Antifungal Effect of Magnolol and Honokiol from Magnolia officinalis on Alternaria alternata Causing Tobacco Brown Spot
Abstract
1. Introduction
2. Results and Discussion
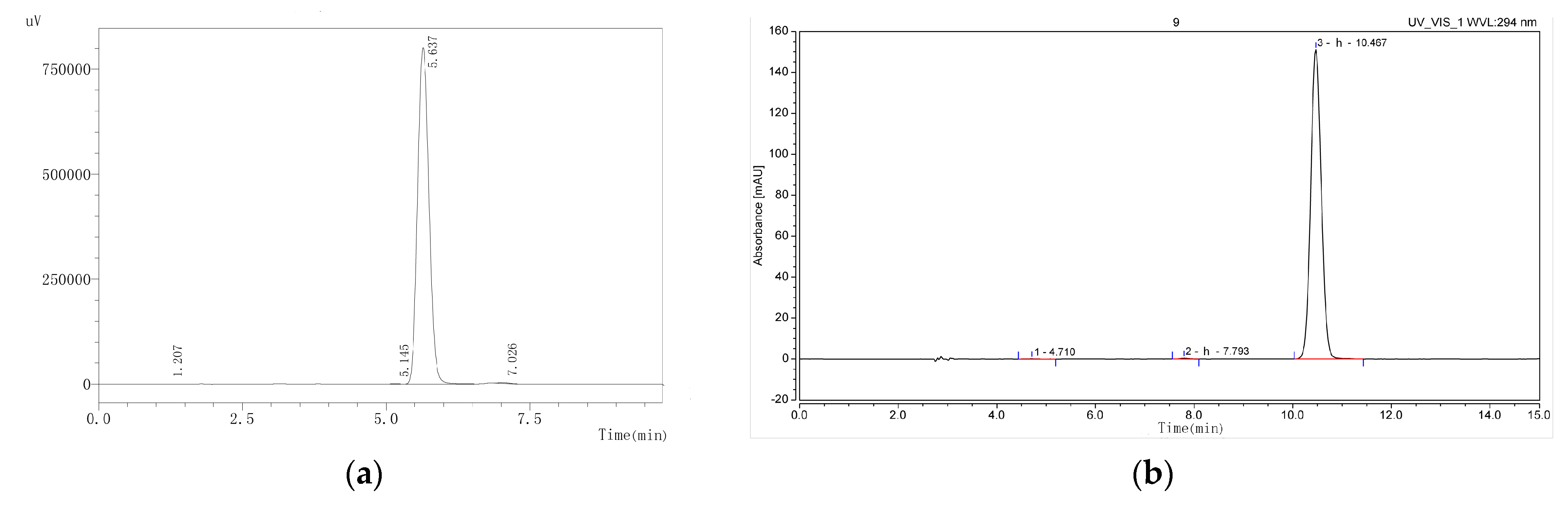
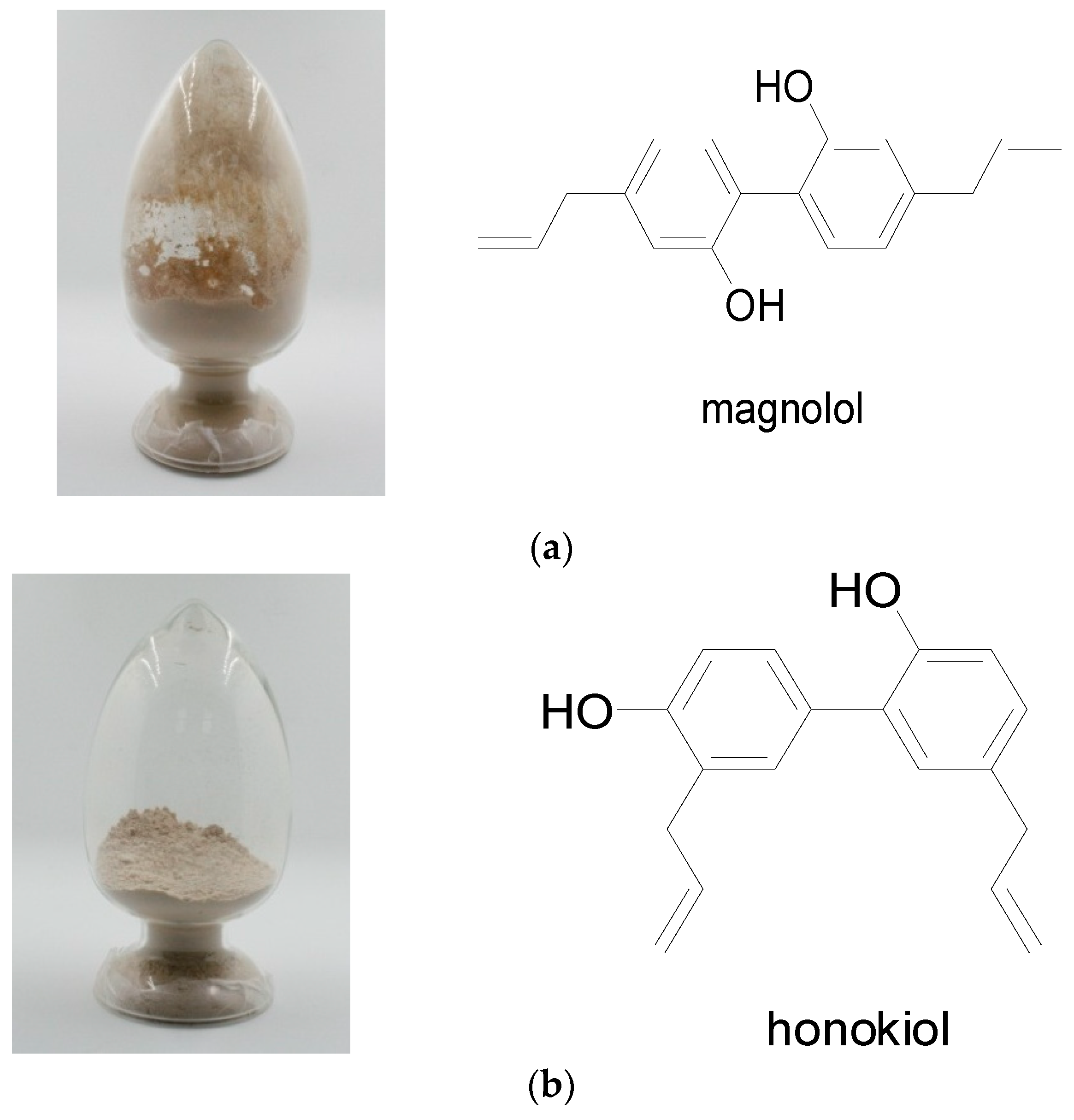
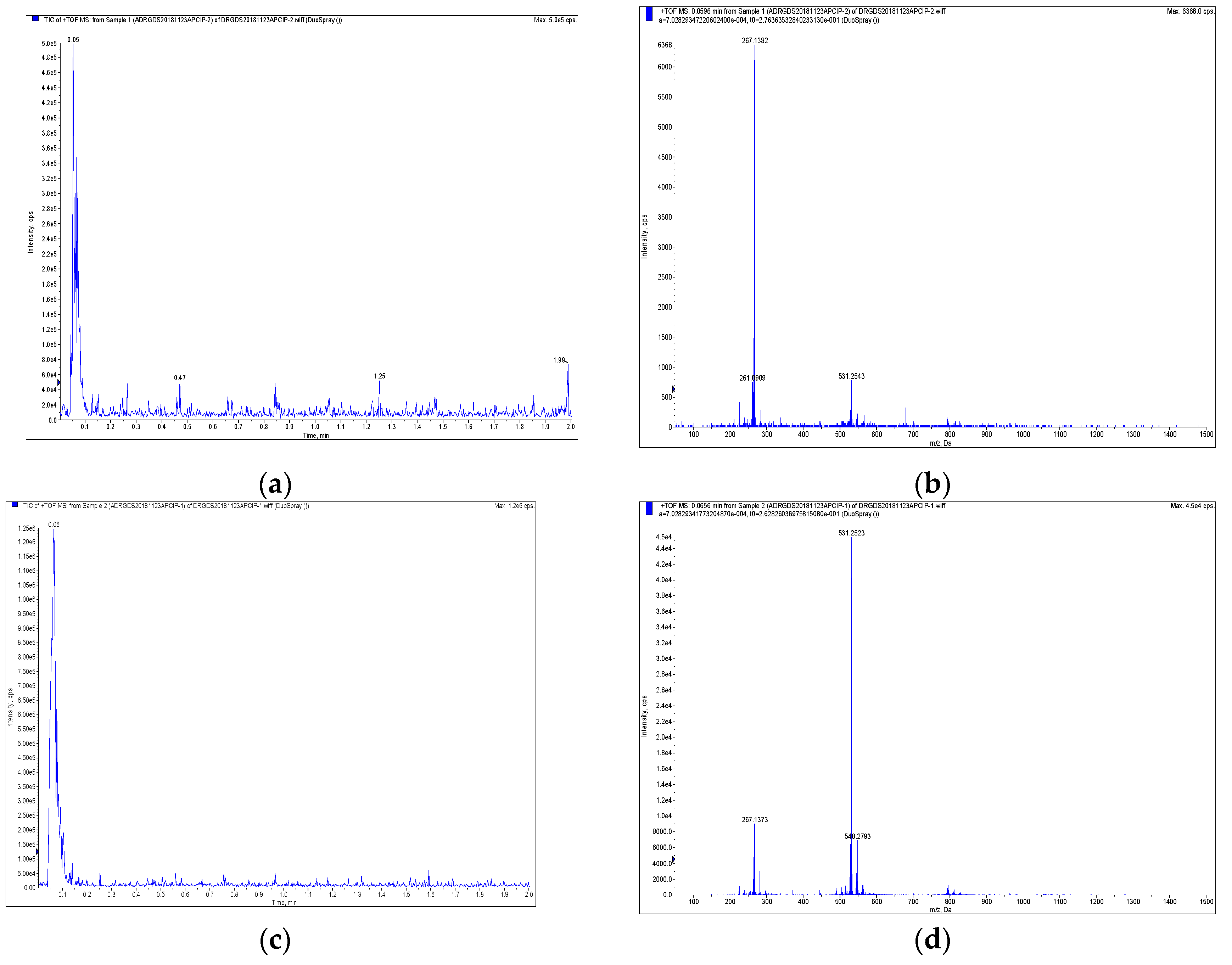
2.1. Identification of Magnolol and Honokiol from M. officinalis
2.2. Antifungal Activity of Magnolol and Honokiol against A. alternata
2.3. Synergistic Effect of Magnolol and Honokiol
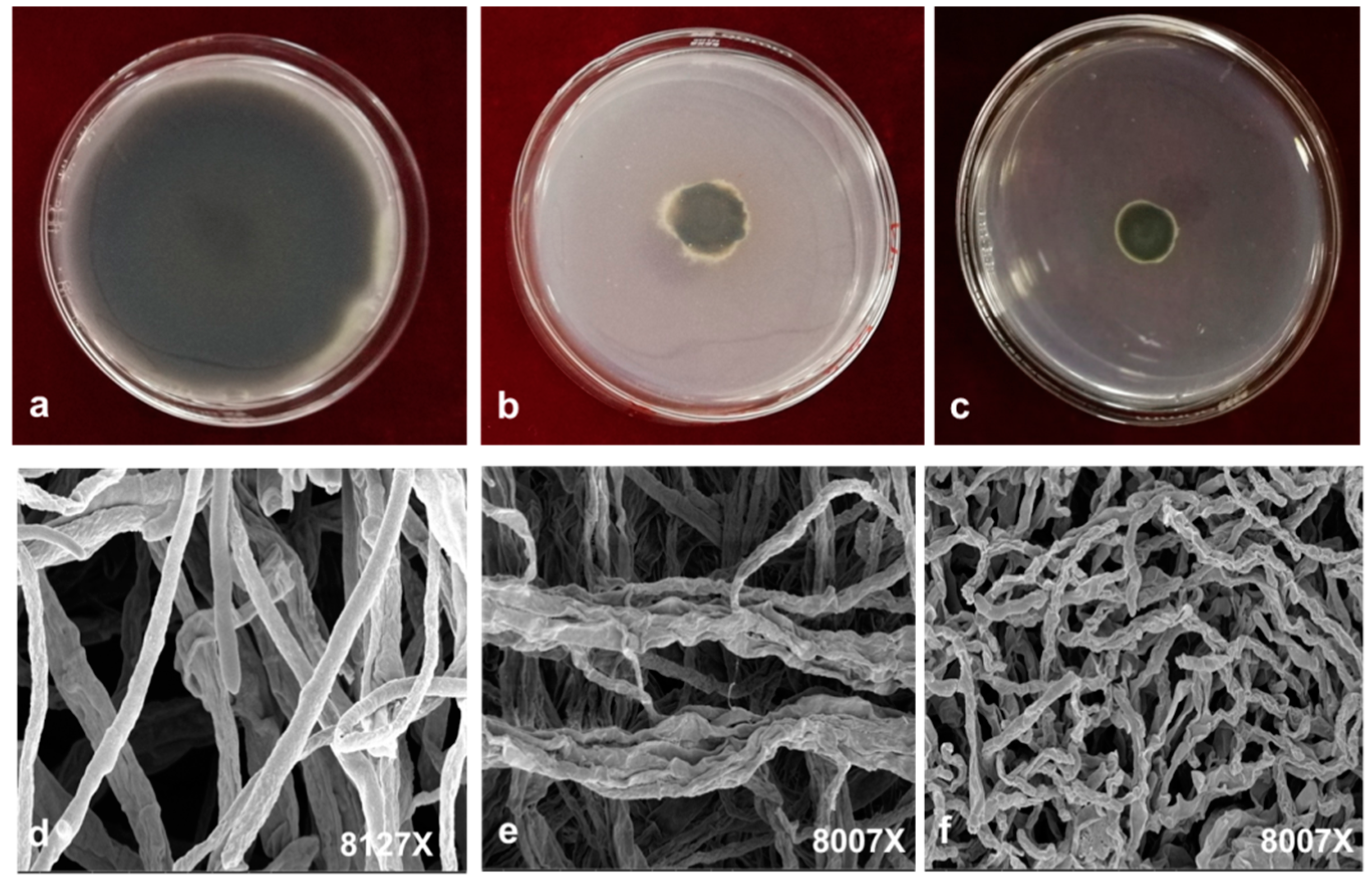
2.4. Growth Inhibition and the Effect on Morphology
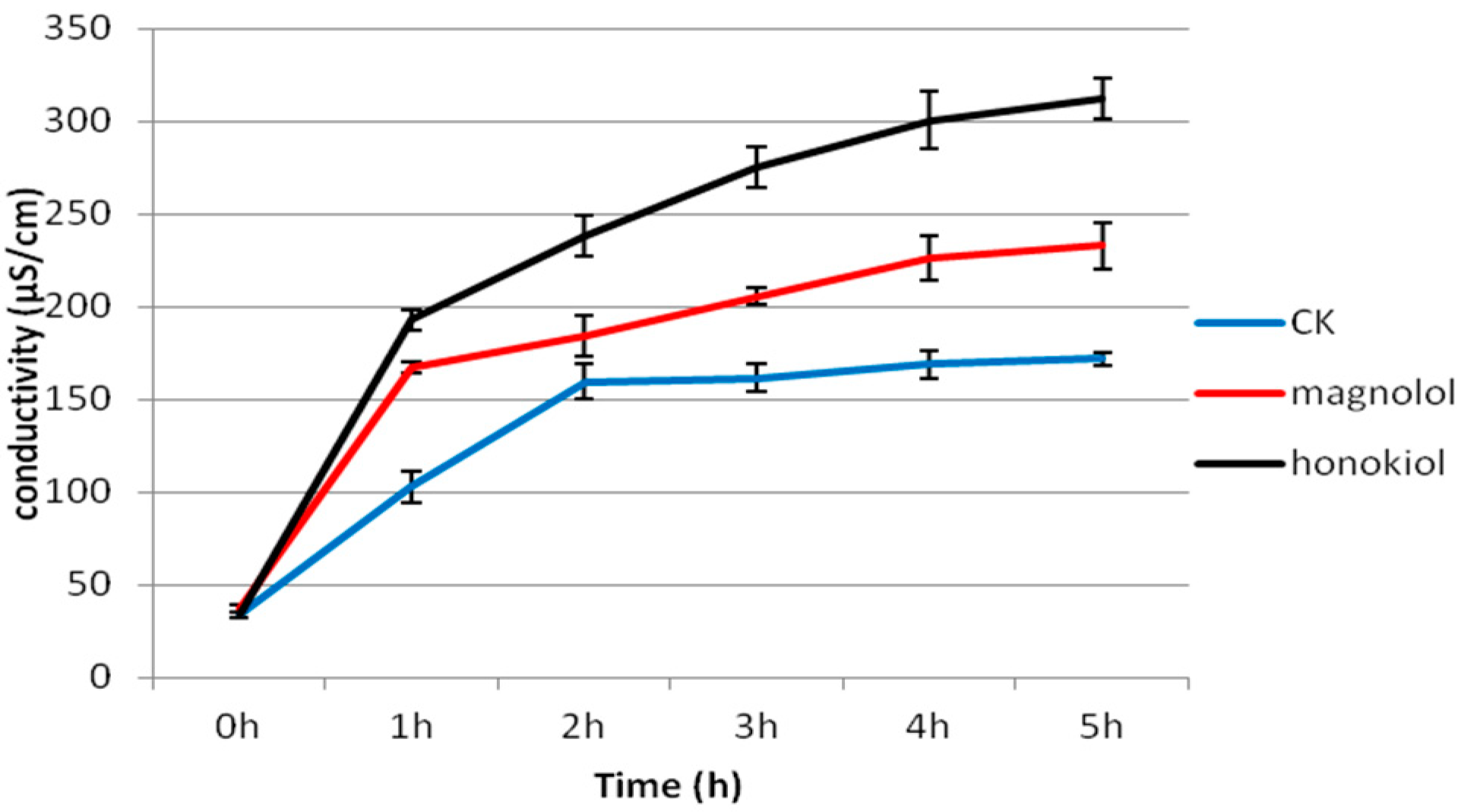
2.5. Effects of Magnolol and Honokiol on Cell Membrane Permeability of A. alternata
2.6. The Inhibitory Efficiency of Magnolol and Honokiol on Six Phytopathogens
3. Materials and Methods
3.1. Chemicals and Media
3.2. Fungal Strains
3.3. Isolation and Purification of Active Compounds and Structure Analysis
3.4. Antifungal Activity of Magnolol and Honokiol on A. alternata
3.5. Synergistic Effect Test
3.6. Scanning Electron Microscopy (SEM) Analysis
3.7. Cell Membrane Permeability Test
3.8. Antifungal Activity Test
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lei, Z.Q. The wet medicine. In The Chinese Materia Medica, 1st ed.; Shanghai Science and Technology Press: Shanghai, China, 1995; p. 128. [Google Scholar]
