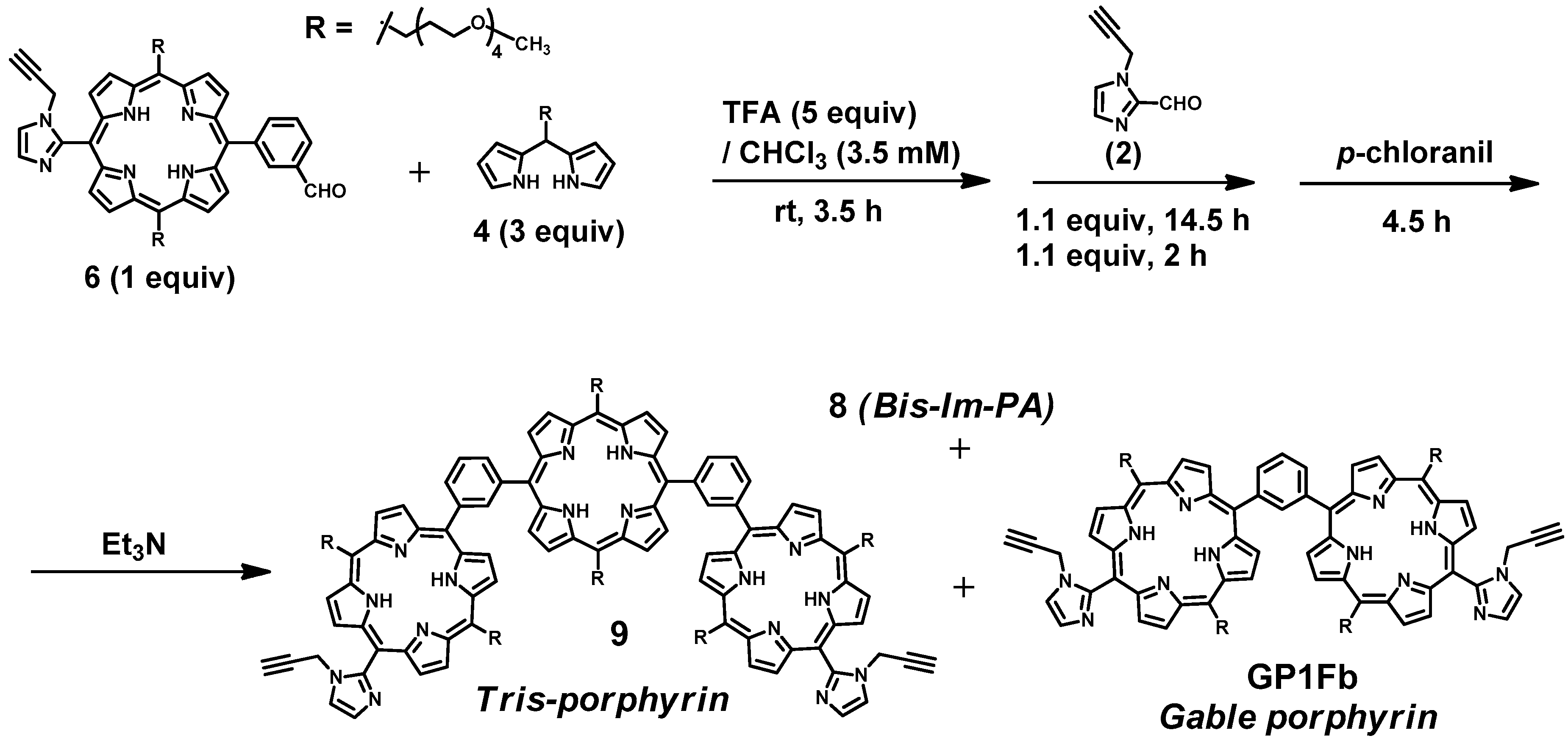
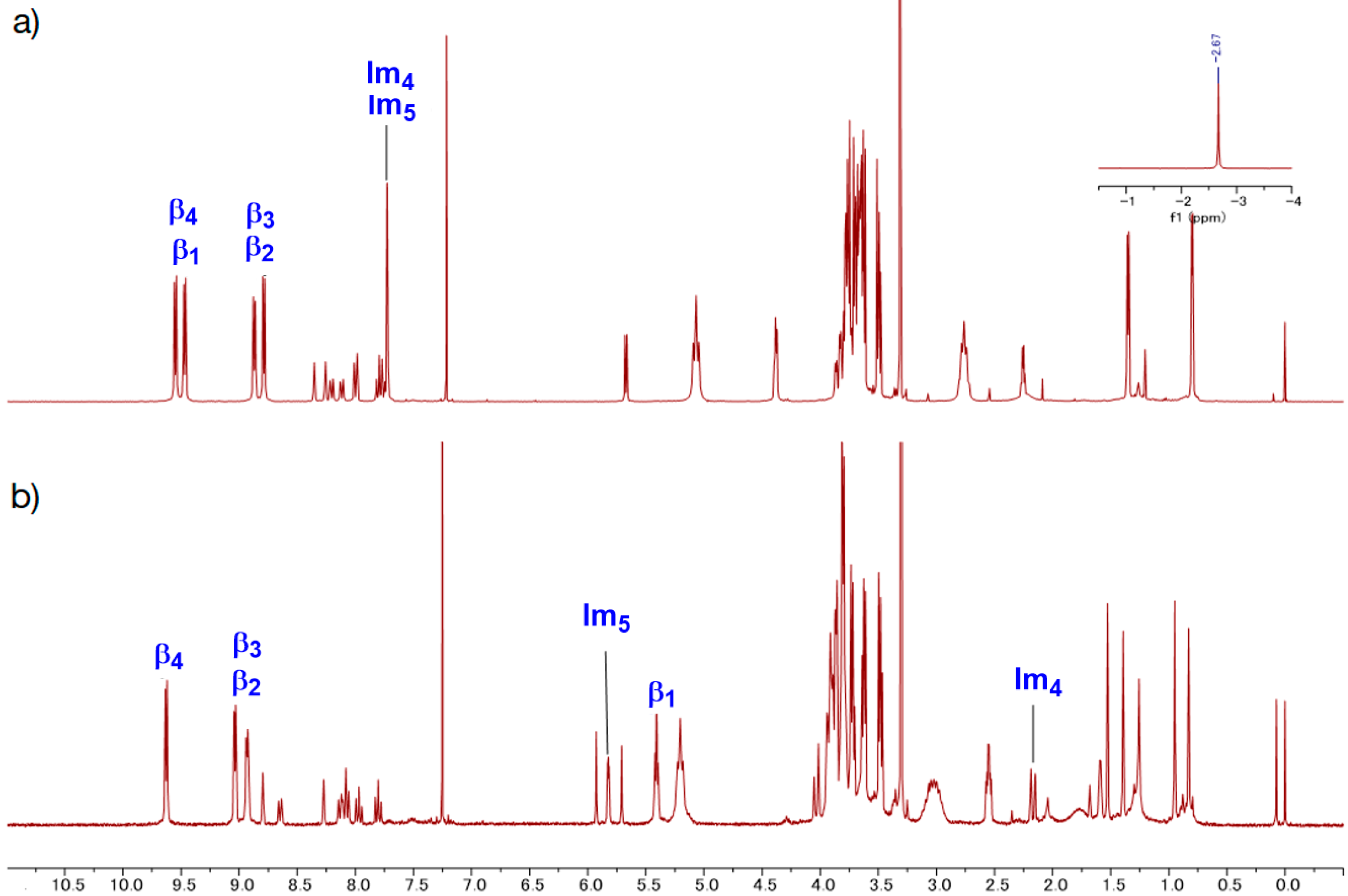
GablePorphyrin (GP 1Fb). A 300 mL three-necked flask was charged with 6 (400 mg, 4.31 × 10−4 mol), dipyrromethane 4 (463 mg, 1.32 × 10−3 mol), and chloroform (123 mL). After the solution was degassed by bubbling with Ar gas for 15 min, TFA (165 μL, 2.16 × 10−3 mol) diluted with chloroform was added, and the mixture was stirred under dark. After 3.5 h, 1-(prop-2-yn-1-yl)-1H-imidazole-2-carbaldehyde (2) (65.2 mg, 4.86 × 10−4 mol) was added. After 14.5 h, further 1-(prop-2-yn-1-yl)-1H-imidazole-2-carbaldehyde (2) (65.2 mg, 4.86 × 10−4 mol) was added. The reaction progress was monitored by MALDI-TOF mass. After 2 h, p-chloranil (583 mg, 2.37 × 10−3 mol) was added, and the mixture was stirred for 4.5 h. The mixture was neutralized with triethylamine (330 μL, 2.37 × 10−3 mol) and the solvent was evaporated to dryness. The black residue was purified by an alumina column (φ 5 cm × 12 cm, chloroform:acetone = 1:1). The red band was collected, and the fraction was concentrated to give a purple solid (392 mg). The GPC