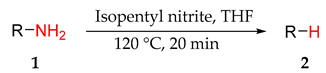Reagents and solvents were purchased from Fischer Scientific, Sigma Aldrich™, Fluorochem™, or Alfa Aesar™, were of analytical reagent grade and were used as received. 1H and 13C-NMR spectra were recorded, in specified deuterated solvents, (purchased from Apollo Scientific™), at room temperature on Bruker™ Avance-400 (1H, 13C) (operating at 400.13 MHz) spectrometers and are reported as follows: chemical shift δ (ppm) (number of protons, multiplicity, coupling constant J (Hz) (if applicable), assignment). Multiplicities are reported using the following abbreviations: s (singlet), d (doublet), t (triplet), q (quartet) and m (unresolved multiplet). All 13C-NMR spectra were proton-decoupled and carbons are numbered according to the IUPAC systematic name. The 1H and 13C chemical shifts are reported using the residual signal of deuterated solvent as the internal reference (for CDCl3: δH = 7.26 ppm; δC = 77.16 ppm and for deuterated d6-DMSO: δH = 2.50 ppm; δC = 39.51 ppm). All chemical shifts are quoted in parts per million, relative to tetramethylsilane (δH, δC = 0.00 ppm). All coupling constants are 3JHH unless otherwise stated. Electrospray Ionisation (ESI) mass spectra, for LC-MS results were obtained using a TQD mass spectrometer (Waters UK Ltd., Manchester, UK). High-resolution mass spectra were obtained with an LCT Premier XE mass spectrometer (Waters UK Ltd., Manchester, UK); all were obtained by the Durham University Mass Spectrometry service. ASAP mass spectra were obtained using a Waters™ Synapt G2s apparatus. Thin-Layer Chromatography was performed using Merck TLC Aluminium oxide 60 F254 with glass backing. Plates were stained with potassium permanganate solution, where required and visualised using UV light. Column chromatography refers to purification by applying the mixture, dissolved in a minimum amount of dichloromethane, onto silica gel (40–63 µm mesh size) with the stated solvent system.