- Choi, J.H.; Ha, J.; Park, J.H.; Lee, J.Y.; Lee, Y.S.; Park, H.J. Costunolide triggers apoptosis in human leukemia U937 cells by depleting intracellular thiols. Cancer Sci. 2002, 93, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Lee, K.H.; Han, M.H.; Lee, H.; Ahn, J.M.; Han, S.B.; Han, G.; Lee, K.; Park, S.K.; Kim, H.M. Antiinflammatory activity of methanol extract isolated from stem bark of Magnolia kobus. Phytother. Res. 2008, 22, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.W.; Tsai, K.; Chin, J.H.; Chan, W.L.; Hong, C.Y. Magnolol attenuates peroxidative damage and improves survival of rats with sepsis. Shock 2000, 13, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Lee, B.J.; Nam, S.Y.; Lee, S.I.; Kim, Y.H.; Kim, K.H.; Oh, K.W.; Hong, J.T. Inhibitory effect of ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on memory impairment and neuronal toxicity induced by beta-amyloid. Pharmacol. Biochem. Behav. 2009, 95, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Bae, K.H.; Tsunezuka, M.; Namba, T. Studies on dental caries prevention by traditional Chinese medicines (part I): Screening of crude drugs for antibacterial action against streptococcus mutans. Jpn. J. Pharmacogn. 1981, 35, 295–302. [Google Scholar]
- Tan, Z.X.; Yang, L.X. Biological control of tobacco brown spot disease: Present and future. Acta Tab. Sin. 2005, 11, 34–38. [Google Scholar]
- Tachikawa, E.; Takahashi, M.; Kashimoto, T. Effects of extract and ingredients isolated from Magnolia obovata thunberg on catecholamine secretion from bovine adrenal chromaffin cells. Biochem. Pharmacol. 2000, 60, 433–440. [Google Scholar] [CrossRef]
