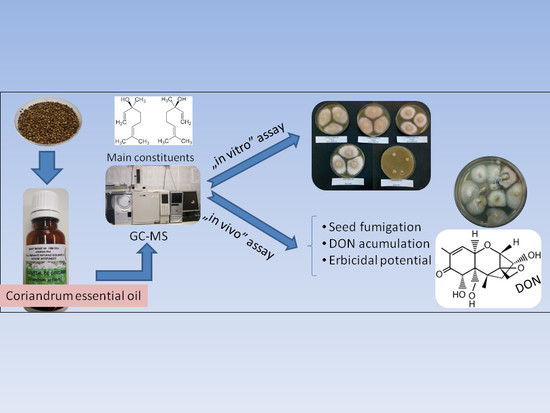
Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil
Abstract
1. Introduction
2. Results and Discussions
2.1. The Chemical Composition of CEO
2.2. The Antifungal Activity Effectiveness
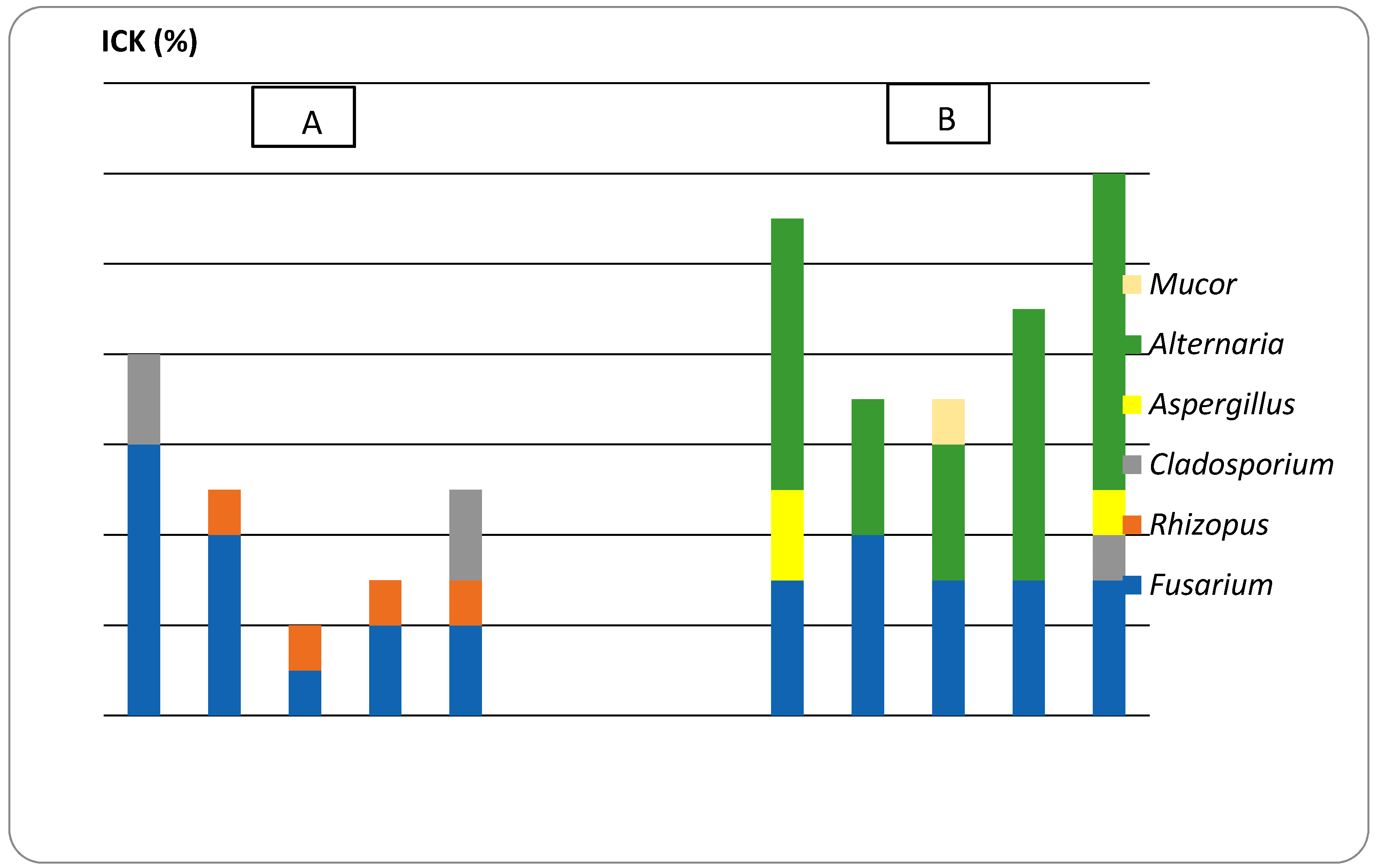
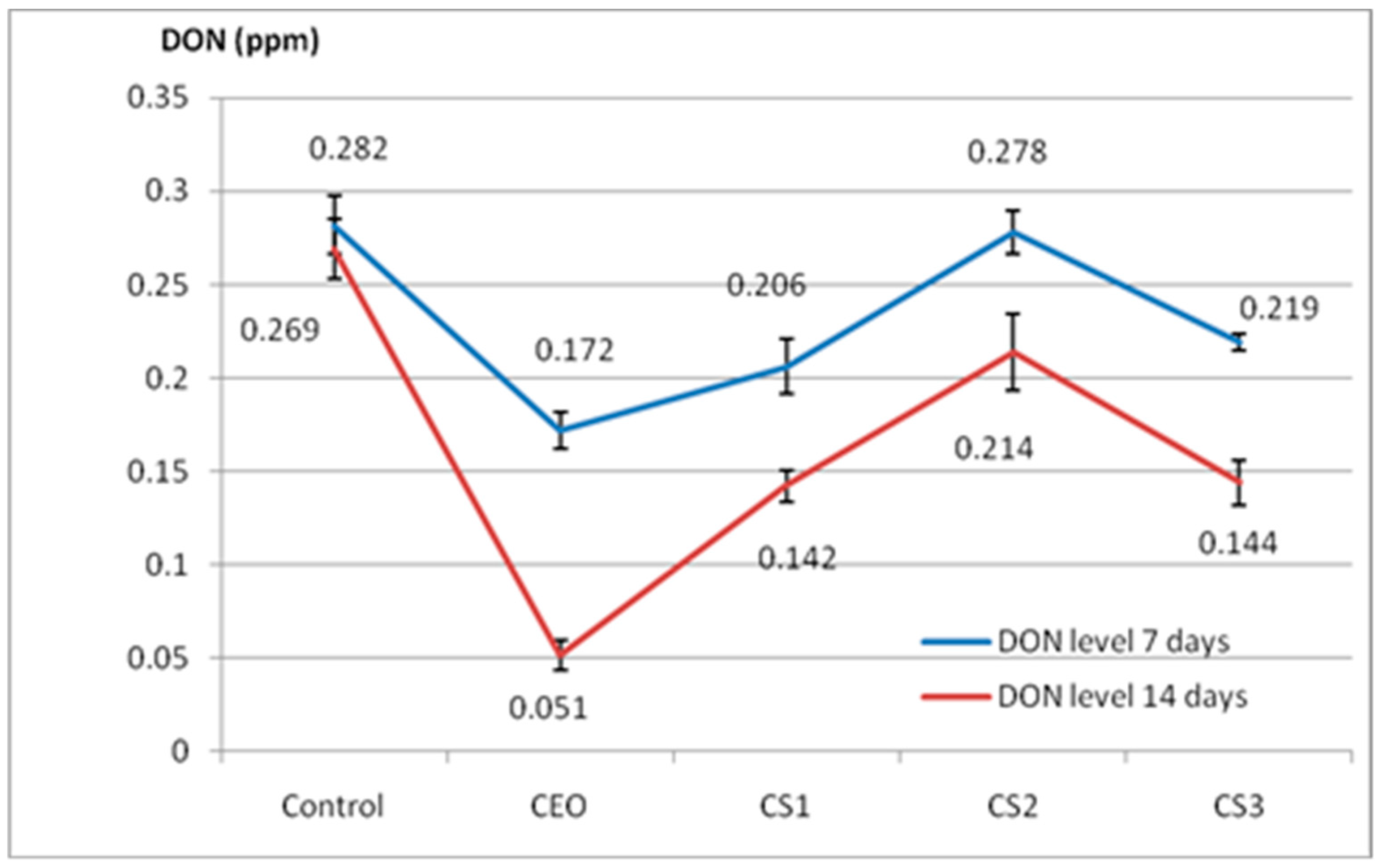
2.3. Effect of Augmented CEO against the Growth of Mycotoxigenic Fungi and Deoxynivalenol (DON) Accumulation
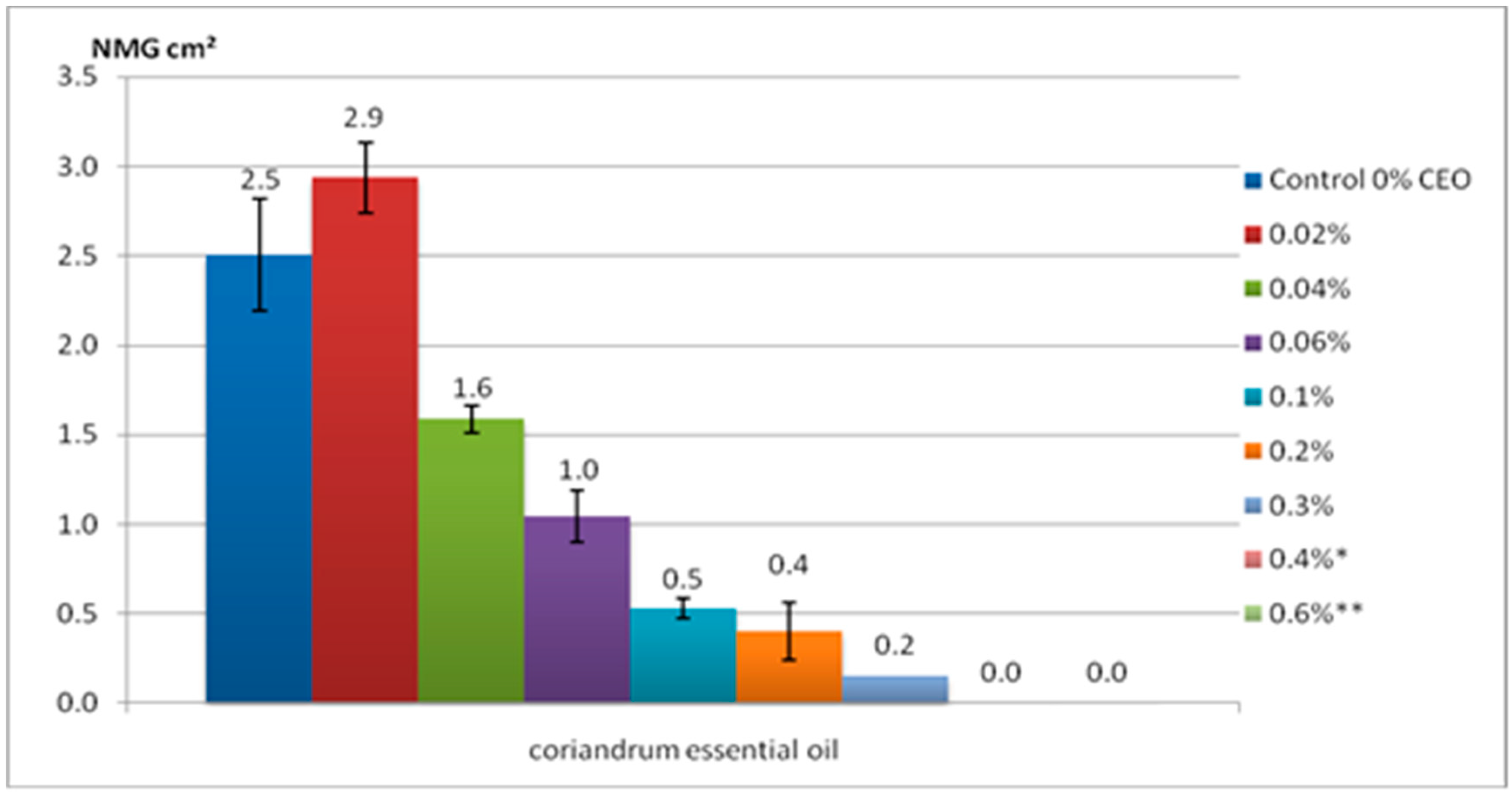
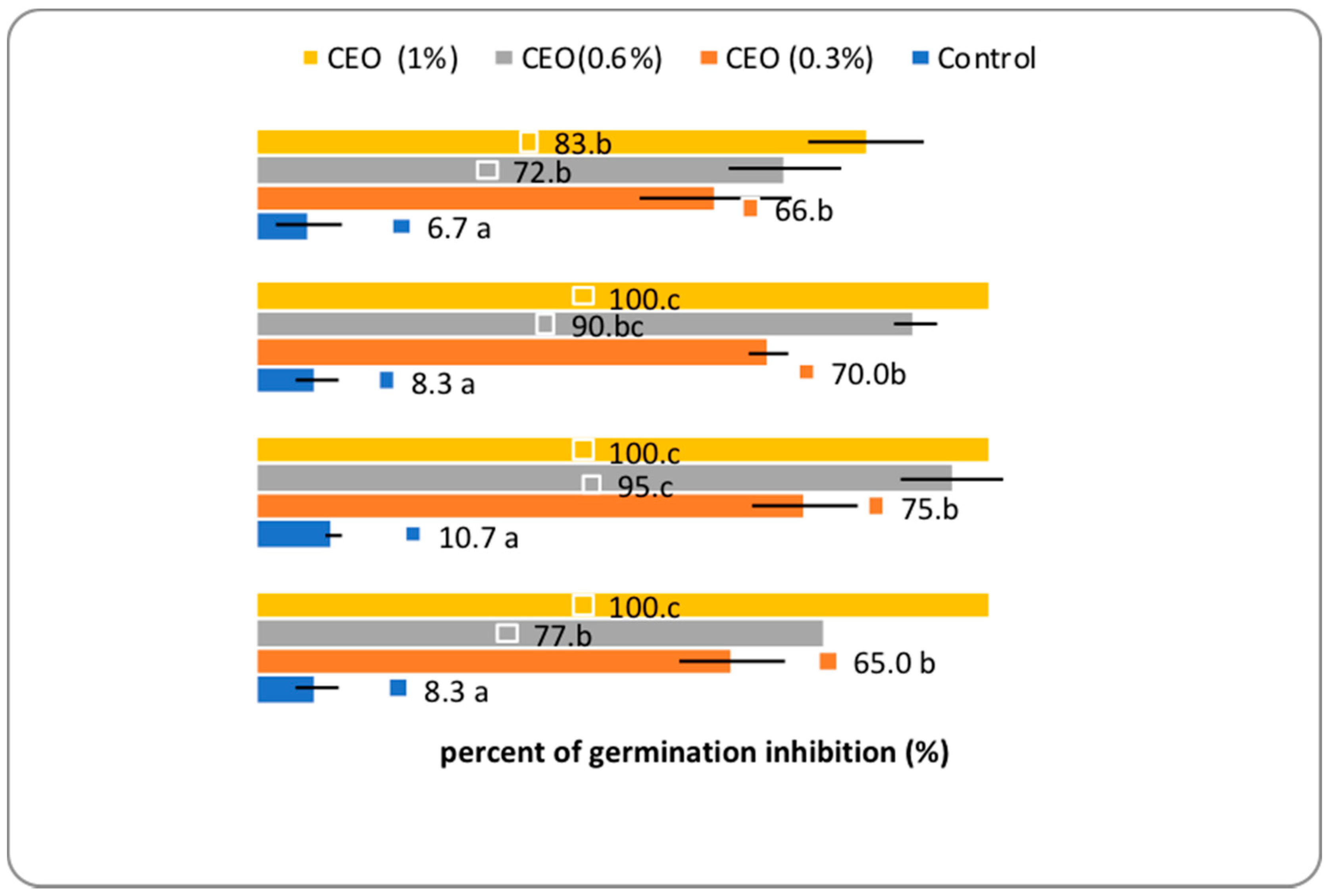
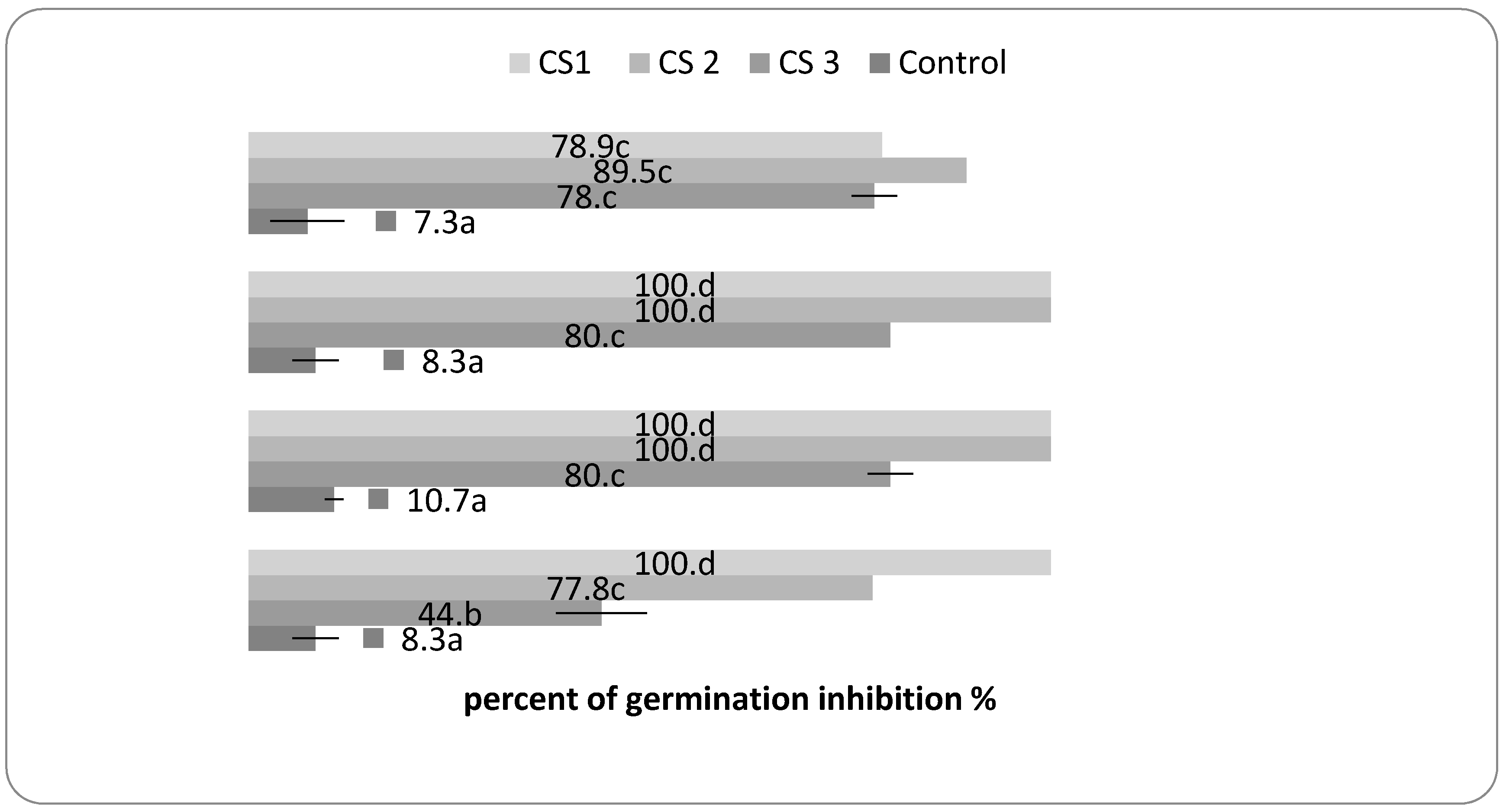
2.4. The Herbicidal Potential Assay
3. Materials and Methods
3.1. Extraction and GC-MS Characterization of CEO
3.2. In Vitro Antifungal Activity Assay
3.3. In Vivo CEO Potential against Mycotoxigenic Fungi and DON Accumulation
3.4. The Herbicidal Potential Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fleurat-Lessard, F. Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins—An update. J. Stored Prod. 2017, 71, 22–40. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Flamini, G. Natural Herbicides as a Safer and More Environmentally Friendly Approach to Weed Control: A Review of the Literature Since 2000. Struct. Chem. 2012, 38, 353–396. [Google Scholar]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Singletary, K. Coriander: Overview of Potential Health Benefits. Nutr. Today 2016, 51, 151–161. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonça, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef]