4.6. General Procedure for Hydrodeazoniation of Substrates
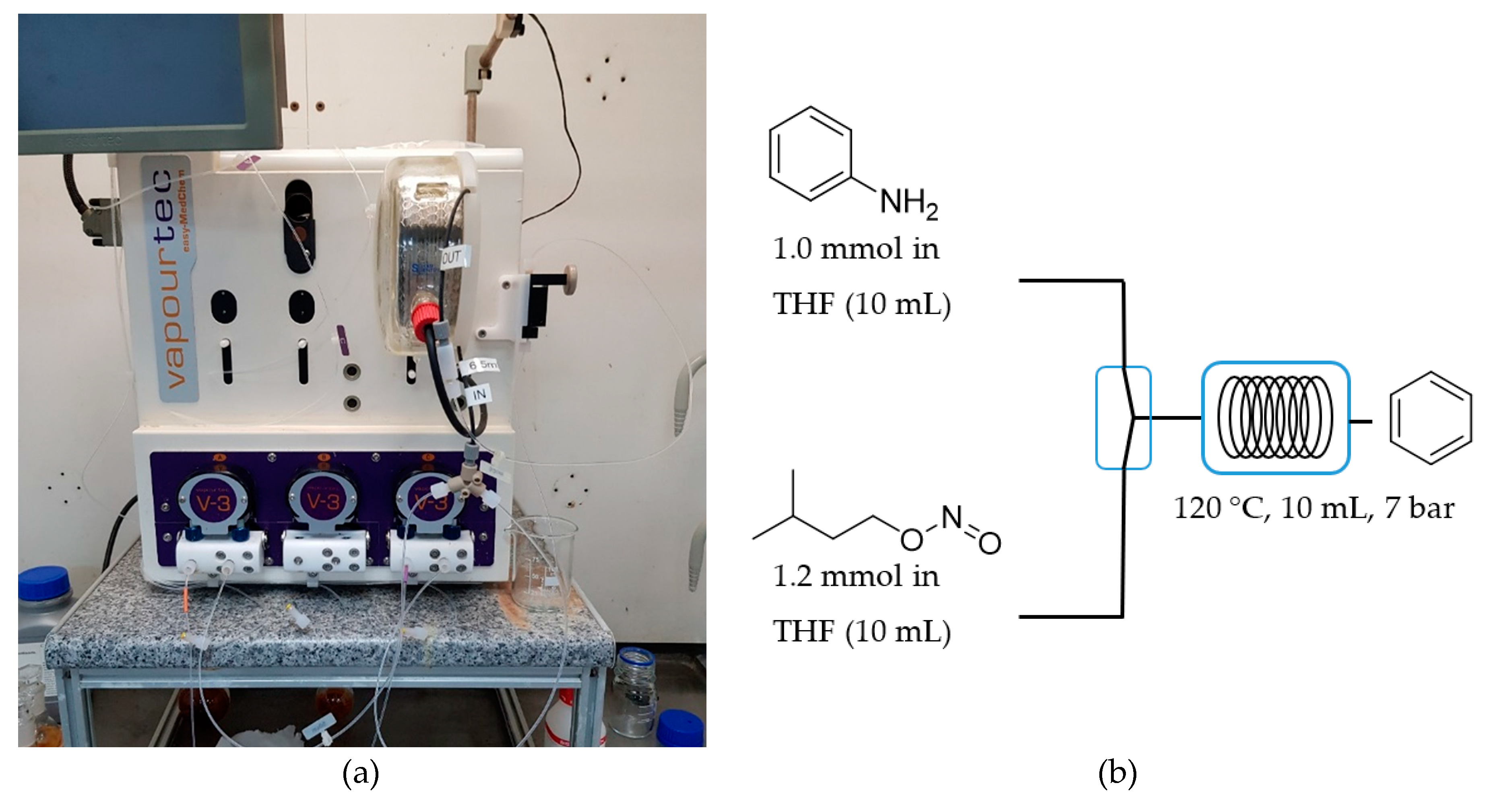
A solution of the selected heterocyclic starting material (1.00 mmol) in THF (10 mL) and a solution of isopentyl nitrite (141 mg, 1.20 mmol) in THF (10 mL) were both pumped at a flow rate of 0.25 mL min−1 with a Vapourtech ‘Easy MedChem V3’ system, meeting at a PTFE T-piece and the output flowing through a 10.0 mL coil reactor maintained at 120 °C, giving a residence time of 20 min. The pressure of the system was maintained at 7 bar with a back-pressure regulator. For compounds where an isolated yield was reported: the output mixture was concentrated under reduced pressure to give an oil (or powder). The oil (or powder) was purified using column chromatography with various mixtures of ethyl acetate and hexane as the eluent, or by recrystallisation using methanol, to give isolated compounds that showed no impurities by NMR spectroscopy. For compounds where a conversion was reported (due to volatility of products), the output mixture was carefully concentrated under a reduced pressure of 100 mbar for 10 min and the conversion was calculated by integration of product peaks to a quantified internal standard (nitrobenzene).
Starting materials
1a–
d,
1f–
s, 1al–
ar were obtained from Alfa Asear and were used as supplied without additional purification. Other starting materials were synthesised, with spectra provided in the
supplementary information.