- LaMondia, J.A. Outbreak of brown spot of tobacco caused by Alternaria alternata in Connecticut and Massachusetts. Plant Dis. 2001, 85, 230. [Google Scholar] [CrossRef]
- Chen, M.C.; Lee, C.F.; Huang, W.H.; Chou, T.C. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1alpha/VEGF signaling pathway in human bladder cancer cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef]
- Chuang, T.C.; Hu, S.C.; Cheng, Y.T.; Shao, W.S.; Wu, K.; Fang, G.S.; Ou, C.C.; Wang, V. Magnolol down-regulates HER2 gene expression, leading to inhibition of HER2-mediated metastatic potential in ovarian cancer cells. Cancer Lett. 2011, 311, 11–19. [Google Scholar] [CrossRef]
- Sun, L.M.; Liao, K.; Liang, S.; Yu, P.-H.; Wang, D.-Y. Synergistic activity of magnolol with azoles and its possible antifungal mechanism against, Candida albicans. J. Appl. Microbiol. 2015, 118, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.H.; Kim, Y.K.; Min, B.S.; Na, M.K.; Rhee, Y.H.; Lee, J.P.; Bae, K.H. Antifungal activity of magnolol and honokiol. Arch. Pharm. Res. 2000, 23, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.H.; Choi, G.J.; Min, B.S.; Jang, K.S.; Choi, Y.H.; Kang, M.S.; Park, M.S.; Choi, J.E.; Bae, B.K.; Kim, J.C. Effects of neolignans from the stem bark of Magnolia obovata on plant pathogenic fungi. J. Appl. Microbiol. 2010, 106, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Li, D.Z. Researching advance of tobacco brown spot caused by Alternaria alternata. J. Northeast Agric. Univ. 2000, 1, 80–85. [Google Scholar]