- Freires, I.D.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; De Alencar, S.M.; Figueira, G.M.; Rodrigues, J.A.D.O.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (Coriander) essential oil: antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef] [PubMed]
- Žabka, M.; Pavela, R.; Prokinova, E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere 2014, 112, 443–448. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Tolueinia, B.; Momeni, Z.; Hasani, Z.; Hasani, M.; Branch, Y. Analysis of antibacterial and antifungal activity of crude extracts from seeds of Coriandrum sativum. Gomal J. Med. Sci. 2012, 10, 167–171. [Google Scholar]
- Morais, W.; Lima, M.; Zanuncio, J.; Oliveira, M.; Bragança, M.; Serrão, J.; Della Lucia, T.; Lima, M.A. Extracts of Ageratum conyzoides, Coriandrum sativum and Mentha piperita inhibit the growth of the symbiotic fungus of leaf-cutting ants. Ind. Crop. Prod. 2015, 65, 463–466. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R.; McPhee, D.J. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.; Regnier, T.; Olivier, E. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S. Afr. J. Bot. 2015, 99, 115–121. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crop. Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Seseni, L.; Regnier, T.; Der Merwe, M.R.-V.; Mogale, E.; Badenhorst, J. Control of Fusarium spp. causing damping-off of pine seedlings by means of selected essential oils. Ind. Crop. Prod. 2015, 76, 329–332. [Google Scholar] [CrossRef]