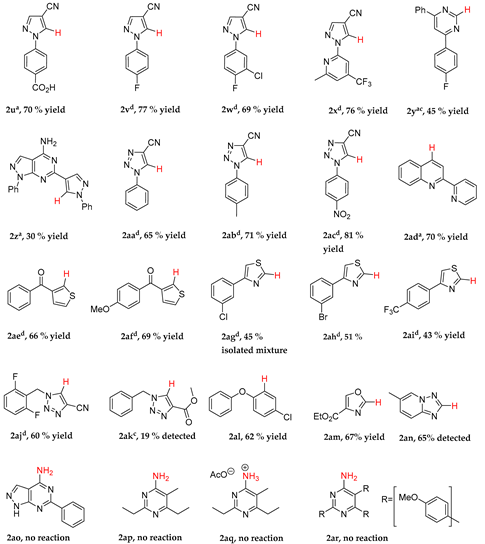
6-Chlorobenzo[1,3]thiazole (2d): Eluent: hexane/ethyl acetate (100:1)→(4:1) yellow crystals, 68% yield. 1H-NMR (400 MHz, CDCl3) δ 8.98 (s, 1H), 8.05 (d, J = 8.7 Hz, 1H), 7.95 (d, J = 2.1 Hz, 1H), 7.49 (dd, J = 8.7, 2.1 Hz, 1H). 13C-NMR (101 MHz, CDCl3) δ 154.51 (CH), 151.72 (C), 135.06 (C), 131.95 (C), 127.34 (CH), 124.46 (CH), 121.66 (CH). ASAP-MS (MeCN) Rt = 0.50 min [M + H]+ = 170.0.
3-(1-Methylethyl)benzoic acid (2e): Eluent: hexane/ethyl acetate (100:1)→(4:1). Yellow needles, 96% yield. 1H-NMR (400 MHz, CDCl3) δ 8.00 (d, J = 1.8 Hz, 1H), 7.95 (dt, J = 7.6, 1.4 Hz, 1H), 7.48 (d, J = 1.3 Hz, 1H), 7.40 (t, J = 1.3 Hz, 1H), 2.99 (p, J = 7.3 Hz, 1H), 1.29 (d, J = 7.3 Hz, 6H). 13C-NMR (101 MHz, CDCl3) δ 172.87 (C), 149.44 (C), 132.29 (CH), 129.46 (C), 128.63 (CH), 128.34 (CH), 127.93 (CH), 34.16 (CH), 24.01 (CH3). LC-MS (MeCN) Rt = 2.25 min [M − H]− = 163.2. HRMS C10H11O2 calc. 163.0759, found 163.0754, (Δ = −3.1 ppm).
Methyl thiophene-3-carboxylate (2r): Eluent: hexane/ethyl acetate (100:1)→(10:1). 1H-NMR (400 MHz, CDCl3) δ 8.12 (dd, J = 3.0, 1.2 Hz, 1H), 7.54 (dd, J = 5.1, 1.2 Hz, 1H), 7.41–7.28 (m, 1H), 3.95–3.85 (m, 3H). 13C-NMR (101 MHz, CDCl3) δ 161.28, 133.50, 132.70, 127.88, 126.04, 51.84. LC-MS (MeOH) Rt = 0.87 min [M + H]+ = 143.1.
Benzo[1,3]thiazole (2s): Eluent: hexane/ethyl acetate (100:1)→(10:1). Brown, volatile oil, 99% yield. 1H-NMR (400 MHz, CDCl3) δ = 9.00 (s, 1H), 8.35 (d, J = 8.2 Hz, 1H), 7.95 (d, J = 8.2 Hz, 1H), 7.51 (t, J = 7.7 Hz, 1H), 7.42 (t, J = 7.7 Hz, 1H). GC-MS M+ 135.1.
5-(2-Bromophenyl)-1,3,4-thiadiazol-2-amine (
1t): Starting material
1t was prepared following the procedure of Mullick et al. [
19] and isolated as a white solid in 85% yield.
1H-NMR (400 MHz, CDCl
3) δ 8.01 (dd,
J = 7.8, 1.7 Hz, 1H), 7.70 (dd,
J = 7.9, 1.3 Hz, 1H), 7.43 (td,
J = 7.6, 1.3 Hz, 1H), 7.37–7.29 (m, 1H), 6.06 (br. s, 2H).
