Seed Characteristics and Nutritional Composition of Pine Nut from Five Populations of P. cembroides from the States of Hidalgo and Chihuahua, Mexico
Abstract
1. Introduction
2. Results
2.1. Pine Nut Morphometric Analyses
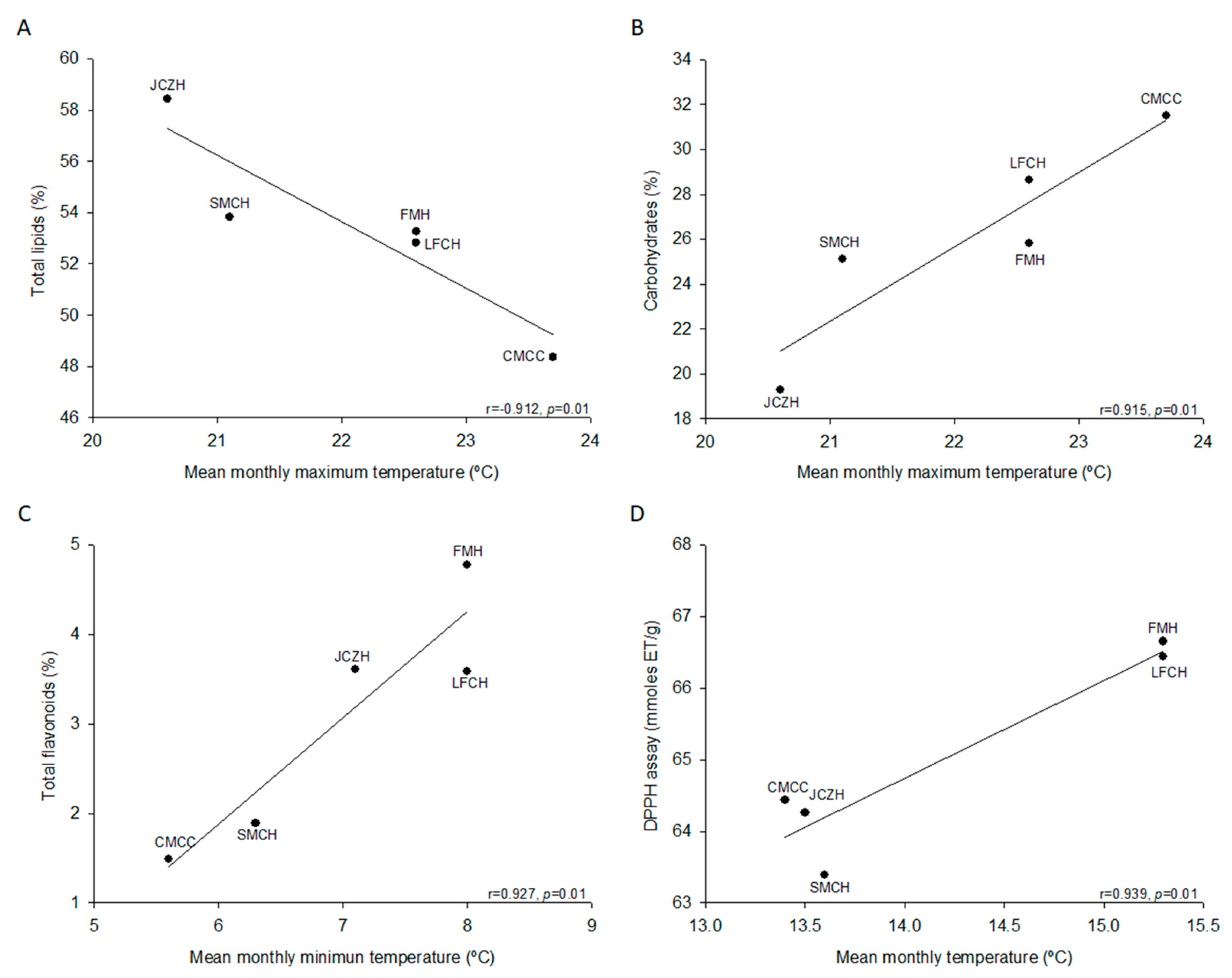
2.2. Chemical Composition of Pine Nut
2.3. Total Phenolics and Flavonoids
2.4. Antioxidant Activity
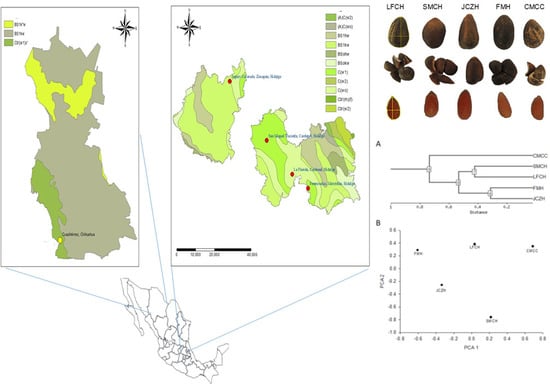
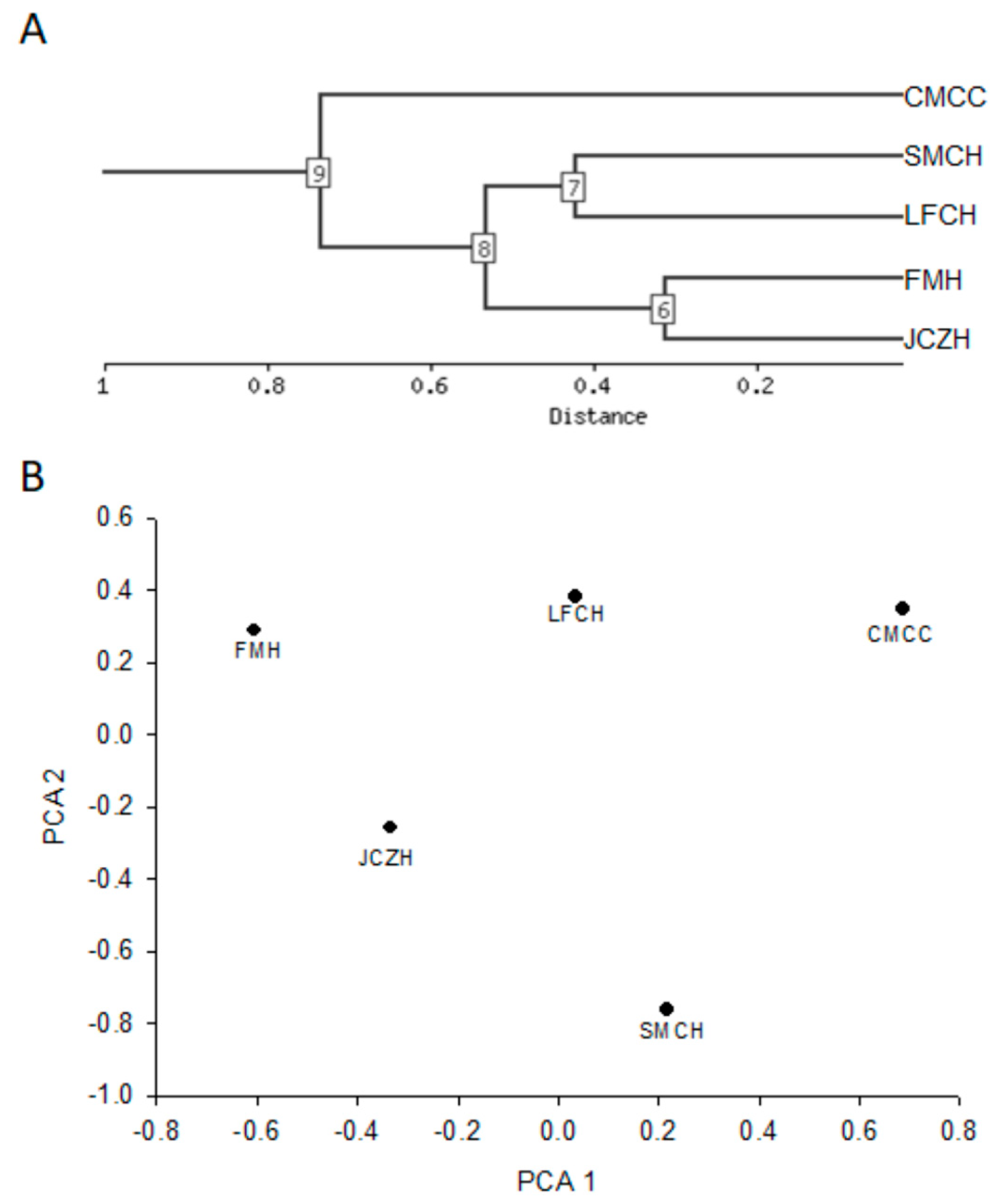
2.5. Cluster Analysis
3. Discussion
4. Materials and Methods
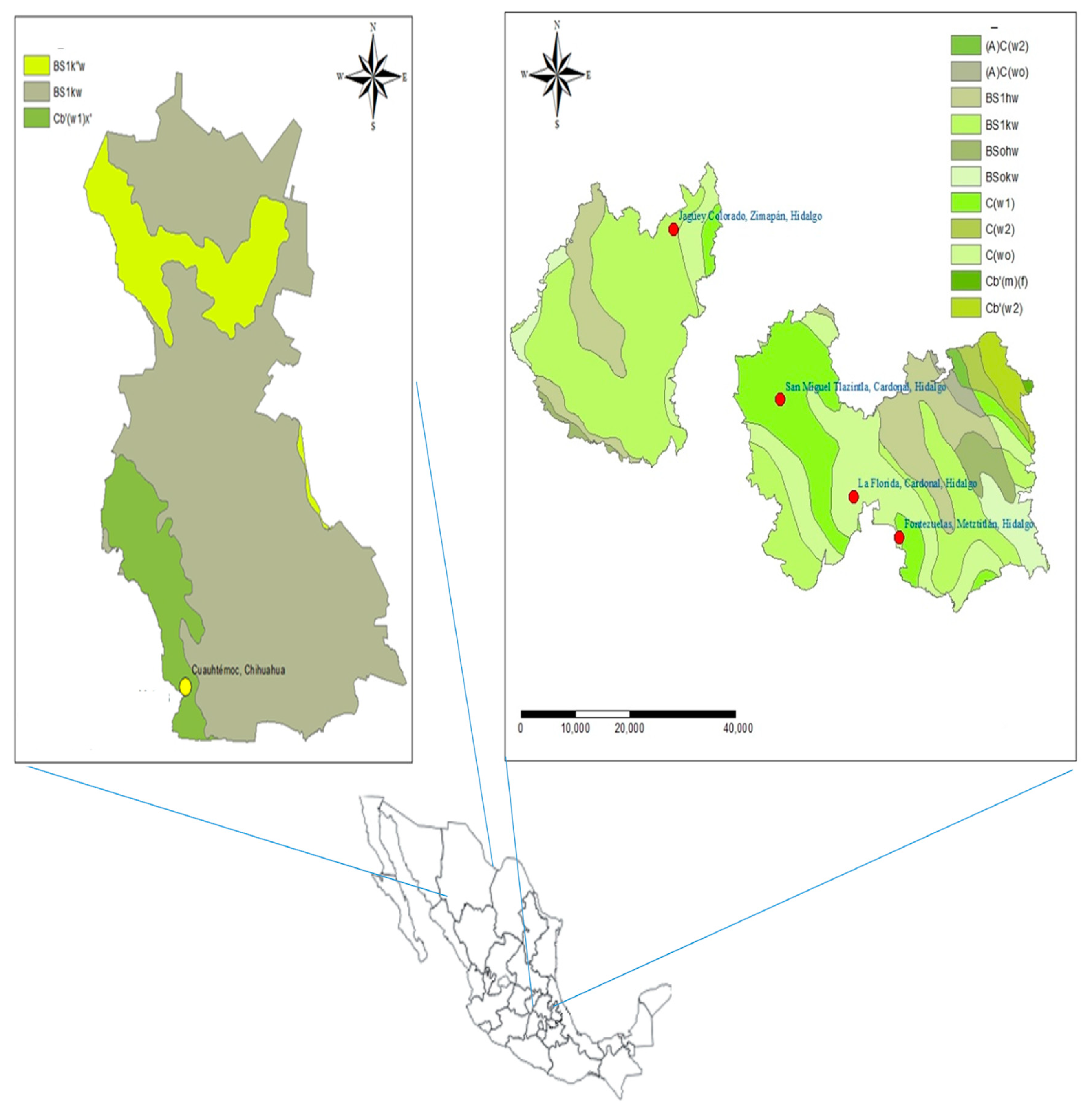
4.1. Biological Material and Seed Collection
4.2. Morphometric Analyses
4.3. Chemical Analyses
4.4. Total Phenolics and Flavonoids
4.5. Antioxidant Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero, A.; García, E.; Marie-Françoise, P. Pinus cembroides s. l. y Pinus johannis del Altiplano Mexicano: una síntesis. Acta Botánica Gálica 1996, 143, 681–693. [Google Scholar]
- Farjon, A.; Pérez de la Rosa, J.A.; Styles, B. A field guide to the pines of Mexico and Central America, 1st ed.; The Royal Botanical Garden, Kew and the University of Oxford: Oxford, UK, 1997; p. 146. [Google Scholar]
- Gernandt, D.S.; Pérez-De La Rosa, J.A. Biodiversidad de Pinophyta (coníferas) en México. Revista Mexicana Biodivesidad 2014, 85 (Suppl. 1), S126–S133. [Google Scholar] [CrossRef]
- Perry, J. The pines of Mexico and Central America, 1st ed.; Timber Press: Portland, OR, USA, 1991; p. 231. [Google Scholar]
