Phagocytosis of a PFOB-Nanoemulsion for 19F Magnetic Resonance Imaging: First Results in Monocytes of Patients with Stable Coronary Artery Disease and ST-Elevation Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. Properties of the Applied PFOB-NE
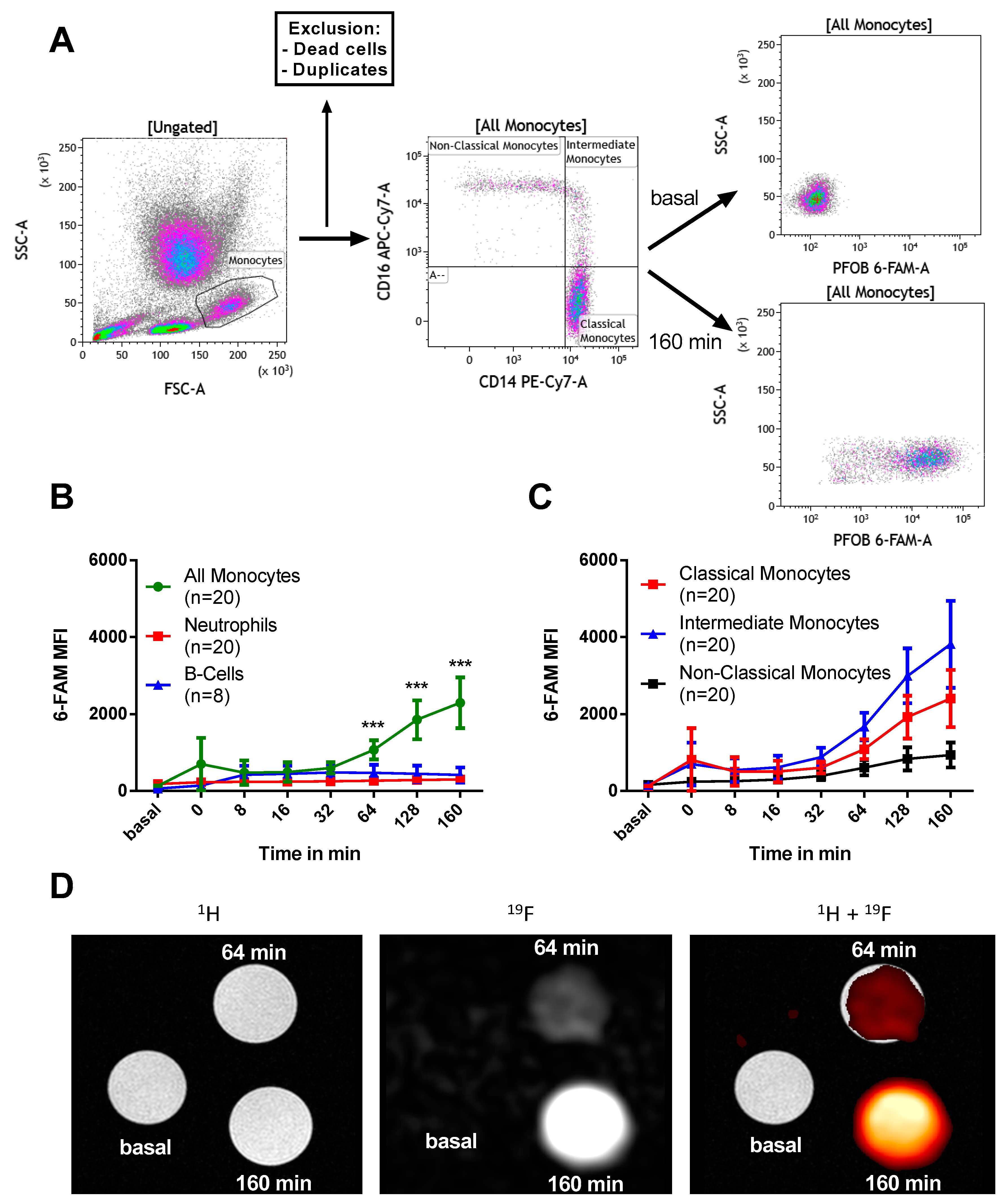
2.2. PFOB-NE is Mainly Ingested by Human Monocytes
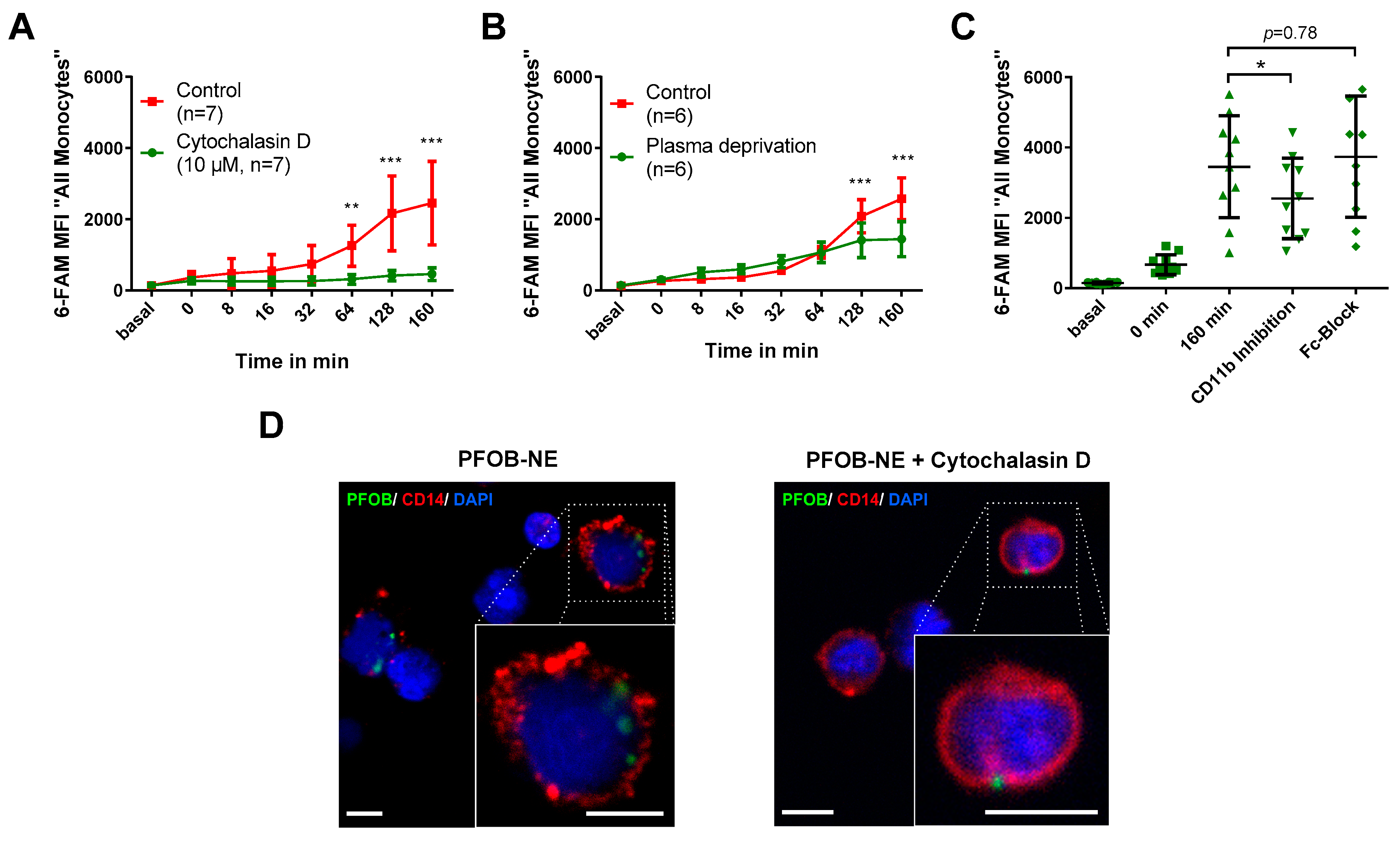
2.3. PFOB-NE is Ingested by is Active Phagocytosis
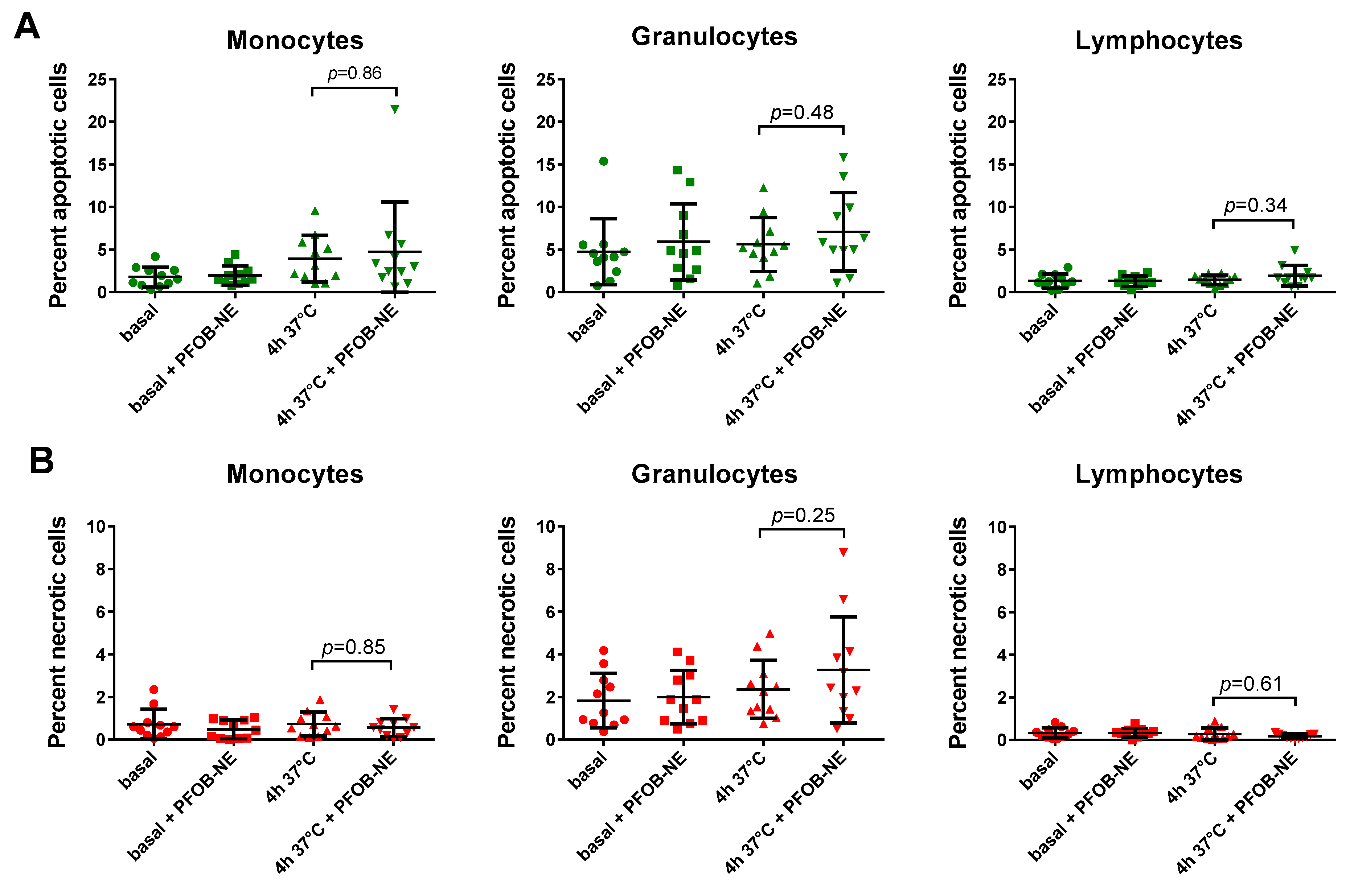
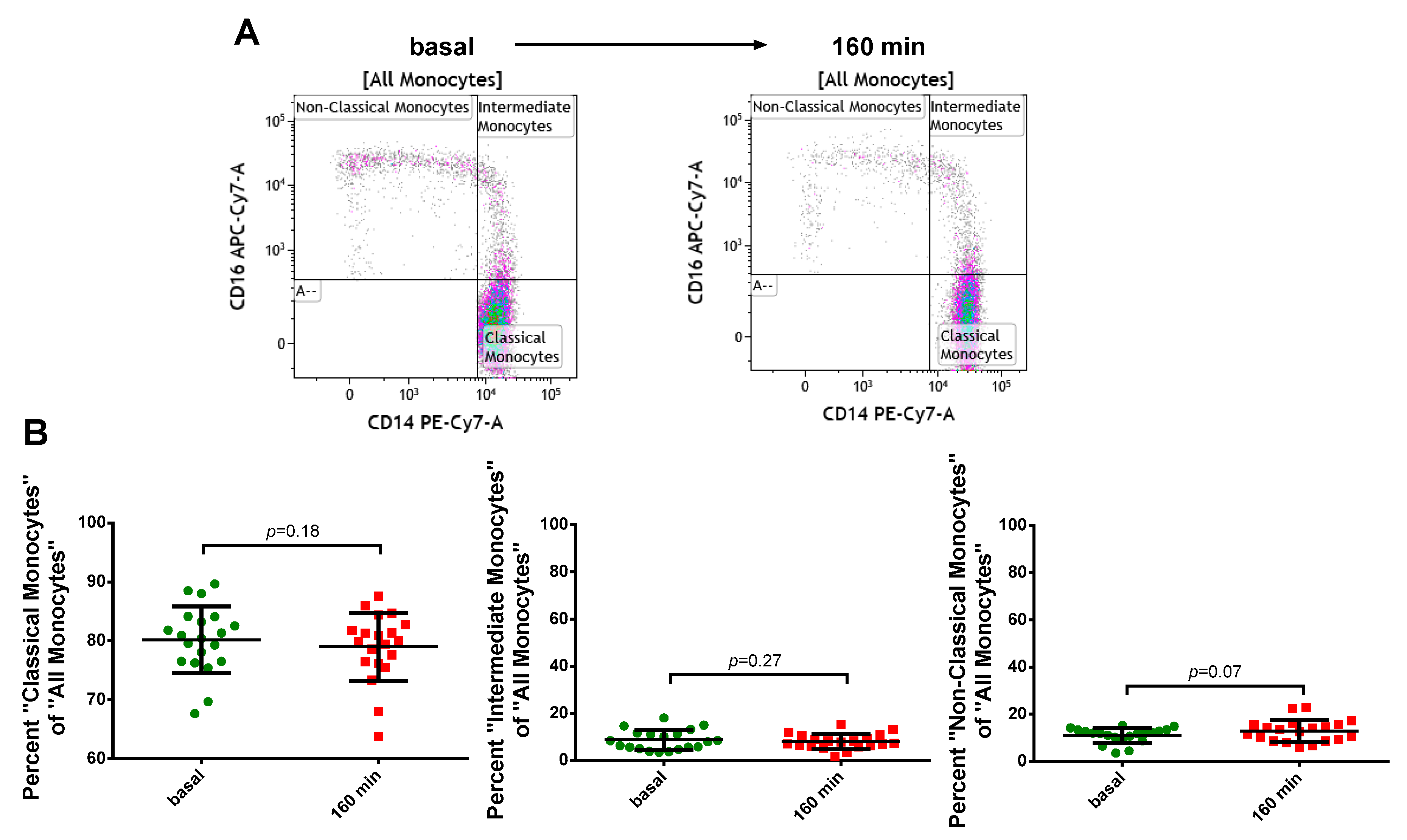
2.4. Viability and Subset Reclassification after PFOB-Ingestion
2.5. Monocyte Function after PFOB-NE Exposure
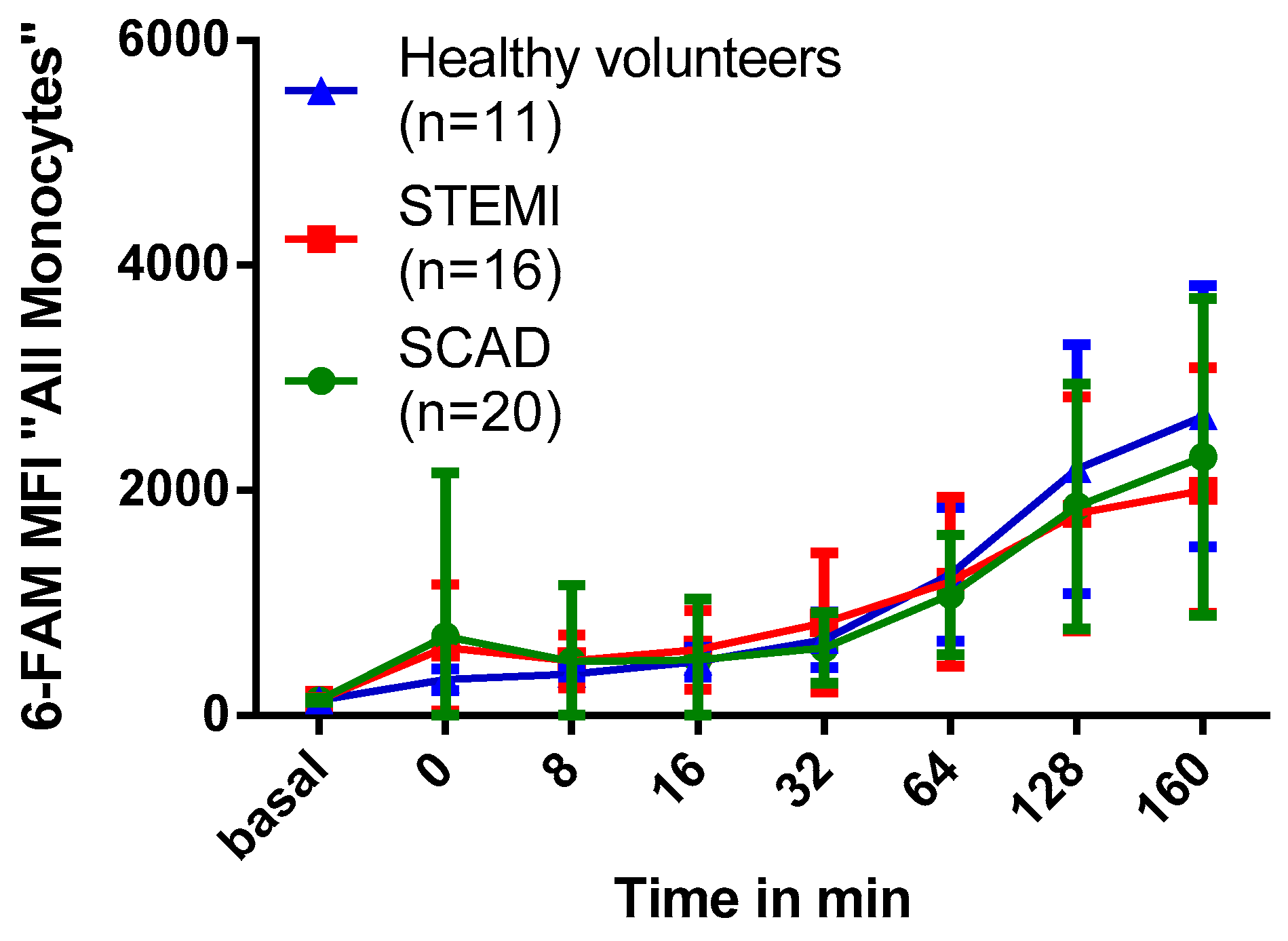
2.6. Association of Myocardial Infarction with Phagocytosis of PFOB-NE by Human Monocytes
3. Discussion
3.1. Cell Type and Subset Specific PFOB-NE Uptake
3.2. Monocyte Function, Viability and Subgroup Distribution after PFOB-NE Ingestion
3.3. Mechanism of PFOB-NE Ingestion
3.4. Impact of Myocardial Infarction on Monocyte Phagocytic Activity
3.5. Future Prospects
4. Materials and Methods
4.1. Collection of Blood Samples and Study Population
4.2. Preparation of PFOB-NE
4.3. In Vitro Uptake of PFOB-NE by Human Leukocytes
4.4. Determination of Apoptotic and Necrotic Leukocytes after PFOB-NE Ingestion
4.5. 19F MRI of Isolated Human Monocytes after PFOB-NE Ingestion