analysis showed that the solid contained the byproducts, tris-porphyrin and bis-imidazolyl-porphyrin 8, along with ca 29% of GP1Fb. The mixture was purified further using preparative GPC system to afford GP1Fb as as a purple solid (130 mg, 17.5%). MALDI-TOF MS (matrix: DCTB) found m/z ([M + H]+) 1741.8659, calcd for [C98H118N12O16 + H]+ 1741.8686; 1H-NMR (400 MHz, CDCl3, observed as atropisomers) δ (ppm) 9.67 (d, J = 4 Hz, 4H, β), 9.57 (d, J = 4 Hz, 4H, β), 9.40 (d, J = 4 Hz, 4H, β), 9.08 (dd, J = 5.2 Hz, J = 4 Hz, 1H, Ph2), 8.80 (d, J = 4 Hz, 4H, β), 8.69, 8.62, 8.58 (d, J = 8 Hz, J = 4 Hz, 4H, Ph4, Ph6), 8.18 (m, 1H, Ph5), 7.72, 7.73 (s, 4H, Im4, Im5), 5.13 (t, J = 4 Hz, 8H, TEG), 3.39 (d, J = 20 Hz, 4H, propargyl CH2), 3.69 (m, 56H, TEG), 3.28 (s,12H, TEG-CH3), 2.81 (m, 8H, TEG), 2.25 (d, J = 20 Hz, 2H, propargyl CH), −2.59 (s, 4H, Hinner); 13C-NMR (100 MHz, CDCl3)δ (ppm) 148.40, 140.98, 140.92, 139.92, 139.81, 134.08-133.96, 132.27, 130.66, 129.60, 128.28, 128.92, 124.97, 120.58, 119.82, 119.75, 103.35, 74.42, 74.39, 72.01, 70.91, 70.86, 70.83, 70.65, 70.48, 70.14, 37.84, 31.39, 59.09, 37.17.