- Granados, R.; Granados, D.; Sánchez, A. Caracterización y ordenación de los bosques de pino piñonero (Pinus cembroides subsp. orizabensis) de la Cuenca Oriental (Puebla, Tlaxcala y Veracruz). Madera y Bosques 2015, 21, 23–42. [Google Scholar] [CrossRef][Green Version]
- Hernández-Moreno, M.; Islas-Gutiérrez, J.; Guerra De La Cruz, V. Márgenes de comercialización del piñón (Pinus cembroides subesp. orizabensis) en Tlaxcala, México. Revista Mexicana de Ciencias Agrícolas 2011, 2, 265–279. [Google Scholar]
- Pompa-García, M.; Yerena-Yamalliel, J.I. Concentración de carbono en Pinus cembroides Zucc: Fuente potencial de mitigación del calentamiento global. Revista Chapingo Serie Ciencias Forestales y Del ambiente 2014, 20, 169–175. [Google Scholar] [CrossRef]
- Nergiz, C.; Dönmez, I. Chemical composition and nutritive value of Pinus pinea L. Seeds. Food Chem. 2004, 86, 365–368. [Google Scholar] [CrossRef]
- Kadri, N.; Khettal, B.; Aid, Y.; Kherfellah, S.; Sobhi, W.; Barragan-Montero, V. Some physicochemical characteristics of pinus (Pinus halepensis Mill., Pinus pinea L., Pinus pinaster and Pinus canariensis) seeds from North Algeria. Their lipid profiles and volatile contents. Food Chem. 2015, 188, 184–192. [Google Scholar] [CrossRef]
- López, M.L. Proteins, amino acids and fatty acids composition of nuts from the Mexican endemic rarity. Pinus maximartinezii, and its conservation implications. Interciencia 2001, 26, 606–610. [Google Scholar]
- Nasri, N.; Triki, S. Lipid analysis of Tunisia pine seed: Pinus halepensis Mill, and Pinus pinea L. La Rivista Italiana Delle Sostanze Grasse 2004, 81, 244–247. [Google Scholar]