- Ullah, N.; Parveen, A.; Bano, R.; Zulfiqar, I.; Maryam, M.; Jabeen, S.; Liaqat, A.; Ahmad, S. In vitro and in vivo protocols of antimicrobial bioassay of medicinal herbal extracts: A review. Asian Pac. J. Trop. 2016, 6, 660–667. [Google Scholar] [CrossRef]
- Ferdes, M.; Al Juhaimi, F.; Özcan, M.; Ghafoor, K. Inhibitory effect of some plant essential oils on growth of Aspergillus niger, Aspergillus oryzae, Mucor pusillus and Fusarium oxysporum. S. Afr. J. Bot. 2017, 113, 457–460. [Google Scholar] [CrossRef]
- Sumalan, R.-M.; Alexa, E.; Poiana, M.-A. Assessment of inhibitory potential of essential oils on natural mycoflora and Fusarium mycotoxins production in wheat. Chem. Cent. J. 2013, 7, 32. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic Activities of Mediterranean Essential Oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C.; O’Bryan, C.A. Essential oils as antimicrobials in food systems – A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils – A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Zamindar, N.; Sadrarhami, M.; Doudi, M. Antifungal activity of coriander (Coriandrum sativum L.) essential oil in tomato sauce. J. Food Meas. Charact. 2016, 10, 589–594. [Google Scholar] [CrossRef]
- Momin, A.; Acharya, S.; Gajjar, A. Coriandrum sativum. Review of advances in phytophaymacology. Intern. J. Pharm. Sci. Res. 2012, 2, 1233–1239. [Google Scholar] [CrossRef]