4.6. Migration Assay
4.7. Imaging in Vitro Uptake of PFOB-NE in CD14+ Cells
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swirski, F.K.; Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013, 339, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Nahrendorf, M. Monocytes in myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Flögel, U.; Ding, Z.; Hardung, H.; Jander, S.; Reichmann, G.; Jacoby, C.; Schubert, R.; Schrader, J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 2008, 118, 140–148. [Google Scholar] [CrossRef]
- Flögel, U.; Su, S.; Kreideweiss, I.; Ding, Z.; Galbarz, L.; Fu, J.; Jacoby, C.; Witzke, O.; Schrader, J. Noninvasive detection of graft rejection by in vivo (19) F MRI in the early stage. Am. J. Transplant 2011, 11, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, C.; Borg, N.; Heusch, P.; Sauter, M.; Bönner, F.; Kandolf, R.; Klingel, K.; Schrader, J.; Flögel, U. Visualization of immune cell infiltration in experimental viral myocarditis by (19)F MRI in vivo. MAGMA 2014, 27, 101–106. [Google Scholar] [CrossRef]
- Ebner, B.; Behm, P.; Jacoby, C.; Burghoff, S.; French, B.A.; Schrader, J.; Flögel, U. Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ. Cardiovasc. Imaging 2010, 3, 202–210. [Google Scholar] [CrossRef]