- Loewe-Muñoz, V.; Álvarez, A.; Navarro-Cerrillo, R. Morphometric and chemical fruit variability of selected stone pine trees (Pinus pinea L.) grown in non-native environments. Plant Biosys. 2018, 152, 547–555. [Google Scholar] [CrossRef]
- Lutz, M.; Álvarez, K.; Loewe, V. Chemical composition of pine nut (Pinus pinea L.) grown in three geographical macrozones in Chile. CYTA – J. Food 2016, 15, 284–290. [Google Scholar] [CrossRef]
- Ryan, E.; Galvin, K.; O’connor, T.; Maguire, A.; O’brien, N. Fatty acid profile, tocopherol, squalene and phytosterol content of brazil, pecan, pine, pistachio and cashew nuts. Int. J. Food Sci. Nutr. 2006, 57, 219–228. [Google Scholar] [CrossRef]
- Ros, E.; Mataix, J. Fatty acid composition of nuts–implications for cardiovascular health. Br. J. Nutr. 2006, 96, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Awan, H.U.M.; Pettenella, D. Pine Nuts: A Review of Recent Sanitary Conditions and Market Development. Forests 2017, 8, 367. [Google Scholar] [CrossRef]
- Evaristo, I.; Batista, D.; Correia, I.; Correia, P.; Costa, R. Chemical profiling of Portuguese Pinus pinea nuts and comparative analysis with P. koraiensis commercial kernels. Options Méditerranéennes 2013, 105, 99–104. [Google Scholar]
- Lantto, T.A.; Dorman, H.J.D.; Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P. Chemical composition, antioxidative activity and cell viability effects of Siberian pine (Pinus sibirica Du Tour) extract. Food Chem. 2009, 112, 936–943. [Google Scholar] [CrossRef]