- Yi, L.; Xiao, C.G. Advances in studies on control of tobacco brown spot. Plant Prot. 2003, 5, 10–14. [Google Scholar]
- Li, S.Y.; Guan, B.Q.; Wei, F.J. The major disease of tobacco. In Technical Guide for Tobacco Production, 1st ed.; China Agriculture Press: Beijing, China, 2010; pp. 169–170. [Google Scholar]
- Chen, C.R.; Tan, R.; Qu, W.M.; Wu, Z.; Wang, Y.; Urade, Y.; Huang, Z.L. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, exerts antiepileptic effects via the GABA/benzodiazepine receptor complex in mice. Br. J Pharmacol. 2011, 164, 1534–1546. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.Y.; Gao, M.; Ma, G.H.; Chen, G.K.; Yu, Q.T. Indoor screening and field trial of fungicides against tobacco brown spot. Chin. Tob. Sci. 2017, 38, 73–77. [Google Scholar]
- Jing, C.L.; Zhao, J.; Han, X.B.; Huang, R.H.; Cai, D.S.; Zhang, C.S. Essential oil of Syringa oblata Lindl. as a potential biocontrol agent against tobacco brown spot caused by Alternaria alternata. Crop Prot. 2018, 104, 41–46. [Google Scholar] [CrossRef]
- Mi, Y.Y.; Byeong, J.C.; Jin, C.K. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar]
- Shin, K.S.; Lee, S.; Cha, B. Antifungal activity of plumbagin purified from leaves of Nepenthes ventricosa x maxima against phytopathogenic fungi. Plant Pathol. 2007, 23, 113–115. [Google Scholar] [CrossRef]