- Soares, B.V.; Morais, S.M.; Fontenelle, R.O.D.S.; Queiroz, V.A.; Vila-Nova, N.S.; Pereira, C.M.C.; Brito, E.S.; Neto, M.A.S.; Brito, E.H.S.; Cavalcante, C.S.P.; et al. Antifungal Activity, Toxicity and Chemical Composition of the Essential Oil of Coriandrum sativum L. Fruits. Molecules 2012, 17, 8439–8448. [Google Scholar] [CrossRef]
- Al-Snafi, P.D.A.E. A review on chemical constituents and pharmacological activities of Coriandrum sativum. IOSR J. Pham. 2016, 6, 17–42. [Google Scholar] [CrossRef]
- Darughe, F.; Barzegar, M.; Sahari, M.A. Antioxidant and antifungal activity of Coriander (Coriandrum sativum L.) Essential oil in cake. Intern. Food Res. J. 2012, 19, 1253–1260. [Google Scholar]
- Nurzyńska-Wierdak, R. Essential oil composition of the coriander (Coriandrum sativum L.) herb depending on the development stage. Acta Agrobot. 2013, 66, 53–60. [Google Scholar] [CrossRef]
- Yildiz, H. Chemical composition, antimicrobial, and antioxidant activities of essential oil and ethanol extract of Coriandrum sativum L. leaves from Turkey. Intern. J. Food Prop. 2016, 19, 1593–1603. [Google Scholar] [CrossRef]
- Strobl, H.; Kolodziejczyk, P. 14-Methylpentadecano-15-lactone (Muscolide): A New Macrocyclic Lactone from the Oil of Angelica archangelica L. Chem. Biodivers. 2004, 1, 1880–1887. [Google Scholar]