- González-Ávalos, J.; García-Moya, E.; Vargas-Hernández, J.; Trinidad Santos, A.; Romero-Manzanares, A.; Cetina-Alcalá, V.M. Evaluación de la producción y análisis de conos y semillas de Pinus cembroides Zucc. Revista Chapingo. Serie Ciencias Forestales y Del ambiente 2006, 12, 133–136. [Google Scholar]
- Sagrero-Nieves, L. Fatty acid composition of Mexican pine nut (Pinus cembroides) oil from three seed coat phenotypes. J. Sci. Food Agri. 1992, 59, 413–414. [Google Scholar] [CrossRef]
- Wolff, R.L.; Marpeau, A.M. D5-olefinic acids in the edible seeds of nut pines (Pinus cembroides edulis) from the United States. J. Am. Oil Chem. Soc. 1997, 74, 613–614. [Google Scholar] [CrossRef]
- Sánchez, T.V.; Nieto, P.M.; Mendizábal, L.D. Producción de semillas de Pinus cembroides subsp. orizabensis D.K. Bailey de Altzayanca, Tlaxcala, México. Foresta Veracruzana 2005, 7, 15–20. [Google Scholar]
- Ramírez-Valiente, J.A.; Valladares, F.; Gil, L.; Aranda, I. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Singh, B.; Prakash, S.K.; Bhatt, B.P. Provenance variation in seed and seedlings attributes of Quercus glauca in India. Dendrobiology 2010, 63, 59–63. [Google Scholar]
- Calama, R.; Montero, G. Cone and seed production from Stone pine (Pinus pinea) stands in Central Range (Spain). Eur. J. For. Res. 2007, 126, 23–35. [Google Scholar] [CrossRef]
- González, M.; Loewe, V.; Delard, C. El piñón de Pinus pinea producido en Chile: características nutricionales y organolépticas. Vitae 2012, 19, S317–S319. [Google Scholar]