- Flögel, U.; Schluter, A.; Jacoby, C.; Temme, S.; Banga, J.P.; Eckstein, A.; Schrader, J.; Berchner-Pfannschmidt, U. Multimodal assessment of orbital immune cell infiltration and tissue remodeling during development of graves disease by (1) H(19) F MRI. Magn. Reson. Med. 2018, 80, 711–718. [Google Scholar] [CrossRef]
- Flögel, U.; Burghoff, S.; van Lent, P.L.; Temme, S.; Galbarz, L.; Ding, Z.; El-Tayeb, A.; Huels, S.; Bönner, F.; Borg, N.; et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 2012, 4, 146ra108. [Google Scholar] [CrossRef]
- Temme, S.; Grapentin, C.; Quast, C.; Jacoby, C.; Grandoch, M.; Ding, Z.; Owenier, C.; Mayenfels, F.; Fischer, J.W.; Schubert, R.; et al. Noninvasive Imaging of Early Venous Thrombosis by 19F Magnetic Resonance Imaging With Targeted Perfluorocarbon Nanoemulsions. Circulation 2015, 131, 1405–1414. [Google Scholar] [CrossRef]
- Bönner, F.; Merx, M.W.; Klingel, K.; Begovatz, P.; Flögel, U.; Sager, M.; Temme, S.; Jacoby, C.; Salehi Ravesh, M.; Grapentin, C.; et al. Monocyte imaging after myocardial infarction with 19F MRI at 3 T: A pilot study in explanted porcine hearts. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 612–620. [Google Scholar] [CrossRef]
- Rothe, M.; Jahn, A.; Weiss, K.; Hwang, J.H.; Szendroedi, J.; Kelm, M.; Schrader, J.; Roden, M.; Flögel, U.; Bönner, F. In vivo (19)F MR inflammation imaging after myocardial infarction in a large animal model at 3 T. MAGMA 2018. [Google Scholar] [CrossRef]