1H-NMR (400 MHz, DMSO-
d6) δ 7.87 (dd,
J = 7.8, 1.8 Hz, 1H), 7.75 (dd,
J = 7.8, 1.2 Hz, 1H), 7.53–7.41 (m, 3H), 7.37 (td,
J = 7.8, 1.8 Hz, 1H).
13C-NMR (101 MHz, DMSO-
d6) δ 170.47 (C), 153.62 (C), 134.17 (C), 132.07 (C), 131.65 (C), 128.53 (C), 121.38 (C).
13C-NMR (101 MHz, DMSO
-d6) δ 134.17, 131.66, 131.64, 128.53. LC-MS (MeCN) R
t = 1.69 min [M + H]
+ = 258.2. HRMS C
8H
6N
3S
79Br calc. 255.9544, found 255.9551, (Δ = 2.7 ppm).
2-(2-Bromophenyl)-1,3,4-thiadiazole (2t): Eluent: hexane/ethyl acetate (95:5)→(4:1). Brown oil, 67% yield. 1H-NMR (400 MHz, CDCl3) δ 9.25 (s, 1H), 8.14 (d, J = 7.8 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.36 (t, J = 7.5 Hz, 1H). 13C-NMR (101 MHz, CDCl3) δ 165.25 (C), 152.87 (CH), 134.12 (CH), 132.21 (CH), 132.01 (CH), 130.67 (C), 128.03 (CH), 122.71 (C).
4-(4-Cyano-1H-pyrazol-1-yl)-benzoic acid (
2u): [
20] Starting material
1u was prepared following the procedure of Smith et al. [
21]. The product was obtained via recrystallization from methanol. Purple powder, 70% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 13.19 (s, 1H), 9.46 (s, 1H), 8.43 (s, 1H), 8.10 (d,
J = 8.8 Hz, 2H), 8.00 (d,
J = 8.8 Hz, 2H).
13C-NMR (101 MHz, DMSO-
d6) δ 143.56 (CH), 138.89 (C), 134.24 (CH), 130.34 (CH), 118.24 (CH), 113.65 (C), 93.07 (C), 51.03 (CH). LC-MS (MeCN) R
t = 1.67 min [M − H]
– = 212.2. HRMS C
11H
6N
3O
2 calc. 212.0460, found 212.0456, (Δ = −1.9 ppm).
1-(4-Fluorophenyl)-1H-pyrazole-4-carbonitrile (
2v): [
22,
23] Starting material
1v was prepared following the procedure of Smith et al. [
21]. The product was obtained via recrystallization from ethanol and water. Brown crystals, 77% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 9.28 (s, 1H), 8.33 (s, 1H), 8.00–7.73 (m, 2H), 7.52–7.16 (m, 2H).
13C-NMR (101 MHz, DMSO-
d6) δ161.62 (C, d,
J = 245.8 Hz), 143.96 (CH), 135.47 (C, d,
J = 3.0 Hz), 134.75 (CH), 122.01 (CH, d,
J = 9.0 Hz), 116.93 (CH, d,
J = 23.8 Hz), 113.97 (C), 93.59 (C).
19F NMR (376 MHz, DMSO-
d6) δ −114.22. GC-MS (MeCN) R
t = 0.30 min M
+ = 187.1. HRMS C
10H
7N
3F calc. 188.0624, found 188.0615, (Δ = −4.8 ppm).
1-(3-Chloro-4-fluorophenyl)-1H-pyrazole-4-carbonitrile (
2w): Starting material
1w was prepared following the procedure of Smith et al. [
21]. The product was obtained via recrystallization ethanol and water. Brown solid, 69% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 9.35 (s, 1H), 8.39 (s, 1H), 8.13 (dt,
J = 6.3, 2.0 Hz, 1H), 7.98–7.76 (m, 1H), 7.64 (td,
J = 9.0, 1.2 Hz, 1H)).