- Ateș, D.A.; Erdogrul, O.T. Antimicrobial Activities of Various Medicinal and Commercial Plant Extracts. Turk. J. Biol. 2003, 27, 157–162. [Google Scholar]
- Zare-shehneh, M.; Askarfarashah, M.; Ebrahimi, L. Biological activities of a new antimicrobial peptide from Coriandrum sativum. Intern. J. Biosci. 2014, 4, 89–99. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Ying, G.; Yang, M.; Nian, Y.; Wei, F.; Kong, W. Antifungal evaluation of plant essential oils and their major components against toxigenic fungi. Ind. Crop. Prod. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Andrés, M.; Rossa, G.; Cassel, E.; Vargas, R.; Santana, O.; Díaz, C.; Gonzalez-Coloma, A. Biocidal effects of Piper hispidinervum (Piperaceae) essential oil and synergism among its main components. Food Chem. Toxicol. 2017, 109, 1086–1092. [Google Scholar] [CrossRef]
- Goger, G.; Demirci, B.; Ilgın, S.; Demirci, F. Antimicrobial and toxicity profiles evaluation of the Chamomile (Matricaria recutita L.) essential oil combination with standard antimicrobial agents. Ind. Crop. Prod. 2018, 120, 279–285. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P.; Sattayasamitsathit, S. Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Ind. Crop. Prod. 2017, 97, 558–566. [Google Scholar] [CrossRef]
- Katar, D.; Kacar, O.; Kara, N.; Aytaç, Z.; Göksu, E.; Kara, S.; Elmastaş, M. Ecological variation of yield and aroma components of summer savory (Satureja hortensis L.). J. Appl. Res. Med. Aromat. Plant. 2017, 7, 131–135. [Google Scholar] [CrossRef]