- Riess, J.G. Perfluorocarbon-based oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2006, 34, 567–580. [Google Scholar] [CrossRef]
- Jacoby, C.; Temme, S.; Mayenfels, F.; Benoit, N.; Krafft, M.P.; Schubert, R.; Schrader, J.; Flögel, U. Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: Image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014, 27, 261–271. [Google Scholar] [CrossRef]
- Spahn, D.R.; Waschke, K.F.; Standl, T.; Motsch, J.; Van Huynegem, L.; Welte, M.; Gombotz, H.; Coriat, P.; Verkh, L.; Faithfull, S.; et al. Use of perflubron emulsion to decrease allogeneic blood transfusion in high-blood-loss non-cardiac surgery: results of a European phase 3 study. Anesthesiology 2002, 97, 1338–1349. [Google Scholar] [CrossRef]
- Ribes, S.; Ebert, S.; Regen, T.; Agarwal, A.; Tauber, S.C.; Czesnik, D.; Spreer, A.; Bunkowski, S.; Eiffert, H.; Hanisch, U.K.; et al. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect. Immun. 2010, 78, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Taylor, L.; Lee, H.; Cowley, S.A.; James, W.S.; Iqbal, A.J.; Greaves, D.R. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem. Pharmacol. 2016, 116, 107–119. [Google Scholar] [CrossRef]