- Su, X.Y.; Wang, Z.Y.; Liu, J.L. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: comparison of low-density lipoprotein oxidation assay, DPPH radical scavening assay and Folin-Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Hoon, L.Y.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Evaluation of the total antioxidant capacity and antioxidant compounds of different solvent extracts of Chilgoza pine nuts (Pinus gerardiana). J. Func. Food. 2015, 18, 1014–1021. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, O.C.-Y.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and Vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Moreno-Escamilla, J.O.; Alvarez-Parrilla, E.; De La Rosa, L.A.; Núñez-Gastélum, J.A.; González-Aguilar, G.A.; Rodrigo-Garcia, J. Effect of different elicitors and pre-harvest day application on the content of phytochemicals and antioxidant activity of butterhead lettuce (Lactuca sativa var capitata) produced under hydroponic conditions. J. Agric. Food Chem. 2017, 65, 5244–5254. [Google Scholar] [CrossRef]
- Sharov, A.A.; Dudekula, D.B.; Ko, M.S.H. A web-based tool for principal component and significance analysis of microarray data. Bioinformatics 2005, 21, 2548–2549. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
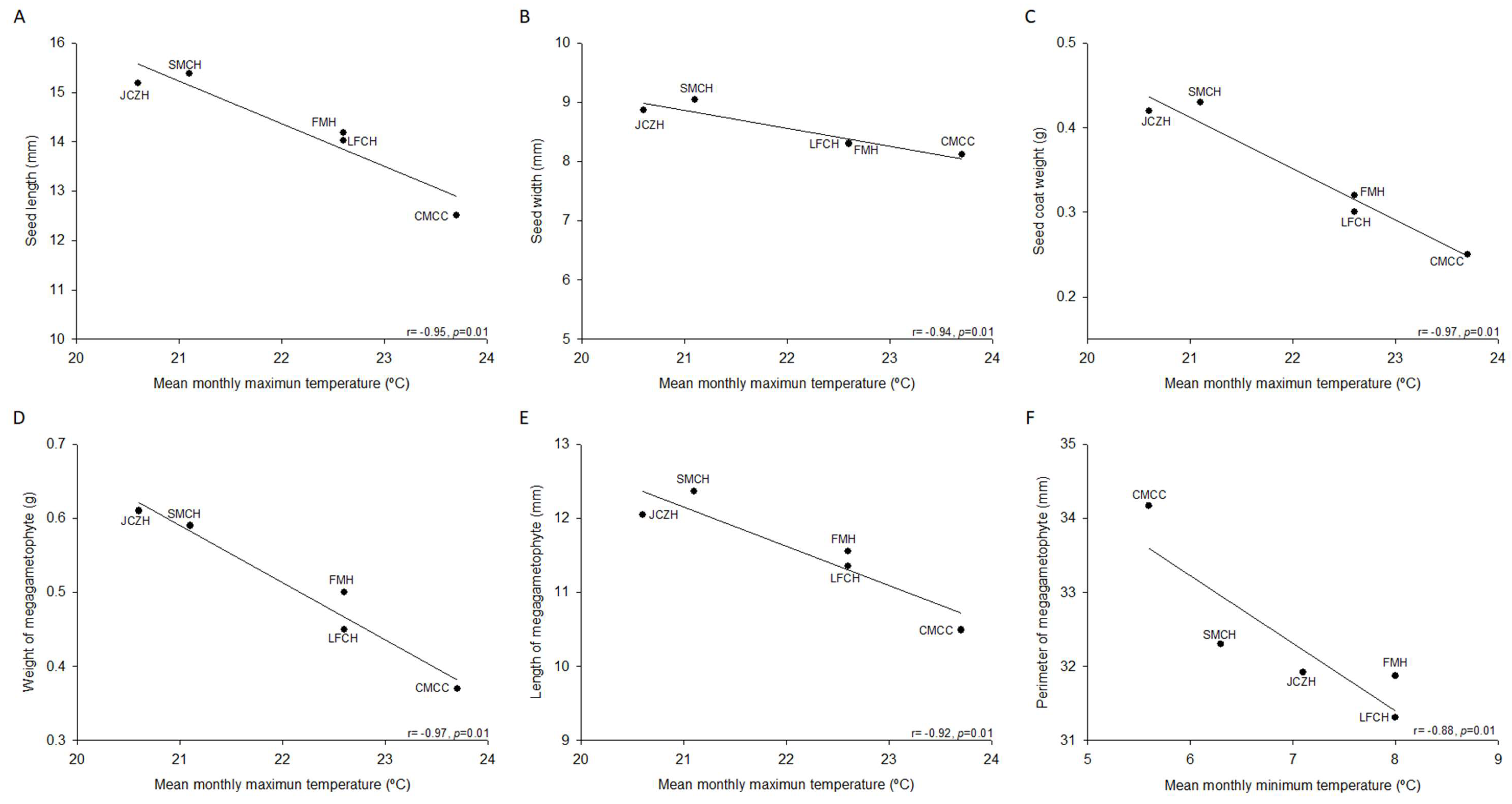
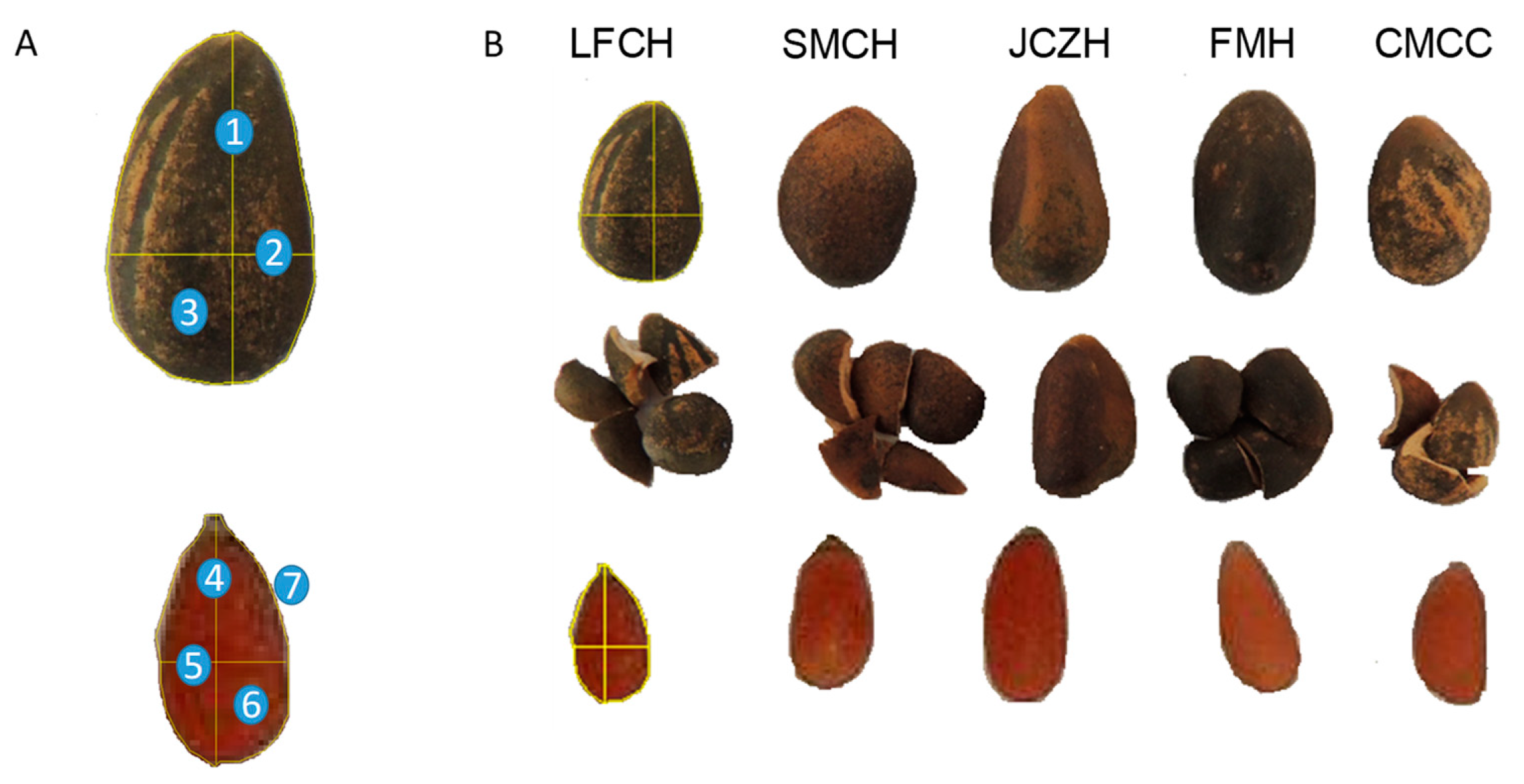
| Seed Morphology | SMCH | JCZH | FMH | LFCH | CMCC |
|---|---|---|---|---|---|
| Seed weight (g) | 0.59 ± 0.03 a | 0.61 ± 0.02 a | 0.50 ± 0.02 b | 0.50 ± 0.02 b | 0.37 ± 0.01 c |
| Seed length (mm) | 15.4 ± 0.3 a | 15.2 ± 0.7 a, b | 14.2 ± 0.2 b, c | 14.0 ± 0.2 c | 12.5 ± 0.2 d |
| Seed width (mm) | 9.0 ± 0.2 a | 8.9 ± 0.1 a | 8.3 ± 0.2 b | 8.3 ± 0.2 b | 8.1 ± 0.2 b |
