Benefits of the Hydrophobic Surface for CH3NH3PbI3 Crystalline Growth towards Highly Efficient Inverted Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Measurements and Characterization
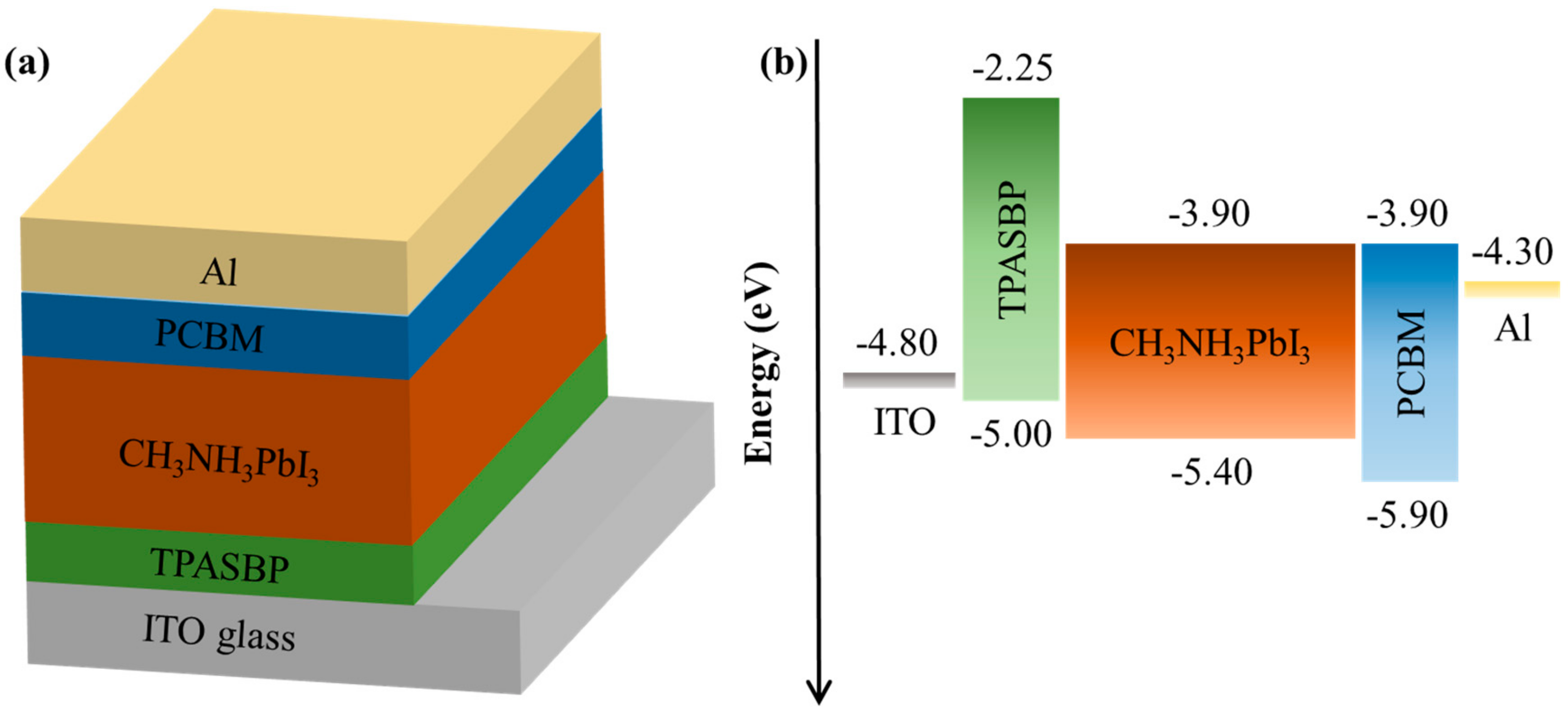
3. Results and Discussion
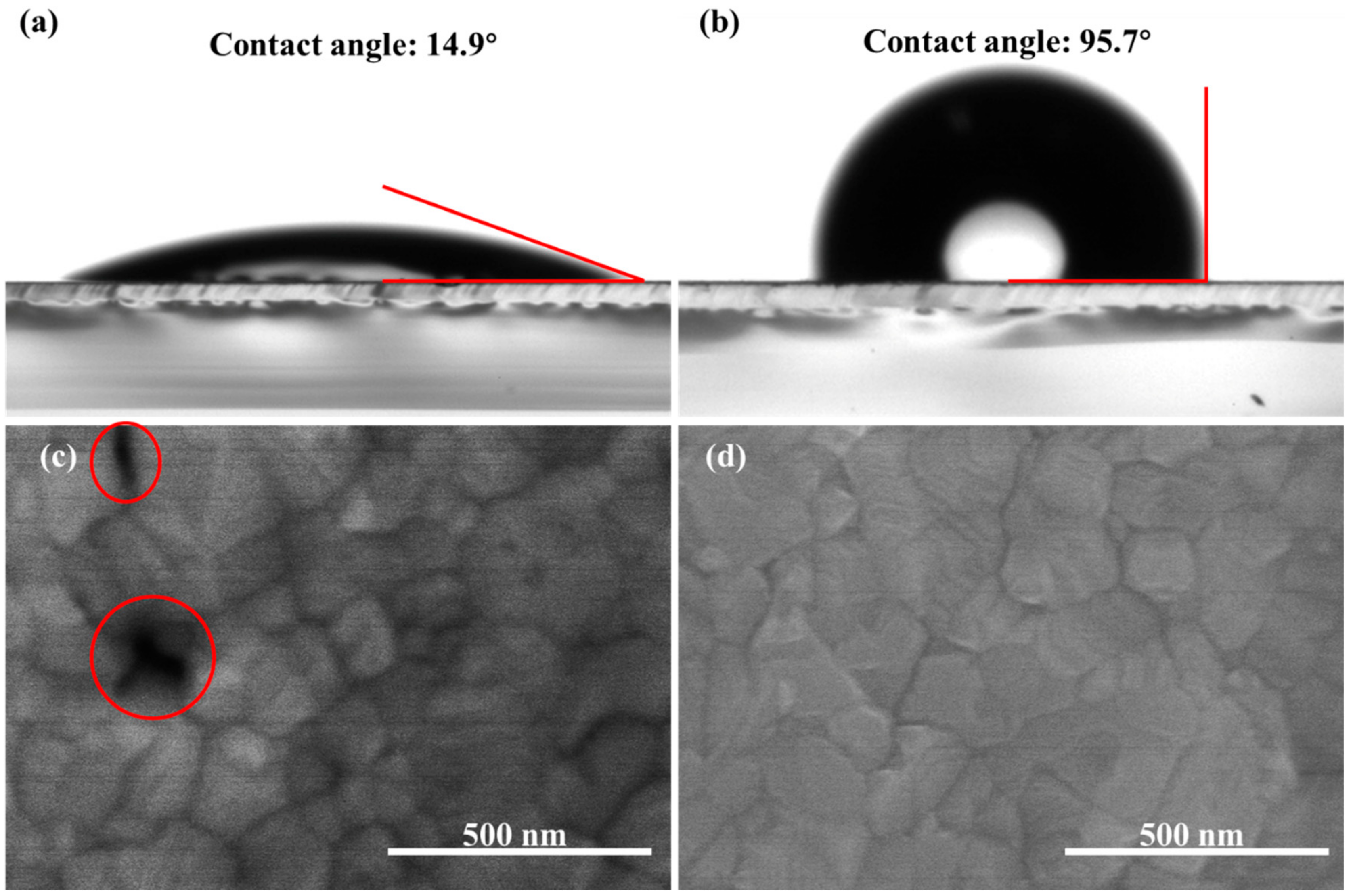
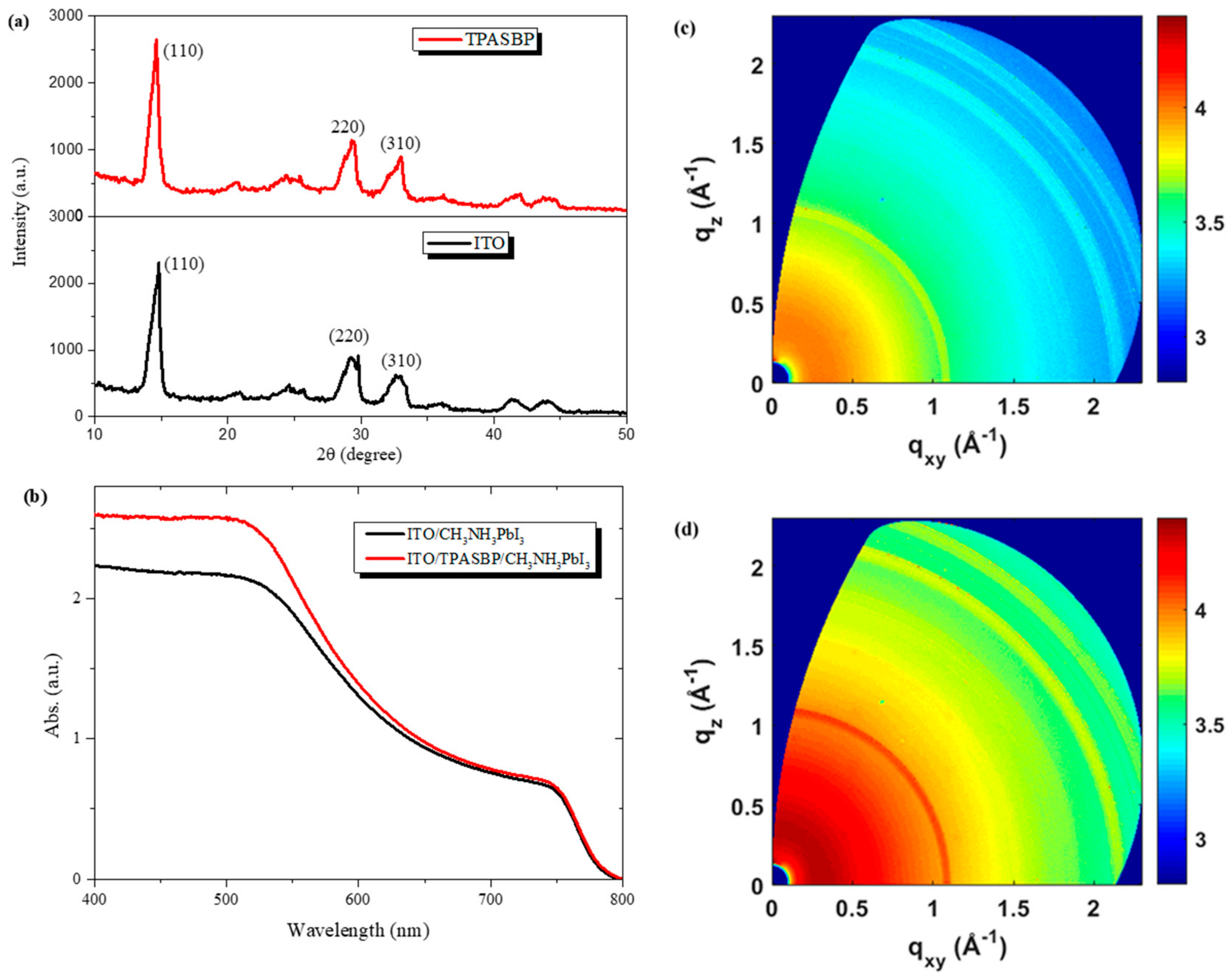
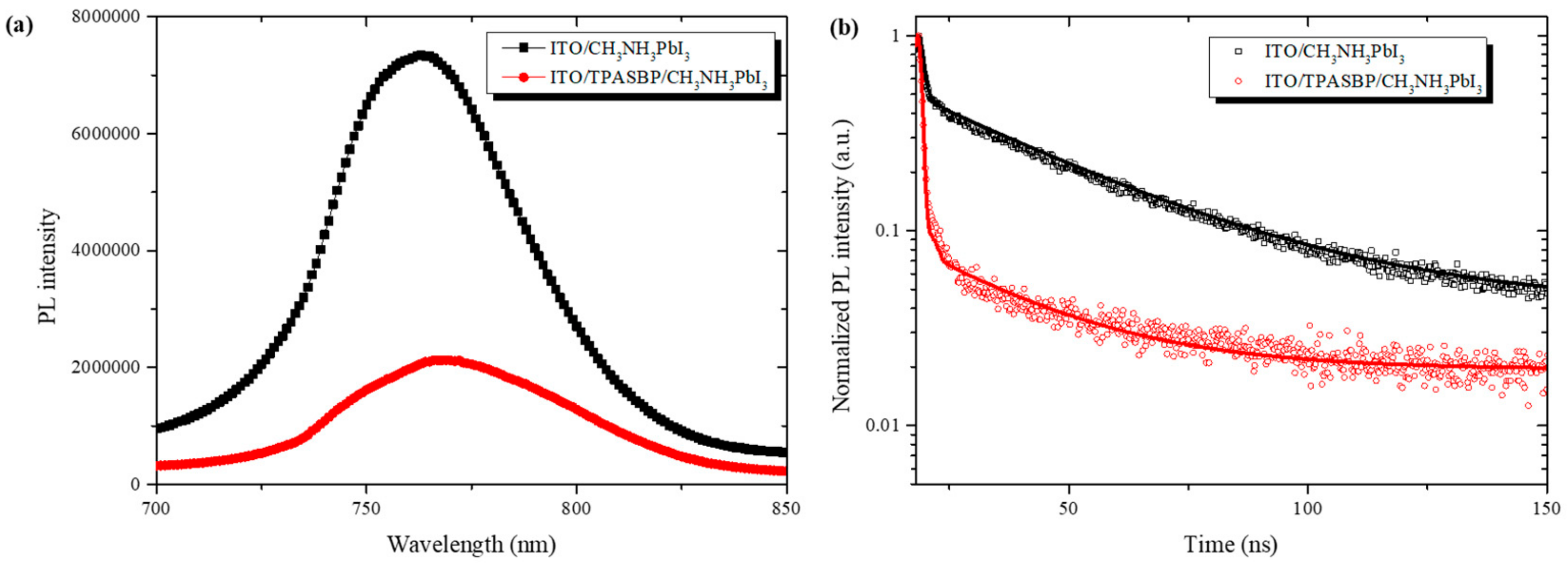
3.1. Characterization of the As-Prepared Perovskite Films
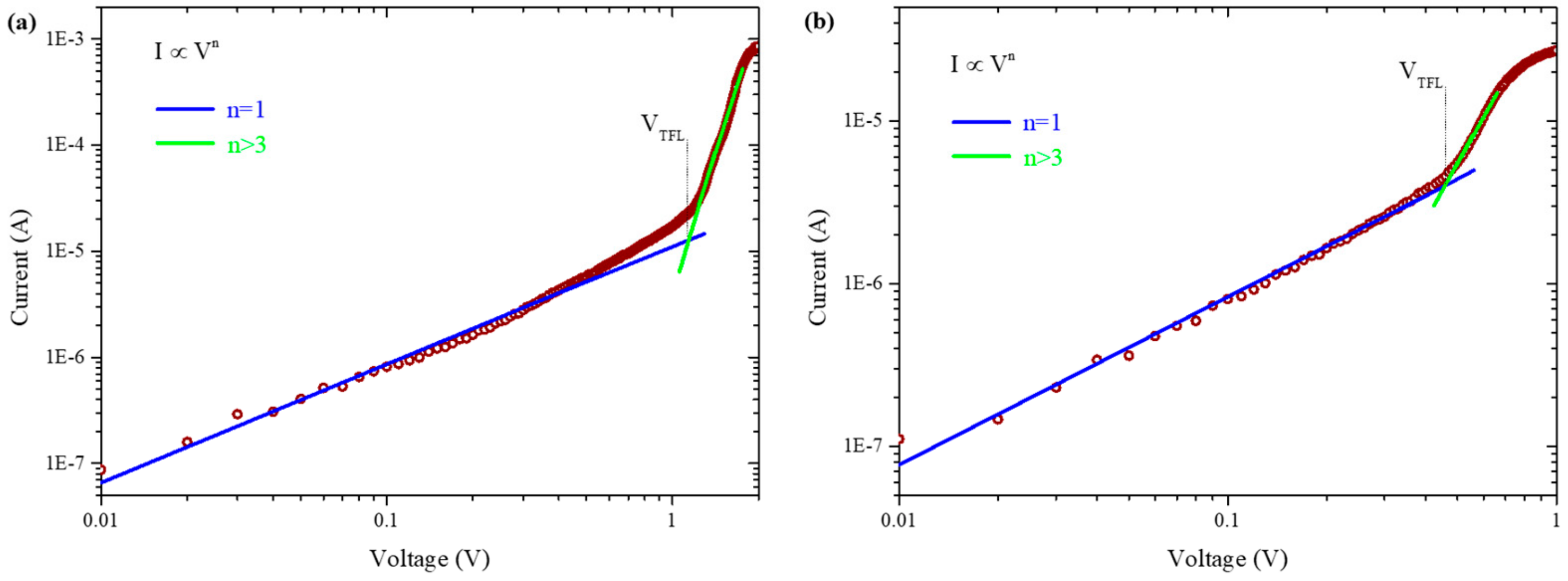
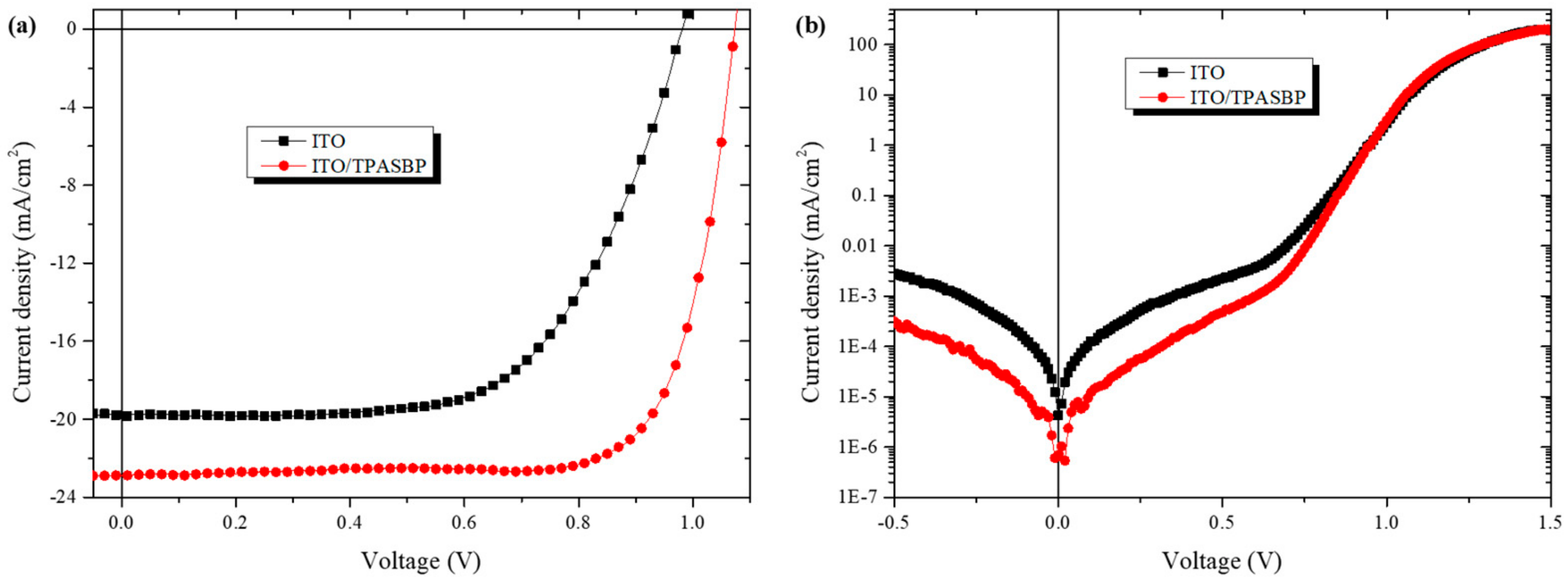
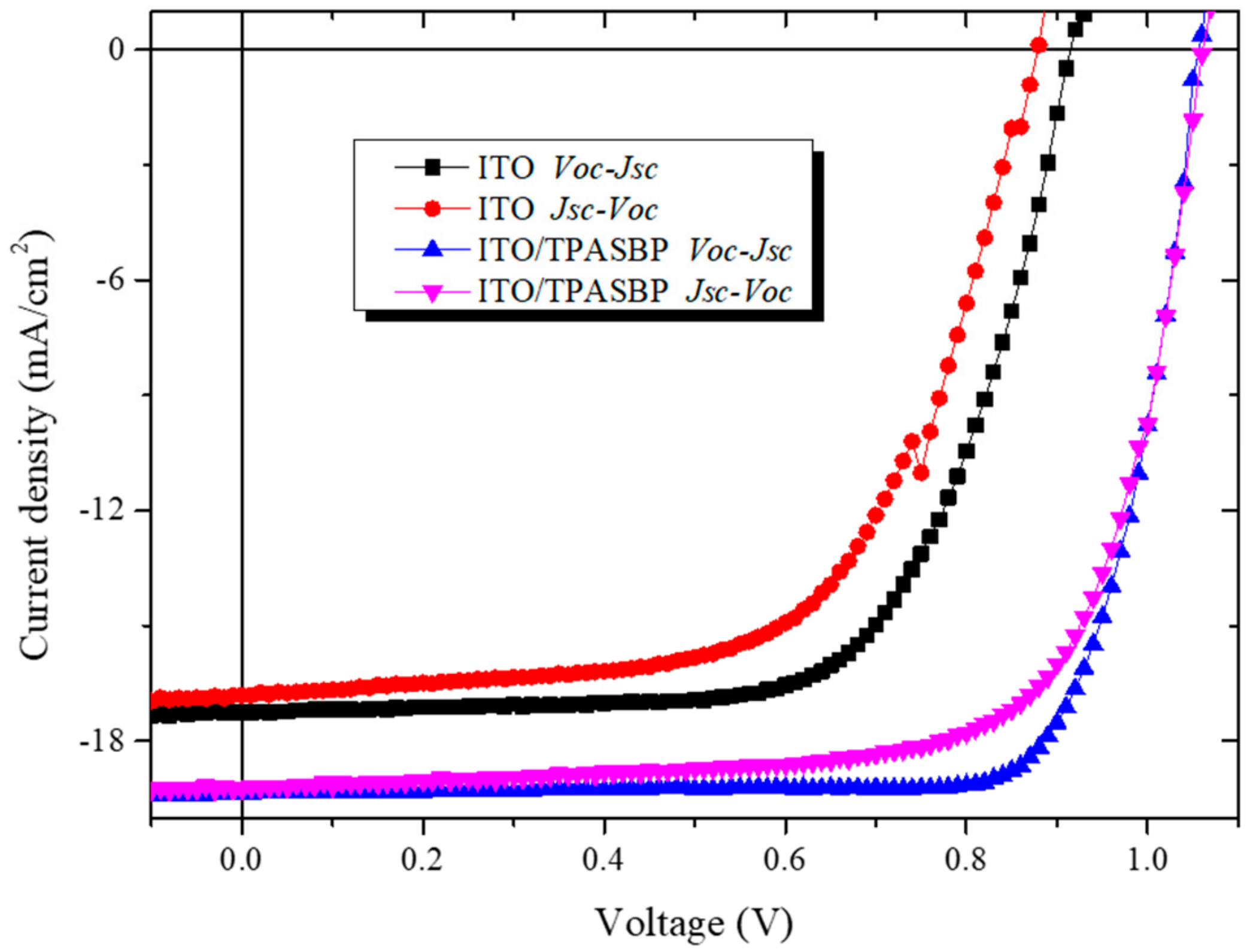
3.2. Photovoltaic Performance of PSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect Passivation in Hybrid Perovskite Solar Cells Using Quaternary Ammonium Halide Anions and Cations. Nat. Energy 2017. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface Passivation of Perovskite Film for Efficient Solar Cells. Nat. Photonics 2019. [Google Scholar] [CrossRef]
- NREL Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-reserch-cell-efficiencies.20190411.pdf (accessed on 17 April 2019).