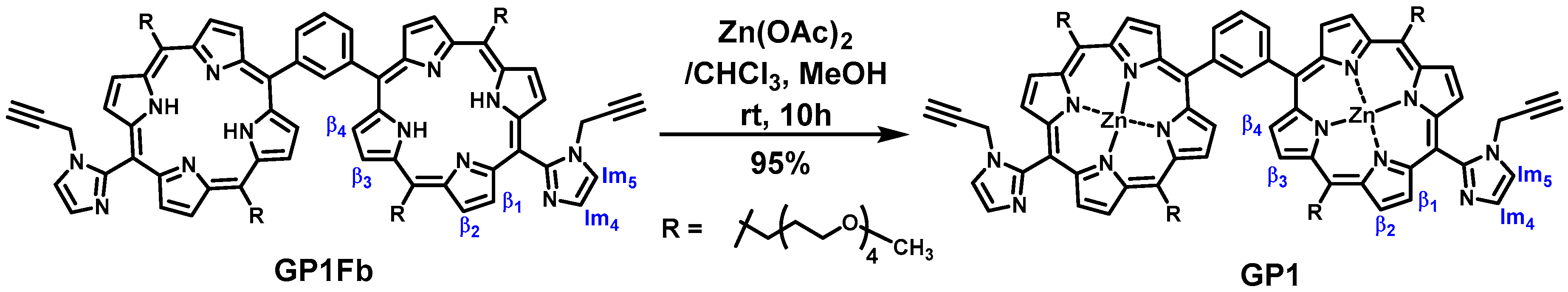
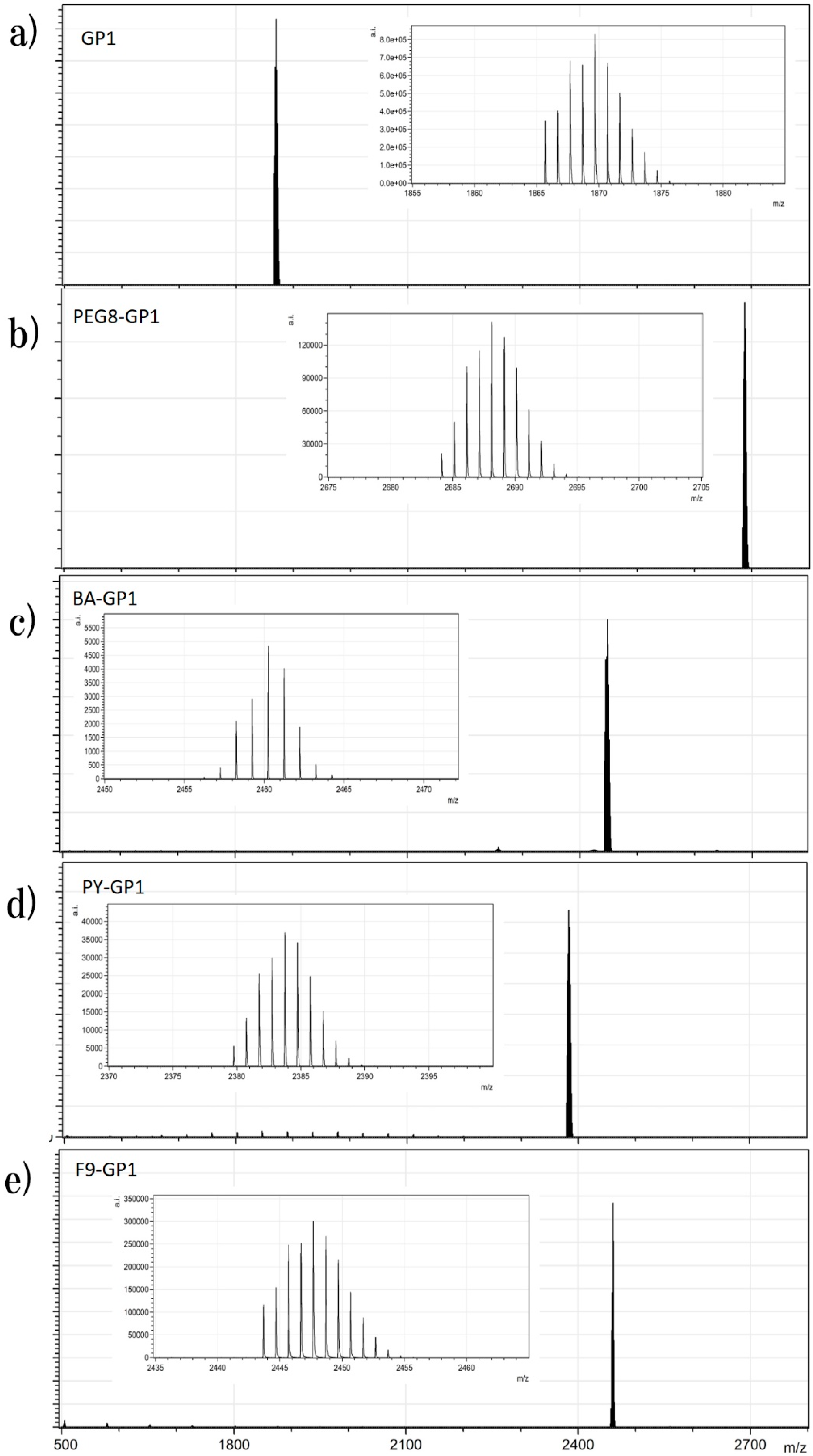
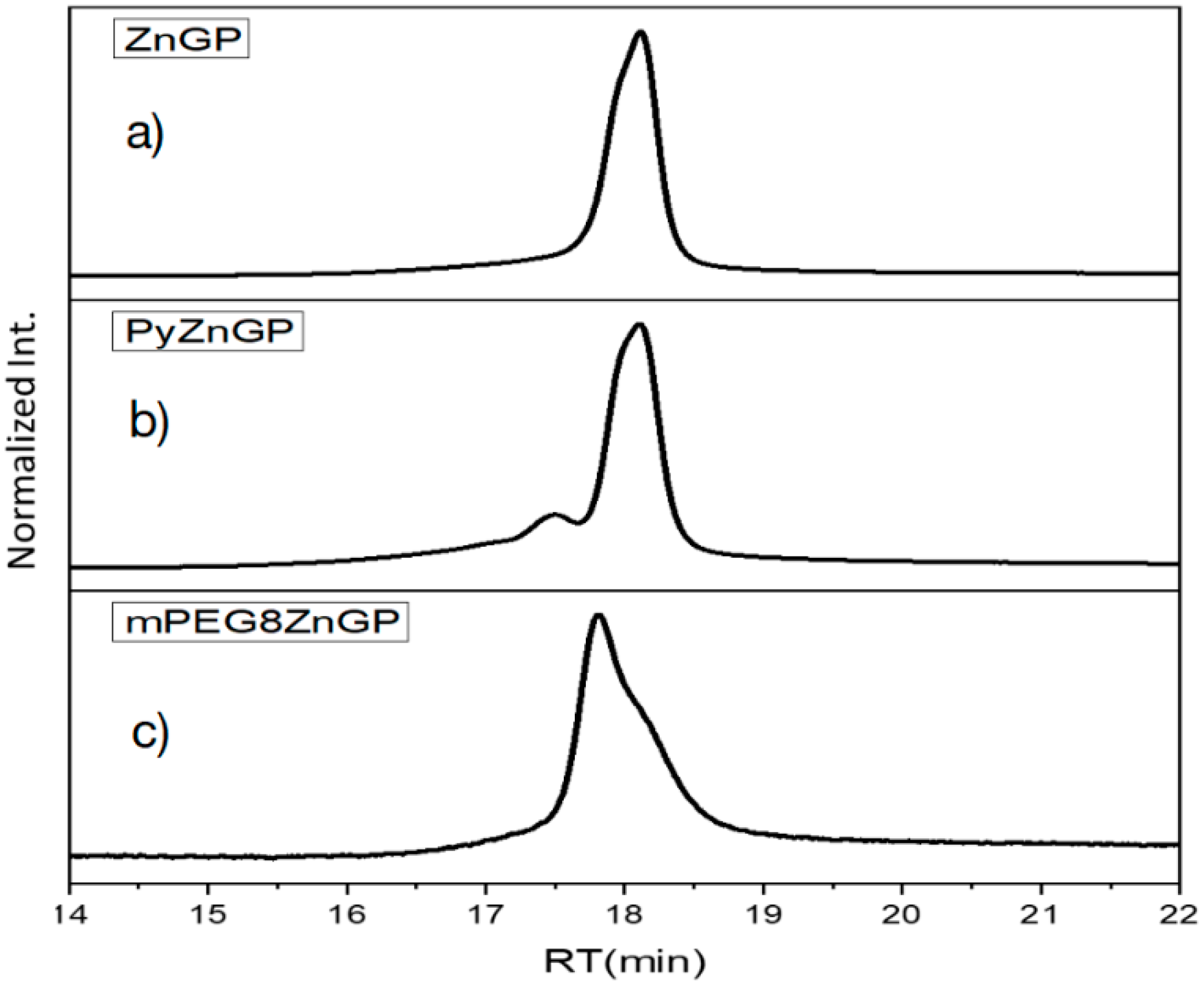
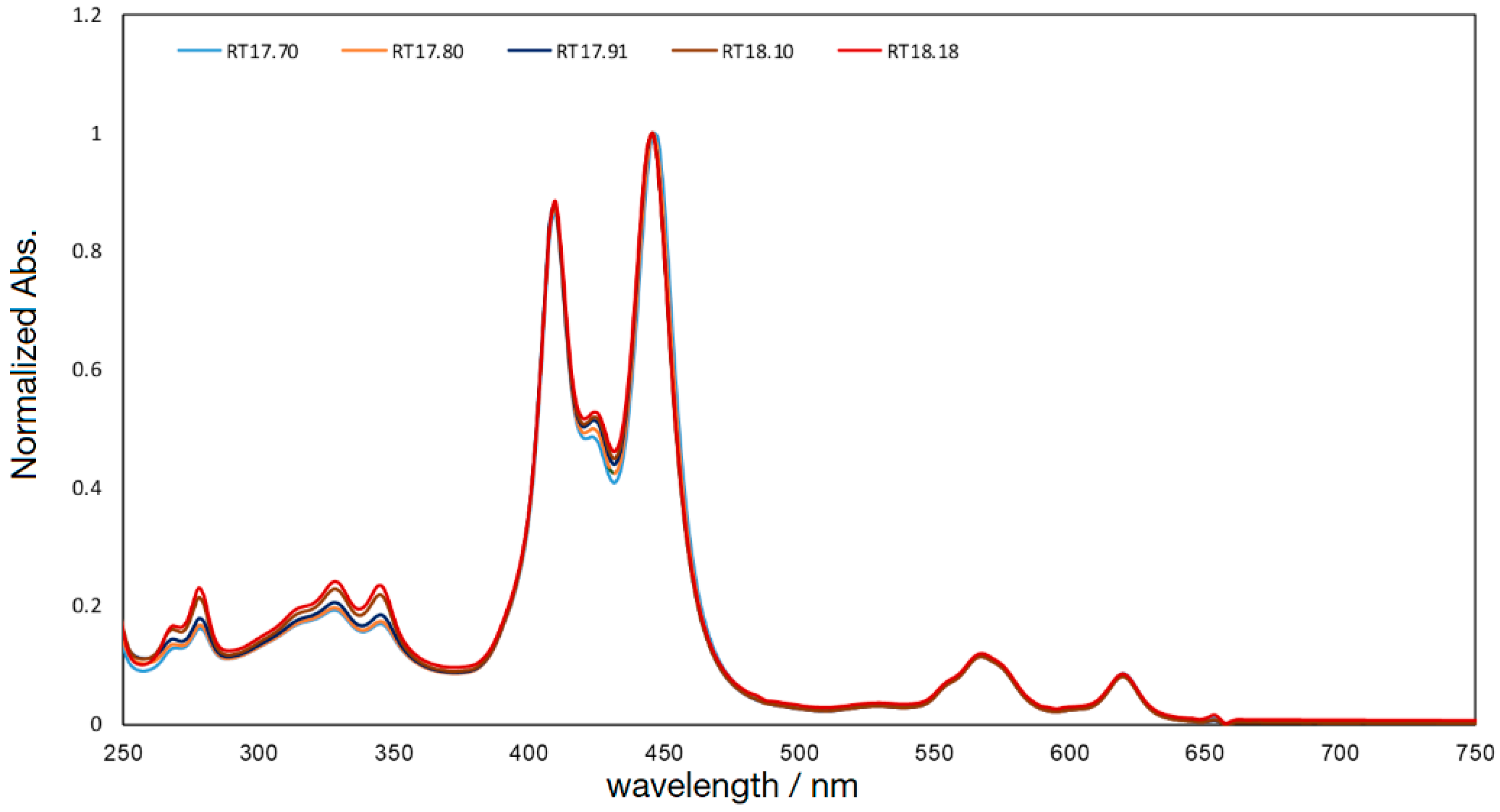
GablePorphyrin-Zn (GP1). GP 1Fb (130 mg, 7.56 × 10−5 mol) was dissolved in chloroform (6 mL) in a 25 mL flask. A saturated methanol solution of Zn(OAc)2 (100 mg, 5.45 × 10−4 mol) was added to the solution, and the mixture was stirred for 10 h at rt in the dark. The resulting solution was washed with water (×3) and passed through Phase Separator paper (Whatman). The solvent was evaporated to dryness, giving GP1 as a purple solid (132 mg, 95%). HPLC-PDA (System 1, flow rate 1.0 mL/min) 21.6 min; MALDI-TOF-MS (matrix: DCTB) found m/z [M]+ 1865.6761, calcd for [C98H114N12O16Zn2]+ 1865.6956.