| Seed coat weight (g) | 0.43 ± 0.02 a | 0.42 ± 0.02 a | 0.32 ± 0.02 b | 0.30 ± 0.01 b | 0.25 ± 0.01 c |
| Megagametophyte weight (g) | 0.16 ± 0.01 a | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.16 ± 0.02 a | 0.12 ± 0.01 b |
| Megagametophyte length (mm) | 12.4 ± 0.3 a | 12.1 ± 0.3 a, b | 11.6 ± 0.3 a, b | 11.35 ± 0.2 b | 10.5 ± 0.3 c |
| Megagametophyte width (mm) | 5.9 ± 0.1 a | 5.8 ± 0.1 a, b, c | 5.9 ± 0.2 a, b | 5.5 ± 0.2 b, c | 5.4 ± 0.1 c |
| Megagametophyte area (mm2) | 57.8 ± 1.8 a | 56.3 ± 2.0 a | 56.2 ± 1.9 a | 50.4 ± 2.3 b | 47.1 ± 1.7 b |
| Megagametophyte perimeter (mm) | 32.3 ± 0.6 a | 31.9 ± 0.8 a | 31.9 ± 0.8 a | 31.3 ± 1.4 a | 34.2 ± 3.2 a |
| Chemical Composition | FMH | JCZH | SMTH | LFCH | CMCC |
|---|---|---|---|---|---|
| Water content (%) | 29.9 ± 0.1 a | 19.2 ± 0.1 b | 11.1 ± 0.2 c | 9.0 ± 0.1 d | 3.7 ± 0.1 e |
| Ash content (%) | 3.3 ± 0.0 b | 3.1 ± 0.0 c | 3.0 ± 0.0 d | 2.8 ± 0.0 e | 3.4 ± 0.0 a |
| Total Lipids (%) | 53.3 ± 0.6 a, b | 58.4 ± 0.2 a | 53.8 ± 3.2 a, b | 52.8 ± 3.1 a, b | 48.4 ± 3.0 b |
| Protein content (%) | 17.7 ± 0.1 a, b, c | 19.2 ± 0.1 a | 18.1 ± 0.4 a, b | 15.7 ± 0.1 c | 16.7 ± 1.3 b, c |
| Carbohydrates (%) * | 25.8 ± 0.6 a, b | 19.3 ± 0.2 a | 25.1 ± 3.5 a, b | 28.6 ± 3.1 a | 31.5 ± 4.1 a |
| Total phenolic ** | 4.8 ± 0.1 c | 4.9 ± 0.1 c | 7.8 ± 0.1 a | 4 ± 0.1 d | 5.9 ± 0.2 b |
| Total flavonoids *** | 4.8 ± 0.1 a | 3.6 ± 0.1 b | 1.9 ± 0.0 c | 3.6 ± 0.1 b | 1.5 ± 0.0 d |
| DPPH assay ****/% inhibition | 66.6 ± 0.9 a/71.1 | 64.3 ± 1.5 a, b/78.2 | 63.4 ± 0.7 b/74.3 | 66.4 ± 0.8 a/78.4 | 64.4 ± 0.5 a, b/75.8 |