- Paek, S.; Zimmermann, I.; Gao, P.; Gratia, P.; Rakstys, K.; Grancini, G.; Nazeeruddin, M.K.; Rub, M.A.; Kosa, S.A.; Alamry, K.A.; et al. Donor-π-Donor Type Hole Transporting Materials: Marked π-Bridge Effects on Optoelectronic Properties, Solid-State Structure, and Perovskite Solar Cell Efficiency. Chem. Sci. 2016, 7, 6068–6075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hua, Y.; Xu, B.; Yang, L.; Liu, P.; Johansson, M.B.; Vlachopoulos, N.; Kloo, L.; Boschloo, G.; Johansson, E.M.J.; et al. The Role of 3D Molecular Structural Control in New Hole Transport Materials Outperforming Spiro-OMeTAD in Perovskite Solar Cells. Adv. Energy Mater. 2016. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-Wetting Surface-Driven High-Aspect-Ratio Crystalline Grain Growth for Efficient Hybrid Perovskite Solar Cells. Nat. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Jhuo, H.-J.; Yeh, P.-N.; Liao, S.-H.; Li, Y.-L.; Sharma, S.; Chen, S.-A. Inverted Perovskite Solar Cells with Inserted Cross-Linked Electron-Blocking Interlayers for Performance Enhancement. J. Mater. Chem. A 2015, 3, 9291–9297. [Google Scholar] [CrossRef]
- Yan, W.; Li, Y.; Li, Y.; Ye, S.; Liu, Z.; Wang, S.; Bian, Z.; Huang, C. High-Performance Hybrid Perovskite Solar Cells with Open Circuit Voltage Dependence on Hole-Transporting Materials. Adv. Energy Mater. 2015, 16, 428–437. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Guo, T.-F.; Yang, Y. Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells. Acc. Chem. Res. 2015, 49, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Ye, S.; Li, Y.; Sun, W.; Rao, H.; Liu, Z.; Bian, Z.; Huang, C. Hole-Transporting Materials in Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2016. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Chiang, Y.F.; Lee, M.H.; Peng, S.R.; Guo, T.F.; Chen, P.; Wen, T.C. CH3NH3PbI3 Perovskite/Fullerene Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-Less Inverted CH3NH3PbI3 Planar Perovskite Hybrid Solar Cells with 18.1% Power Conversion Efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Wang, Z.; Pathak, S.; Zhang, W.; Wisnivesky-Rocca-Rivarola, F.; Huang, J.; Nayak, P.K.; Patel, J.B.; Yusof, H.A.M.; Vaynzof, Y. Efficient Perovskite Solar Cells by Metal Ion Doping. Energy Environ. Sci. 2016, 9, 2892–2901. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced Photovoltage for Inverted Planar Heterojunction Perovskite Solar Cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M. Efficient and Stable Large-Area Perovskite Solar Cells with Inorganic Charge Extraction Layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Ye, S.; Sun, W.; Yan, W.; Li, Y.; Peng, H.; Liu, Z.; Bian, Z.; Li, Y.; Huang, C. A 19.0% Efficiency Achieved in CuOx-Based Inverted CH3NH3PbI3−xClx Solar Cells by an Effective Cl Doping Method. Nano Energy 2016, 27, 51–57. [Google Scholar] [CrossRef]
- Xu, B.; Bi, D.; Hua, Y.; Liu, P.; Cheng, M.; Grätzel, M.; Kloo, L.; Hagfeldt, A.; Sun, L. A Low-Cost Spiro [fluorene-9, 9′-xanthene]-Based Hole Transport Material for Highly Efficient Solid-State Dye-Sensitized Solar Cells and Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 873–877. [Google Scholar] [CrossRef]
- Huang, C.; Fu, W.; Li, C.-Z.; Zhang, Z.; Qiu, W.; Shi, M.; Heremans, P.; Jen, A.K.-Y.; Chen, H. Dopant-Free Hole-Transporting Material with a C3h Symmetrical Truxene Core for Highly Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 2528–2531. [Google Scholar] [CrossRef]
- Wang, Q.; Bi, C.; Huang, J. Doped Hole Transport Layer for Efficiency Enhancement in Planar Heterojunction Organolead Trihalide Perovskite Solar Cells. Nano Energy 2015, 15, 275–280. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Kunwu, F.; Boix, P.P.; Li, H.; Koh, T.M.; Leong, W.L.; Powar, S.; Grimsdale, A.; Grätzel, M.; Mathews, N. A Swivel-Cruciform Thiophene Based Hole-Transporting Material for Efficient Perovskite Solar Cells. J. Mater. Chem. A 2014, 2, 6305–6309. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, Z.; Zhu, L.; Kou, C.; Liu, Y.; Li, C.; Meng, Q.; Li, W.; Bo, Z. Elimination of the J-V Hysteresis of Planar Perovskite Solar Cells by Interfacial Modification with a Thermo-Cleavable Fullerene Derivative. J. Mater. Chem. A 2016, 4, 17649–17654. [Google Scholar] [CrossRef]
- Wang, Z.K.; Gong, X.; Li, M.; Hu, Y.; Wang, J.M.; Ma, H.; Liao, L.S. Induced Crystallization of Perovskites by a Perylene Underlayer for High-Performance Solar Cells. ACS Nano 2016, 10, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-Structure Perovskite Solar Cells with Efficiency beyond 21%. Adv. Mater. 2017. [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Li, X.; Zhu, L.; Meng, Q.; Xiao, Y.; Li, D. Novel Hole Transporting Materials with a Linear π-Conjugated Structure for Highly Efficient Perovskite Solar Cells. Chem. Commun. 2014, 50, 5829–5832. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Park, S.; Kim, Y.C.; Jeon, N.J.; Noh, J.H.; Yoon, S.C.; Seok, S.I. Benefits of very Thin PCBM and LiF Layers for Solution-Processed p–i–n Perovskite Solar Cells. Energy Environ. Sci. 2014, 7, 2642–2646. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent Engineering for High-Performance Inorganic–Organic Hybrid Perovskite Solar Cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Dong, Q.; Bi, C.; Shao, Y.; Yuan, Y.; Huang, J. Solvent Annealing of Perovskite-Induced Crystal Growth for Photovoltaic-Device Efficiency Enhancement. Adv. Mater. 2014, 26, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K. Low Trap-State Density and Long Carrier Diffusion in Organolead Trihalide Perovskite Single Crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, R.; Ren, X.; Zhu, X.; Yang, Z.; Li, C.; Liu, S. Hysteresis-Suppressed High-Efficiency Flexible Perovskite Solar Cells Using Solid-State Ionic-Liquids for Effective Electron Transport. Adv. Mater. 2016, 28, 5206–5213. [Google Scholar] [CrossRef] [PubMed]
- Laban, W.A.; Etgar, L. Depleted Hole Conductor-Free Lead Halide Iodide Heterojunction Solar Cells. Energy Environ. Sci. 2013, 6, 3249–3253. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Zhao, S.; Qiao, B.; Huang, D.; Zhao, L.; Zhao, J.; Wang, P.; Zhu, Y.; Li, X.; et al. Highly Efficient p-i-n Perovskite Solar Cells Utilizing Novel Low-Temperature Solution-Processed Hole Transport Materials with Linear π-Conjugated Structure. Small 2016, 12, 4902–4908. [Google Scholar] [CrossRef] [PubMed]
- Wetzelaer, G.J.A.; Scheepers, M.; Sempere, A.M.; Momblona, C.; Ávila, J.; Bolink, H.J. Trap-Assisted Non-Radiative Recombination in Organic–Inorganic Perovskite Solar Cells. Adv. Mater. 2015, 27, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Ji, J.; Li, Y.; Li, G.; Li, M.; Wang, T.; Wei, D.; Cui, P.; He, Y.; Mbengue, J.M. Degradation of Organometallic Perovskite Solar cells Induced by Trap States. Appl. Phys. Lett. 2016. [CrossRef]
- Shi, J.; Dong, J.; Lv, S.; Xu, Y.; Zhu, L.; Xiao, J.; Xu, X.; Wu, H.; Li, D.; Luo, Y. Hole-Conductor-Free Perovskite Organic Lead Iodide Heterojunction Thin-Film Solar Cells: High Efficiency and Junction Property. Appl. Phys. Lett. 2014. [CrossRef]
| PSCs | Voc [V] | Jsc [mA/cm2] | FF [%] | PCE [%] | Average PCE a [%] |
|---|---|---|---|---|---|
| ITO | 0.98 | 19.72 | 62.35 | 12.05 | 11.07± 0.97 |
| ITO/TPASBP | 1.07 | 22.88 | 76.47 | 18.72 | 17.23 ± 0.79 |
| PSCs | A1 a | A2 b | RS (Ωcm2) | J0 (mAcm−2) |
|---|---|---|---|---|
| ITO | 2.27 | 2.22 | 5.68 | 2.27 × 10-4 |