PEG8-5Zn. To an acetonitrile solution (1 mL) containing 5Zn (4.3 mg, 4.0 × 10−6 mol) and PEG8-N3 (10, 10 mg, 2.4 × 10−5 mol) in a 10 mL flask was added CuI (~1 mg). The mixture was stirred for 8 days at rt in the dark under Ar atmosphere. Disappearance of the starting materials was confirmed with TLC. The mixture was passed through a Celite pad with methanol. The filtrate was evaporated to dryness, and the residue was purified with a silica gel column (φ1 cm × 9 cm, chloroform:methanol = 10:1). Fractions containing target compound was concentrated to dryness, and residue was reprecipitated from chloroform and hexane, giving a purple solid (4.8 mg, 81%). MALDI-TOF-MS (matrix: DCTB) found m/z [M]+ 1483.6199, calcd for [C75H105N9O18Zn]+ 1483.6869; 1H-NMR (500 MHz, CDCl3) δ (ppm) 9.61 (d, J = 2.7 Hz, 4H, β), 9.03 (d, J = 2.4 Hz, 4H, β), 8.93 (d, J = 2.7 Hz, 4H, β), 8.76 (s, 1H, Ph2), 8.63 (d, J = 3.9 Hz, 1H, Ph6), 8.24 (s, 1H, Ph2), 8.12 (d, J = 4.2 Hz, 1H, Ph6), 8.08 (t, J = 4.5 Hz, 2H, Ph4), 7.98 (t, J = 4.5 Hz, 1H, Ph5), 7.82 (t, J = 4.5 Hz, 1H, Ph5), 5.93, 7.92 (each s, 2H, acetal-CH), 5.87, 5.83 (each s, 2H, Im5), 5.26 (d, J = 2.4 Hz, 4H, β), 5.21–5.10 (m, 8H, TEG), 4.30–4.27 (m, 2H, TEG), 4.10–2.95 (m, TEG, triazole, trazole-CH2), 2.71–2.68 (m, 2H, TEG), 2.33–2.87 (m, 4H, TEG), 2.12–2.05 (m, 2H, Im4,), 1.53, 1.39 (each s, 6H, acetal- CH3), 0.95, 0.84 (each s, 6H, acetal-CH3), 1.70–1.12 (m, TEG).
Py-5Zn. In a 10 mL flask, Py-N3[33] (11, 10 mg, 2.4 × 10−5 mol), 5Zn (5.2 mg, 4.6 × 10−6 mol), and CuI (~1 mg) were dissolved in chloroform (ca. 1 mL) and 2,6-lutidine (a few drops). The mixture was stirred for 3 days at rt in the dark under Ar atmosphere. The target compound Py-5Zn was confirmed with MALDI-TOF MS. The mixture was washed with 0.5 M ethylenediaminetetraacetic acid (EDTA) aqueous solution (pH 8) and water subsequently, and the organic layer was passed through Phase Separator paper (Whatman). The filtrate was evaporated to dryness and the residue was purified with a silica gel column (φ1 cm × 20 cm, chloroform → chloroform:methanol = 10:1). The green band eluted with chloroform:methanol = 10:1 was collected, and the fraction was concentrated, giving a purple solid (5.8 mg, 95%). MALDI-TOF-MS (matrix: dithranol) found m/z [M + Na]+ 1354.5297, calcd for [C55H68N6O10 + Na]+ 1354.5296; 1H-NMR (400 MHz, CDCl3) δ (ppm) 9.68(d, J = 4.4 Hz, 2H, β), 9.15 (d, J = 4.8 Hz, 2H, β), 8.89 (s, 0.5H, Ph2), 8.78 (d, J = 4.8 Hz, 2H, β), 8.75 (d, J = 7.6 Hz, 0.5H, Ph6), 8.38 (s, 0.5H, Ph2), 8.20 (d, J = 7.2 Hz, 0.5H, Ph6), 8.12 (d, J = 7.6 Hz, 1H, Ph4), 8.04 (t, J = 7.6 Hz, 0.5H, Ph5), 7.86-7.82 (m, 1H, Ph5), 7.84-7.82 (m, 1.5H, Py), 7.71–7.62 (m, 2.5H, Py), 7.47 (d, J = 7.2 Hz, 1H, Py), 7.36–7.31 (m, 1H, Py), 6.31–6.14 (m, 2H, Py), 5.98, 5.71 (each s, 2H, acetal-CH), 5.86–5.78 (m, 1H, Im5, Py), 5.21 (dd, J = 4.4 Hz, 2H, β), 5.22–5.08 (m, 4H, TEG), 4.97–4.82 (m, 1H, Py), 4.08–3.49 (m, TEG), 3.31 (s, 6H, TEG-CH3), 3.23 (m, 4H, triazol- CH2), 3.07–3.02 (m, TEG), 2.14–2.20 (m, 1H, Im4), 2.09, 2.07, 2.04 (each s, 1H, triazole), 1.56, 1.34 (each s, 3H, acetal-CH3), 0.98, 0.82 (each s, 3H, acetal-CH3).