- Indik, Z.K.; Park, J.G.; Hunter, S.; Schreiber, A.D. Structure/function relationships of Fc gamma receptors in phagocytosis. Semin. Immunol. 1995, 7, 45–54. [Google Scholar] [CrossRef]
- Sandor, N.; Kristof, K.; Parej, K.; Pap, D.; Erdei, A.; Bajtay, Z. CR3 is the dominant phagocytotic complement receptor on human dendritic cells. Immunobiology 2013, 218, 652–663. [Google Scholar] [CrossRef]
- Lukacsi, S.; Nagy-Balo, Z.; Erdei, A.; Sandor, N.; Bajtay, Z. The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes. Immunol. Lett. 2017, 189, 64–72. [Google Scholar] [CrossRef]
- Zanoni, I.; Granucci, F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell Infect. Microbiol. 2013, 3, 32. [Google Scholar] [CrossRef]
- Selvaraj, P.; Fifadara, N.; Nagarajan, S.; Cimino, A.; Wang, G. Functional regulation of human neutrophil Fc gamma receptors. Immunol. Res. 2004, 29, 219–230. [Google Scholar] [CrossRef]
- Vallieres, F.; Girard, D. Mechanism involved in interleukin-21-induced phagocytosis in human monocytes and macrophages. Clin. Exp. Immunol. 2017, 187, 294–303. [Google Scholar] [CrossRef]
- Gu, B.J.; Huang, X.; Ou, A.; Rembach, A.; Fowler, C.; Avula, P.K.; Horton, A.; Doecke, J.D.; Villemagne, V.L.; Macaulay, S.L.; et al. Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer’s disease. Acta Neuropathol. 2016, 132, 377–389. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef]