| ITO/TPASBP | 1.56 | 1.72 | 1.19 | 2.38 × 10-6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, Z.; Zhao, S.; Song, D.; Qiao, B.; Zhu, Y.; Meng, J. Benefits of the Hydrophobic Surface for CH3NH3PbI3 Crystalline Growth towards Highly Efficient Inverted Perovskite Solar Cells. Molecules 2019, 24, 2027. https://doi.org/10.3390/molecules24102027
Li Y, Xu Z, Zhao S, Song D, Qiao B, Zhu Y, Meng J. Benefits of the Hydrophobic Surface for CH3NH3PbI3 Crystalline Growth towards Highly Efficient Inverted Perovskite Solar Cells. Molecules. 2019; 24(10):2027. https://doi.org/10.3390/molecules24102027
Chicago/Turabian StyleLi, Yang, Zheng Xu, Suling Zhao, Dandan Song, Bo Qiao, Youqin Zhu, and Juan Meng. 2019. "Benefits of the Hydrophobic Surface for CH3NH3PbI3 Crystalline Growth towards Highly Efficient Inverted Perovskite Solar Cells" Molecules 24, no. 10: 2027. https://doi.org/10.3390/molecules24102027
APA StyleLi, Y., Xu, Z., Zhao, S., Song, D., Qiao, B., Zhu, Y., & Meng, J. (2019). Benefits of the Hydrophobic Surface for CH3NH3PbI3 Crystalline Growth towards Highly Efficient Inverted Perovskite Solar Cells. Molecules, 24(10), 2027. https://doi.org/10.3390/molecules24102027