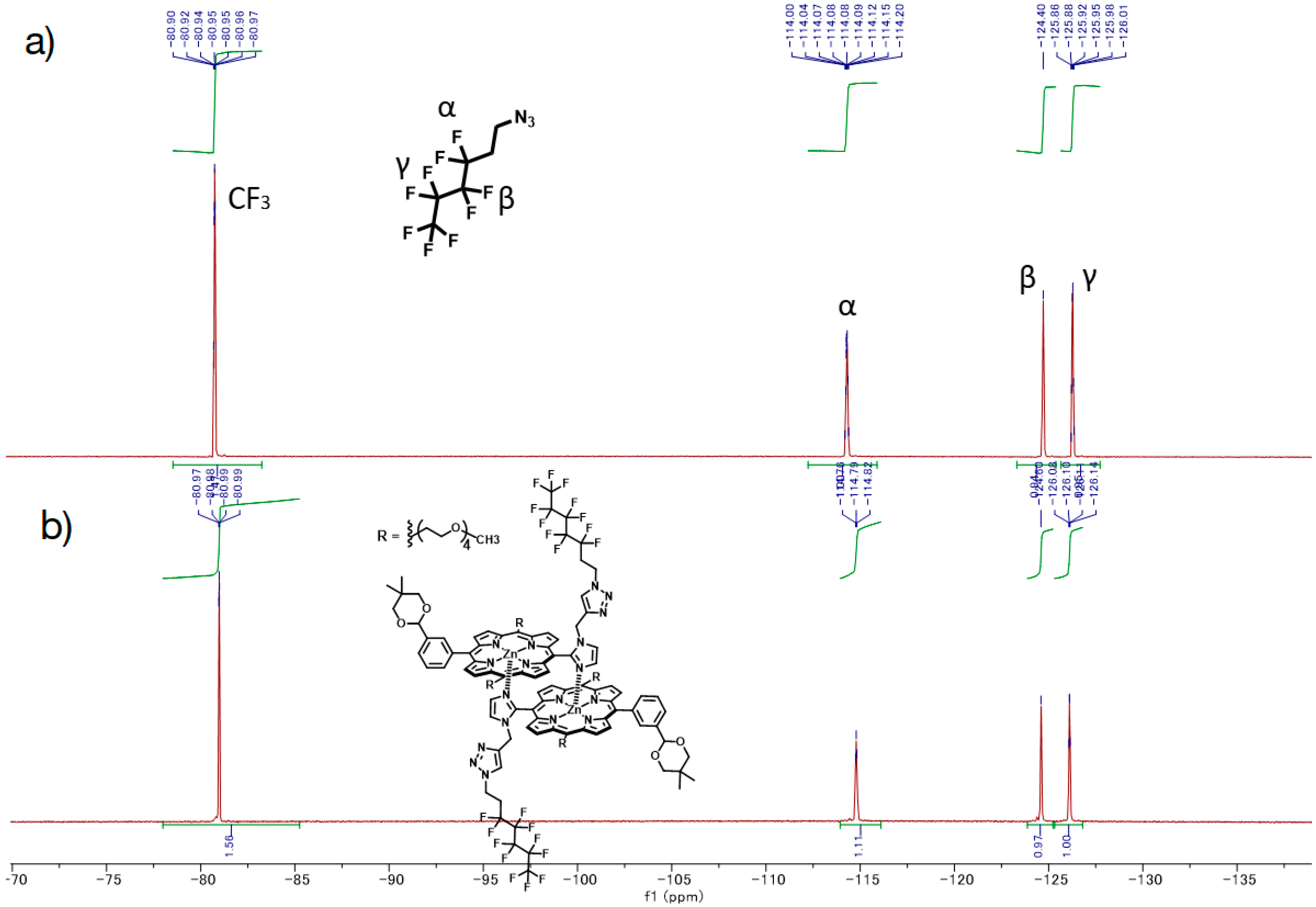
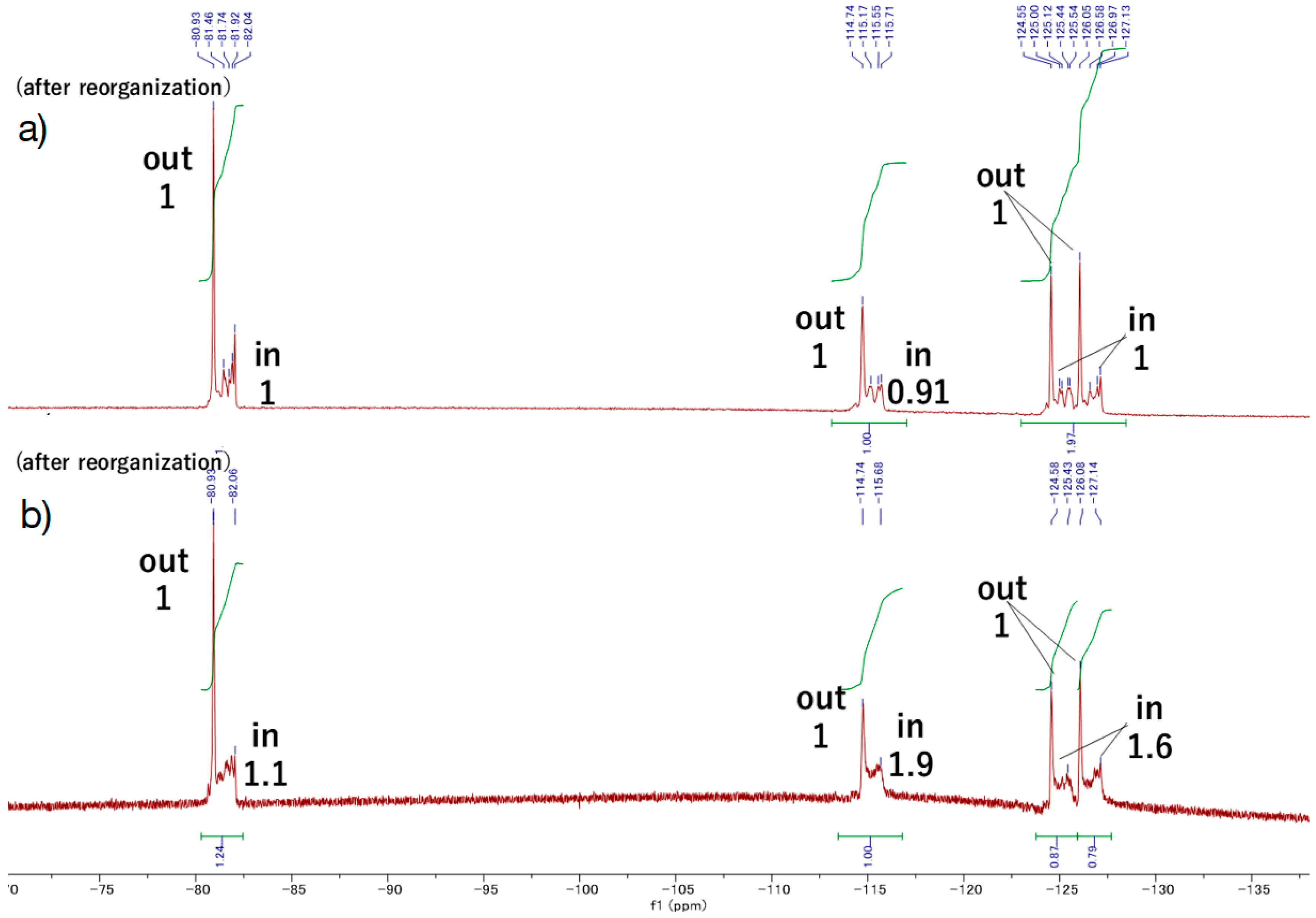
F9-5Zn. In a 10 mL flask, CuI (~1 mg) and 5Zn (7.2 mg, 6.7 × 10−6 mol) were dissolved in chloroform (ca. 1 mL) and 2,6-lutidine (three drops). F9-N3[34] (13, 19 mg, 6.7 × 10−5 mol) was added in the dark, and the mixture was stirred for 3 days at rt in the dark under Ar atmosphere. Disappearance of the starting materials and formation of the target compound were confirmed with MALDI-TOF MS. The mixture was washed with 0.5 M EDTA aqueous solution (pH 8) and water subsequently, and the organic layer was passed through Phase Separator paper (Whatman). The filtrate was evaporated to dryness and the residue was purified with a silica gel column (φ1 cm × 20 cm, ethyl acetate → chloroform:methanol = 5:1). The green band was collected, and the fraction was concentrated, giving F9-5Zn as a purple solid (8.1 mg, 88%). MALDI-TOF-MS (matrix: DCTB) found m/z [M + Na]+ 1386.4079, calcd for [C64H74N9O10 + Na]+ 1386.4604. 