| FRAP assay **** | 26.2 ± 0.1 a | 24.3 ± 0.5 b | 22.0 ± 0.3 c | 22.5 ± 0.1 c | 21.9 ± 0.2 c |
| ABTS assay/% inhibition | 28.2 ± 0.3 a/14.3 | 18.9 ± 0.2 b/23.9 | 10.9 ± 0.2 d/5.3 | 12.6 ± 0.2 c/7.2 | 12.3 ± 0.3 c/6.8 |
| Characteristics | Log10 Change | Correlation | PC Number | Direction | LFCH | SMTCH | JCZH | FMH | CMCC |
|---|---|---|---|---|---|---|---|---|---|
| FRAP assay antioxidant activity | −0.36 | −0.85 | 1 | Negative | 1.09 | 1.03 | 1.27 | 1.45 | 1.08 |
| Flavonoids content | −0.52 | −0.94 | 1 | Negative | 0.55 | 0.27 | 0.55 | 0.67 | 0.17 |
| Water content | −0.87 | −0.97 | 1 | Negative | 0.95 | 1.04 | 1.28 | 1.47 | 0.56 |
| Population | Latitude/Longitude | Altitude (m) | Annual Precipitation (mm) | Mean Monthly Temperature (°C) | Mean Monthly Maximum Temperature (°C) | Mean Monthly Minimum Temperature (°C) | Climate Data |
|---|---|---|---|---|---|---|---|
| La Florida, Cardonal, Hidalgo (LFCH) | 20°31′59.59″/98°58′48.8″ | 1953 | 555.6 | 15.3 | 22.6 | 8.0 | El encino (00013151) |
| San Miguel Tlazintla, Cardonal, Hidalgo (SMCH) | 20°39′20.0″/99°06′4.5″ | 2300 | 482.2 | 13.7 | 21.1 | 6.3 | Santuario (00013070) |
| Jagüey Colorado, Zimapán, Hidalgo (JCZH) | 20°52′10.5″/99°16′37.5″ | 2275 | 1051.0 | 13.8 | 20.6 | 7.1 | Encarnación (00013065) |
| Fontezuelas, Metztitlán, Hidalgo (FMH) | 20°28′56.5″/98°54′17.5″ | 2455 | 555.6 | 15.3 | 22.6 | 8.0 | El encino (00013151) |