- Arfvidsson, J.; Ahlin, F.; Vargas, K.G.; Thaler, B.; Wojta, J.; Huber, K. Monocyte subsets in myocardial infarction: A review. Int. J. Cardiol. 2017, 231, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.L.; Ji, W.J.; Liu, J.X.; Guo, Z.Z.; Ren, D.; Ma, Y.Q.; Zeng, S.; Xu, Z.W.; Li, H.X.; et al. The Kinetics of Circulating Monocyte Subsets and Monocyte-Platelet Aggregates in the Acute Phase of ST-Elevation Myocardial Infarction: Associations with 2-Year Cardiovascular Events. Medicine (Baltimore) 2016, 95, e3466. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, A.M.; ter Horst, E.N.; Delewi, R.; Begieneman, M.P.V.; Krijnen, P.A.; Hirsch, A.; Lavaei, M.; Nahrendorf, M.; Horrevoets, A.J.; Niessen, H.W.; et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 2014, 35, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Temme, S.; Bönner, F.; Schrader, J.; Flögel, U. 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Riess, J.G. Oxygen carriers ("blood substitutes") - Raison d’Etre, chemistry, and some physiology. Chem. Rev. 2001, 101, 2797–2919. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Flores, R.; Xu, H.Y.; Morel, P.A. In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 2005, 23, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Donaldson, J.G. Search for inhibitors of endocytosis: Intended specificity and unintended consequences. Cell Logist. 