13C-NMR (101 MHz, DMSO-
d6) δ 158.84 (C, d,
J = 247.1 Hz), 144.32 (CH), 135.93 (C, d,
J = 3.2 Hz), 135.26 (CH), 122.05 (CH), 121.09 (C, d,
J = 19.3 Hz), 120.58 (CH, d,
J = 8.0 Hz), 118.46 (CH, d,
J = 22.4 Hz), 113.83 (C), 93.92 (C).
19F NMR (376 MHz, DMSO-
d6) δ −117.41. ASAP-MS (MeCN) R
t = 0.30 min [M + H]
+ = 222.0. HRMS C
10H
6Cl
2FN
3 calc. 222.0234, found 222.0228, (Δ = −2.7 ppm).
5-Amino-1-(6-methyl-4-(trifluoromethyl)pyridin-2-yl)-1H-pyrazole-4-carbonitrile (
1x): Starting material
1x was prepared following the procedure of Smith et al. [
21].
1H-NMR (400 MHz, DMSO-
d6) δ 8.09 (s, 2H), 8.01–7.87 (m, 1H), 7.87–7.75 (m, 1H), 7.59 (s, 1H), 2.64 (s, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 159.72 (C), 153.73 (C), 153.58 (C), 143.73 (CH), 140.25 (C, q,
J = 33.9 Hz), 122.90 (C, q,
J = 273.5 Hz), 116.23 (CH, q,
J = 3.2 Hz), 114.65 (C), 106.40 (CH, q,
J = 3.2 Hz), 73.92 (C), 24.09 (CH3).
19F NMR (376 MHz, DMSO-
d6) δ 63.81. LC-MS (MeCN) R
t = 2.22 min [M + H]
+ = 269.2.
1-(6-Methyl-4-(trifluoromethyl)pyridin-2-yl)-1H-pyrazole-4-carbonitrile (2x): Recrystallised from ethanol and water as rose coloured needles in 76% yield. 1H-NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.47 (s, 1H), 7.95 (s, 1H), 7.78 (s, 1H), 2.65 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 160.98 (C), 150.29 (C), 145.27 (CH), 140.48 (C, q, J = 34.6 Hz), 134.47 (CH), 122.84 (C, q, J = 273.5 Hz), 119.01 (CH, q, J = 3.4 Hz), 113.62 (C), 106.15 (CH, q, J = 4.0 Hz), 94.81 (C), 24.30 (CH3). 19F NMR (376 MHz, DMSO-d6) δ −63.60. LC-MS (MeCN) Rt = 4.44 min M+ = 252.1. HRMS C11H8N4F3 calc. 253.0701, found 253.0702, (Δ = 0.4 ppm).
4-(4-Fluorophenyl)-6-phenylpyrimidin-2-amine (
1y): Starting material
1y was prepared following the procedure of Baxendale et al. [
24]. Recrystallised from ethanol and obtained as yellow crystals, 59% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 8.29–8.26 (m, 2H), 8.17–8.15 (m, 2H), 7.67–7.58 (m, 4H), 7.47–7.42 (m, 2H).
13C-NMR (101 MHz, DMSO-
d6) δ 165.81 (C), 164.50 (C, d,
J = 29.7 Hz), 163.32 (C), 157.53 (C), 133.17 (C), 132.22 (CH), 130.68 (CH, d,
J = 9.2 Hz), 130.24 (CH), 128.95 (CH), 127.98 (CH), 116.10 (CH, d,
J = 21.9 Hz), 100.81 (CH), 30.69 (CH).
19F NMR (376 MHz, DMSO-
d6) δ −107.72. ASAP-MS (MeCN) R
t = 0.36 min [M + H]
+ = 267.1. Product 4-(4-fluorophenyl)-6-phenylpyrimidine (
2y) was consistent with the literature data [
25]. LC-MS (MeCN) R
t = 1.96 min [M + H]
+ = 251.3. HRMS C
16H
12N
2F calc. 251.0985, found 251.0982, (Δ = −1.2 ppm).