- Velluti, A. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int. J. Food Microbiol. 2003, 89, 145–154. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd_Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Atak, M.; Mavi, K.; Uremis, I. Bio-herbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11159. [Google Scholar]
- Aslani, F.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Hashemi, F.S.G.; Alam, M.A. Control of weeds in glasshouse and rice field conditions by phytotoxic effects of Tinospora crispa (L.) Hook. f. & Thomson leaves. Chil. J. Agric. Res. 2016, 76, 432–440. [Google Scholar] [CrossRef]
- Paudel, V.R.; Gupta, V.P. Effect of some Essential Oils on Seed Germination and Seedling Length of Parthenium hysterophorous L. Ecoprint: Int. J. Ecol. 2009, 15, 69–73. [Google Scholar] [CrossRef]
- Campiglia, E.; Mancinelli, R.; Cavalieri, A.; Caporali, F. Use of Essential Oils of Cinnamon, Lavender and Peppermint for Weed Control. Ital. J. Agron. 2007, 2, 171. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commission. European Pharmacopoeia; European Directorate for the Quality of Medicines & Healthcare (EDQM): Strasbourg, France, 1975; Volume 3, pp. 89–99. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Sangeeta. Efficacy of some medicinal plant extract against Fusarium oxysporum ssp. ciceri causing chickpea wilt. Asian J. Crop Sci. 2015, 7, 138–146. [Google Scholar] [CrossRef]