1H-NMR (400 MHz, CDCl3) δ (ppm) 9.63 (d, J = 4.4 Hz, 4H, β), 9.05 (d, J = 4.4 Hz, 4H, β), 8.87 (d, J = 4.4 Hz, 4H, β), 8.76 (s, 1H, Ph2), 8.62 (d, J = 7.6 Hz, 1H, Ph6), 8.27 (s, 1H, Ph2), 8.13 (d, J = 7.2 Hz, 1H, Ph6), 8.08 (t, J = 7.6 Hz, 2H, Ph4), 7.98 (t, J = 7.6 Hz, 1H, Ph5), 7.81 (t, J = 7.6 Hz, 1H, Ph5), 5.92, 5.71 (each s, 2H, acetal-CH), 5.84, 5.81 (each s, 2H, Im5), 5.26 (t, J = 4.4 Hz, 4H, β), 5.23-5.11 (m, J = 6.6 Hz, 8H, TEG), 4.04-3.51 (m, 56H, TEG), 3.33 (s,12H, TEG-CH3), 3.08-2.98 (m, 8H, TEG), 3.22 (t, J = 4.8 Hz, 4H, Fluorine-H1), 2.17, 2.12 (each s, 2H, Im4), 1.81-1.65 (m, 4H, Fluorine-H2), 1.52, 1.37 (each s, 6H, acetal-CH3), 0.95, 0.83 (each s, 6H, acetal-CH3); 19F-NMR (376 MHz, CDCl3) δ(ppm) −80.99 (3F, CF3), −114.79 (2F, CF2α), −124.60 (2F, CF2β), -126.11 (2F, CF2γ).