- Kijjoa, A.; Pinto, M.M.M.; Tantisewie, B.; Herz, W. A biphenyl type neolignan and biphenyl ether from Magnolia henryi. Phytochemistry 1989, 28, 1284–1286. [Google Scholar] [CrossRef]
- Ho, K.Y.; Tsai, C.C.; Chen, C.P.; Huang, J.S.; Lin, C.C. Antimicrobial activity of honokiol and magnolol isolated from Magnolia officinalis. Phytother. Res. 2001, 15, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.Q.; Geng, Y.L.; Li, F.W.; Zheng, C.C. Isolation and purification of honokiol and magnolol from cortex Magnoliae officinalis by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1036, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Boudina, A.; Emmelin, C.; Baaliouamer, A.; Grenier-Loustalot, M.F.; Chovelon, J.M. Photochemical behaviour of carbendazim in aqueous solution. Chemosphere 2003, 50, 649–655. [Google Scholar] [CrossRef]
- Holtman, M.A.; Kobayashi, D.Y. Identification of Rhodococcus erythropolis isolates capable of degrading the fungicide carbendazim. Appl. Microbiol. Biotechnol. 1997, 47, 578–582. [Google Scholar] [CrossRef]
- Edris, A.E.; Farrag, E.S. Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Nahrung 2003, 47, 117–121. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control 2011, 22, 1707–1714. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Amin, B.H.; Baka, Z.A. Efficiency of pomegranate (Punica granatum L.) peels extract as a high potential natural tool towards Fusarium dry rot on potato tubers. Postharvest Biol Tec. 2016, 111, 256–263. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Paul, S.; Dubey, R.C.; Maheswari, D.K.; Kang, S.C. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 2011, 22, 725–731. [Google Scholar] [CrossRef]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 114, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Concentration (mg/mL) | Mycelial Growth Inhibition (%) | |||
|---|---|---|---|---|
| Magnolol | Honokiol | Eugenol | Carbendazim | |
| 0 | 0 | 0 | - | - |
| 0.001 | 7 ± 3.18 | 9 ± 0.81 | - | 58 ± 4.64 |
| 0.005 | 16 ± 3.98 | 20 ± 2.29 | - | |
| 0.01 | 23 ± 2.88 | 26 ± 1.63 | - | - |
| 0.1 | 77 ± 2.09 | 91 ± 1.56 | 11 ± 4.02 | - |
| 1 | 90 ± 3.02 | 100 * | - | - |
| 3 | 100 * | 100 * | - | - |
| 5 | 100 * | 100 * | - | - |
| 7 | 100 * | 100 * | - | - |
| The Volume Ration of Magnolol (0.1 mg/mL) and Honokiol (0.1 mg/mL) | Mycelial Growth Inhibition (%) |
|---|---|
| 1:0 | 77 ± 2.09 |
| 1:1 | 84 ± 3.85 |
| 1:4 | 89 ± 2.77 * |
| 1:9 | 88 ± 1.19 |
| 9:1 | 87 ± 1.74 |
| 4:1 | 80 ± 2.11 |
| 0:1 | 91 ± 1.56 |
| Pathogen | Mycelial Growth Inhibition Rate (%) | |
|---|---|---|
| Magnolol | Honokiol | |
| (0.1 mg/mL) | (0.1 mg/mL) | |
| P. expansum (Link) Thom | 80 ± 3.95 * | 81± 0.23 |
| Alternaria dauci f.sp. solani | 70 ± 5.26 | 80 ± 9.23 * |
| F. moniliforme J. Sheld | 79 ± 0.69 | 82 ±0.69 * |