- Alexa, E.; Danciu, C.; Cocan, I.; Negrea, M.; Morar, A.; Obistioiu, D.; Dogaru, D.; Berbecea, A.; Radulov, I. Chemical Composition and Antimicrobial Potential of Satureja hortensis L. in Fresh Cow Cheese. J. Food Qual. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Doolotkeldieva, T. Microbiological Control of Flour-Manufacture: Dissemination of Mycotoxins Producing Fungi in Cereal Products. Microbiol. Insights 2010, 3, 1. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Nr. | Compounds | Type | Retention Time | LRIc * | LRIr ** | % |
|---|---|---|---|---|---|---|
| 1. | α-Pinene | MH | 5.552 | 1011 | 1015 | 11.626 |
| 2. | Camphene | MH | 6.500 | 1057 | 1057 | 1.083 |
| 3. | β-Pinene | MH | 7.445 | 1097 | 1096 | 0.710 |
| 4. | β-Myrcene | MH | 8.684 | 1164 | 1164 | 1.504 |
| 5. | d-limonene | MH | 9.639 | 1188 | 1189 | 9.628 |
| 6. | o-Cymene | MH | 10.394 | 1203 | 1205 | 0.447 |
| 7. | γ-Terpinene | MH | 10.757 | 1238 | 1238 | 5.236 |
| 8. | p-Cymene | MH | 11.344 | 1257 | 1261 | 8.000 |
| 9. | α-Terpinolene | MH | 11.645 | 1278 | 1274 | 0.970 |
| 10. | Methyl-heptenone | MO | 12.789 | 1323 | 1332 | 0.050 |
| 11. | l-Fenchone | MO | 14.352 | 1418 | 1419 | 0.237 |
| 12. | trans-Linalool oxide | MO | 15.292 | 1453 | 1456 | 2.817 |
| 13. | cis-Linalool oxide | MO | 15.940 | 1471 | 1472 | 2.284 |
| 14. | Camphor | MO | 17.086 | 1507 | 1511 | 6.016 |
| 15. | Linalool | MO | 17.549 | 1533 | 1537 | 45.387 |