| Curvas de Malpaso Cuauhtémoc, Chihuahua (CMCC) | 28°24′25.0″/107°0′06″ | 2242 | 447.6 | 14.7 | 23.7 | 5.6 | Cuauhtémoc (00008026) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero-Galván, J.; Reyna-González, M.; Chico-Romero, P.A.; Martínez-Ruiz, N.d.R.; Núñez-Gastélum, J.A.; Monroy-Sosa, A.; Ruiz-May, E.; González Fernández, R. Seed Characteristics and Nutritional Composition of Pine Nut from Five Populations of P. cembroides from the States of Hidalgo and Chihuahua, Mexico. Molecules 2019, 24, 2057. https://doi.org/10.3390/molecules24112057
Valero-Galván J, Reyna-González M, Chico-Romero PA, Martínez-Ruiz NdR, Núñez-Gastélum JA, Monroy-Sosa A, Ruiz-May E, González Fernández R. Seed Characteristics and Nutritional Composition of Pine Nut from Five Populations of P. cembroides from the States of Hidalgo and Chihuahua, Mexico. Molecules. 2019; 24(11):2057. https://doi.org/10.3390/molecules24112057
Chicago/Turabian StyleValero-Galván, José, Margarita Reyna-González, Perla Anneth Chico-Romero, Nina del Rocío Martínez-Ruiz, José Alberto Núñez-Gastélum, Abigail Monroy-Sosa, Eliel Ruiz-May, and Raquel González Fernández. 2019. "Seed Characteristics and Nutritional Composition of Pine Nut from Five Populations of P. cembroides from the States of Hidalgo and Chihuahua, Mexico" Molecules 24, no. 11: 2057. https://doi.org/10.3390/molecules24112057
APA StyleValero-Galván, J., Reyna-González, M., Chico-Romero, P. A., Martínez-Ruiz, N. d. R., Núñez-Gastélum, J. A., Monroy-Sosa, A., Ruiz-May, E., & González Fernández, R. (2019). Seed Characteristics and Nutritional Composition of Pine Nut from Five Populations of P. cembroides from the States of Hidalgo and Chihuahua, Mexico. Molecules, 24(11), 2057. https://doi.org/10.3390/molecules24112057