PEG8-GP1. According to a similar procedure to give Py-5Zn and F9-5Zn, PEG8-GP1 (14.8 mg) was prepared from PEG8-N3 (18 mg, 4.4 × 10−5 mol), GP1 (12 mg, 6.3 × 10−6 mol), CuI (8 mg, 4.0 × 10−5 mol), and 2,6-lutidine (12 mg, 1.0 × 10−4 mol) in a 88% yield as a purple solid. HPLC-PDA (System 1, flow rate 1.0 mL/min) 20.4 min; MALDI-TOF-MS (matrix: DCTB) found m/z [M + Na]+ 2684.1042, calcd for [C132H184N18O32Zn2 + Na]+ 2684.1805.
Py-GP1. According to a similar procedure to give Py-5Zn and F9-5Zn, Py-GP1 (13 mg) was prepared from Py-N3 (7 mg, 2.7 × 10−5 mol) and GP1 (10 mg, 5.4 × 10−6 mol) in a quantitative yield. HPLC-PDA (System 1, flow rate 1.0 mL/min) 21.4 min; MALDI-TOF-MS (matrix: DCTB) found m/z [M + Na]+ 2379.8734, calcd for [C132H136N18O16Zn2Na]+ 2379.8862.
BA-GP1. According to a similar procedure to give Py-5Zn and F9-5Zn, BA-GP1 (5.5 mg) was prepared from BA-N3 (45 mg, 1.5 × 10−5 mol) and GP1 (2.5 mg, 1.4 × 10−6 mol) in a quantitative yield. HPLC-PDA (System 1, flow rate 1.0 mL/min) 20.8 min; MALDI-TOF-MS (matrix: DCTB) found m/z [M + Na]+ 2456.2474, calcd for [C134H188N18O16Zn2Na]+ 2456.2931.
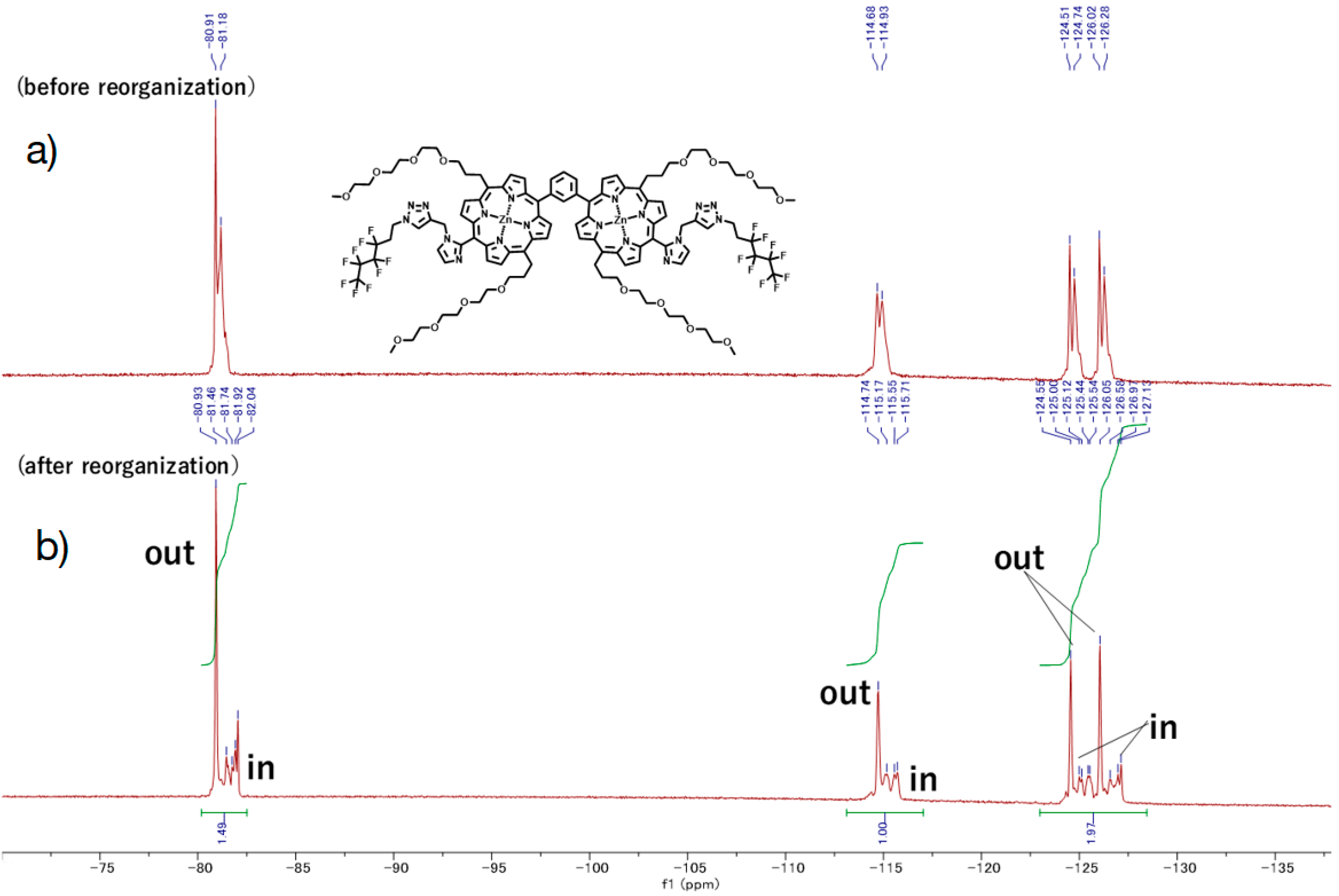
F9-GP1. According to a similar procedure to give Py-5Zn and F9-5Zn, F9-GP1 (5.5 mg) was prepared from GP1 (3.5 mg) and F9-N3 (13, 16.8 mg, 5.8 × 10−5 mol) in a quantitative yield. HPLC-PDA (System 1, flow rate 1.0 mL/min) 21.0 min; MALDI-TOF-MS (matrix: DCTB) found m/z [M + Na]+ 2443.6951, calcd for [C110H122F18N18O16Zn2Na]+ 2443.7479.