| 16. | Bornyl-acetate | MO | 18.419 | 1542 | 1544 | 0.056 |
| 17. | Estragole | MO | 20.128 | 1654 | 1652 | 0.158 |
| 18. | α-Terpineol | MO | 21.116 | 1694 | 1692 | 0.122 |
| 19. | Geranyl acetate | MO | 21.682 | 1763 | 1765 | 1.423 |
| 20. | p-Propenil-anisol | MO | 23.148 | 1817 | 1818 | 1.630 |
| 21. | Thymol | MO | 29.948 | 2160 | 2164 | 0.376 |
| Total of major compounds | 99.760% | |||||
| Monoterpene hidrocarbonates (MH) | 39.204% | |||||
| Monoterpene oxygenate (MO) | 60.556% | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumalan, R.M.; Alexa, E.; Popescu, I.; Negrea, M.; Radulov, I.; Obistioiu, D.; Cocan, I. Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil. Molecules 2019, 24, 2040. https://doi.org/10.3390/molecules24112040
Sumalan RM, Alexa E, Popescu I, Negrea M, Radulov I, Obistioiu D, Cocan I. Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil. Molecules. 2019; 24(11):2040. https://doi.org/10.3390/molecules24112040
Chicago/Turabian StyleSumalan, Renata Maria, Ersilia Alexa, Iuliana Popescu, Monica Negrea, Isidora Radulov, Diana Obistioiu, and Ileana Cocan. 2019. "Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil" Molecules 24, no. 11: 2040. https://doi.org/10.3390/molecules24112040
APA StyleSumalan, R. M., Alexa, E., Popescu, I., Negrea, M., Radulov, I., Obistioiu, D., & Cocan, I. (2019). Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil. Molecules, 24(11), 2040. https://doi.org/10.3390/molecules24112040