2012, 2, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, L.M.; Roth, R.; Heuser, J.E.; Schmid, S.L. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 2000, 1, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Flannagan, R.S.; Jaumouille, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef] [PubMed]
- Djurdjevic, P.M.; Arsenijevic, N.N.; Baskic, D.D.; Djukic, A.L.; Popovic, S.; Samardzic, G. Systemic response of peripheral blood leukocytes and their phagocytic activity during acute myocardial infarction. Exp. Clin. Cardiol. 2001, 6, 159–166. [Google Scholar] [PubMed]
Sample Availability: Samples will be provided on reasonable request. |
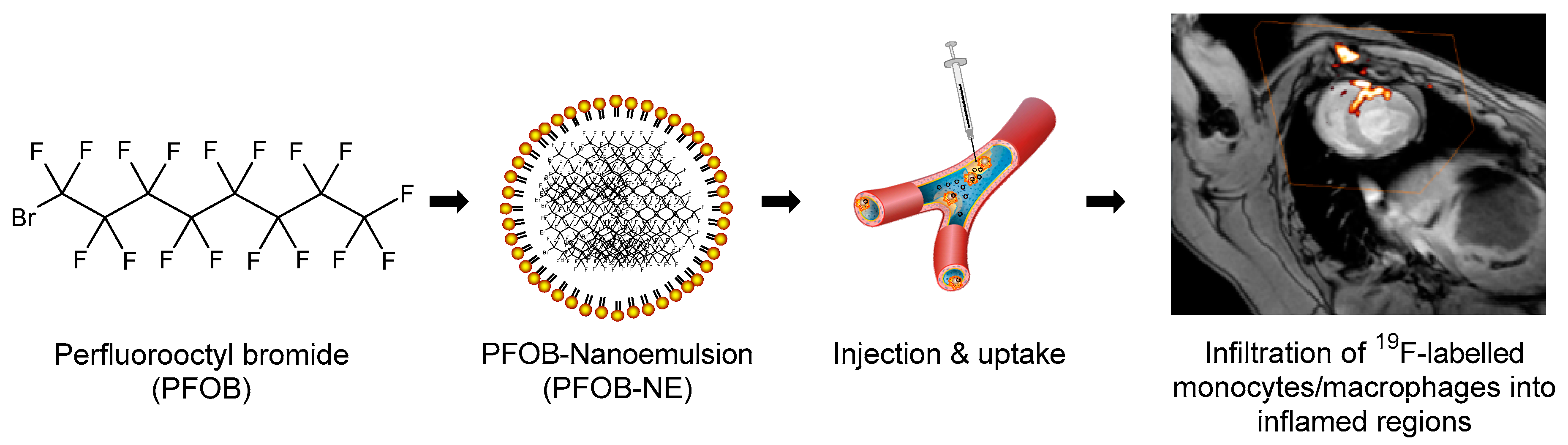

| Parameter | Value (± SD) |
|---|---|
| Particle size | 209.2 ± 17.4 nm |
| Zeta-Potential | −45.4 ± 7.8 mv |
| Polydispersity Index | 0.22 |
| Phospholipid type | E 80 S |
| PFOB fraction | 50.5% [w/w] |
| Buffer fraction | 45.2% [w/w] |
| Lipoid fraction | 2.9% [w/w] |
| Stabilizer fraction | 1.4% [w/w] |
| 19F molarity | 65.69 mol/L |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nienhaus, F.; Colley, D.; Jahn, A.; Pfeiler, S.; Flocke, V.; Temme, S.; Kelm, M.; Gerdes, N.; Flögel, U.; Bönner, F. Phagocytosis of a PFOB-Nanoemulsion for 19F Magnetic Resonance Imaging: First Results in Monocytes of Patients with Stable Coronary Artery Disease and ST-Elevation Myocardial Infarction. Molecules 2019, 24, 2058. https://doi.org/10.3390/molecules24112058