| F. oxysporum Schltdl. | 76 ± 1.54 * | 89 ± 1.55 |
| V. mali Miyabe & G. Yamada | 100 * | 100 * |
| R. solani J.G. Kühn | 57 ± 2.74 | 68 ± 1.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Lu, M.-H.; Guo, D.-S.; Zhai, Y.-Y.; Miao, D.; Yue, J.-Y.; Yuan, C.-H.; Zhao, M.-M.; An, D.-R. Antifungal Effect of Magnolol and Honokiol from Magnolia officinalis on Alternaria alternata Causing Tobacco Brown Spot. Molecules 2019, 24, 2140. https://doi.org/10.3390/molecules24112140
Chen Y-H, Lu M-H, Guo D-S, Zhai Y-Y, Miao D, Yue J-Y, Yuan C-H, Zhao M-M, An D-R. Antifungal Effect of Magnolol and Honokiol from Magnolia officinalis on Alternaria alternata Causing Tobacco Brown Spot. Molecules. 2019; 24(11):2140. https://doi.org/10.3390/molecules24112140
Chicago/Turabian StyleChen, Ya-Han, Mei-Huan Lu, Dong-Sheng Guo, Ying-Yan Zhai, Dan Miao, Jian-Ying Yue, Chen-Hong Yuan, Ming-Min Zhao, and De-Rong An. 2019. "Antifungal Effect of Magnolol and Honokiol from Magnolia officinalis on Alternaria alternata Causing Tobacco Brown Spot" Molecules 24, no. 11: 2140. https://doi.org/10.3390/molecules24112140
APA StyleChen, Y.-H., Lu, M.-H., Guo, D.-S., Zhai, Y.-Y., Miao, D., Yue, J.-Y., Yuan, C.-H., Zhao, M.-M., & An, D.-R. (2019). Antifungal Effect of Magnolol and Honokiol from Magnolia officinalis on Alternaria alternata Causing Tobacco Brown Spot. Molecules, 24(11), 2140. https://doi.org/10.3390/molecules24112140