Nienhaus F, Colley D, Jahn A, Pfeiler S, Flocke V, Temme S, Kelm M, Gerdes N, Flögel U, Bönner F. Phagocytosis of a PFOB-Nanoemulsion for 19F Magnetic Resonance Imaging: First Results in Monocytes of Patients with Stable Coronary Artery Disease and ST-Elevation Myocardial Infarction. Molecules. 2019; 24(11):2058. https://doi.org/10.3390/molecules24112058
Chicago/Turabian StyleNienhaus, Fabian, Denise Colley, Annika Jahn, Susanne Pfeiler, Vera Flocke, Sebastian Temme, Malte Kelm, Norbert Gerdes, Ulrich Flögel, and Florian Bönner. 2019. "Phagocytosis of a PFOB-Nanoemulsion for 19F Magnetic Resonance Imaging: First Results in Monocytes of Patients with Stable Coronary Artery Disease and ST-Elevation Myocardial Infarction" Molecules 24, no. 11: 2058. https://doi.org/10.3390/molecules24112058
APA StyleNienhaus, F., Colley, D., Jahn, A., Pfeiler, S., Flocke, V., Temme, S., Kelm, M., Gerdes, N., Flögel, U., & Bönner, F. (2019). Phagocytosis of a PFOB-Nanoemulsion for 19F Magnetic Resonance Imaging: First Results in Monocytes of Patients with Stable Coronary Artery Disease and ST-Elevation Myocardial Infarction. Molecules, 24(11), 2058. https://doi.org/10.3390/molecules24112058