1-Phenyl-6-(1-phenyl-1H-pyrazol-4-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (
2z): Starting material
1z was prepared following the procedure of Smith et al. [
21]. Eluent: hexane/ethyl acetate (4:1)
→(1:4). Orange solid, 30% yield.
1H-NMR (400 MHz, DMSO-
d6) δ
13C-NMR (101 MHz, DMSO-
d6) δ 158.24, 154.27, 148.34, 141.13, 139.40, 139.28, 134.19, 129.59, 129.32, 129.24, 128.41, 128.07, 126.76, 125.73, 125.35, 123.34, 120.28, 118.81, 100.03. LC-MS (MeCN) R
t = 2.76 min [M + H]
+ = 352.3. HRMS C
22H
19N
5 calc. 354.1470, found 354.1476, (Δ = 1.7 ppm).
1-Phenyl-1H-1,2,3-triazole-4-carbonitrile (
2aa): [
26] Starting material
1aa was prepared following the procedure of Smith et al. [
27]. The product was obtained by recrystallization from ethanol and water. Orange solid, 65% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 8.51 (s, 1H), 7.77–7.74 (m, 2H), 7.64–7.55 (m, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 135.74 (C), 130.21 (CH), 127.59 (CH), 121.96 (C), 120.99 (CH), 111.21 (C).
1-(p-Tolyl)-1H-1,2,3-triazole-4-carbonitrile (
2ab): [
28] Starting material
1ab was prepared following the procedure of Smith et al. [
27]. The product was obtained by recrystallization from ethanol and water. Brown solid, 71% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 9.69 (s, 1H), 7.80 (app. d,
J = 8.5 Hz, 2H), 7.45 (app. d,
J = 8.5 Hz, 2H), 2.40 (s, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 140.22 (C), 133.82 (C), 130.89 (C), 130.85 (CH), 121.22 (CH), 120.80 (C), 112.52 (C), 21.11 (CH
3). ASAP-MS (MeCN) R
t = 0.47 min [M + H]
+ = 185.1. HRMS C
10H
9N
4 calc. 185.0827, found 185.0830, (Δ = 1.6 ppm).
1-(4-Nitrophenyl)-1H-1,2,3-triazole-4-carbonitrile (
2ac): Starting material
1ac was prepared following the procedure of Smith et al. [
27]. The product was obtained by recrystallization from ethanol and water as a brown solid in 81% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 9.94 (s, 1H), 8.51 (d,
J = 8.7 Hz, 2H), 8.23 (d,
J = 8.7 Hz, 2H).
13C-NMR (101 MHz, DMSO-
d6) δ 148.03 (C), 140.27 (C), 131.81 (CH), 126.08 (CH), 122.21 (CH), 121.35 (C), 112.19 (C). ASAP-MS (MeCN) R
t = 0.75 min [M + H]
+ = 216.1. HRMS C
9H
6N
5O
2 calc. 216.0521, found 216.0521, (Δ = 0.0 ppm).
2-(2-Pyridinyl)-quinoline (
2ad): [
29] Starting material
1z was prepared following the procedure of Smith et al. [
30]. The product was obtained by elution with hexane/ethyl acetate (100:1)
→(10:1) as a white solid in 70% yield.
1H-NMR (400 MHz, CDCl
3) δ 8.75 (ddd,
J = 4.8, 1.8, 1.0 Hz, 1H), 8.68 (dt,
J = 8.0, 1.1 Hz, 1H), 8.58 (d,
J = 8.6 Hz, 1H), 8.29 (dd,
J = 8.6, 0.9 Hz, 1H), 8.21 (dq,
J = 8.5, 0.9 Hz, 1H), 7.95–7.81 (m, 2H), 7.76 (ddd,
J = 8.4, 6.9, 1.5 Hz, 1H), 7.57 (ddd,
J = 8.1, 6.9, 1.2 Hz, 1H), 7.37 (ddd,
J = 7.5, 4.8, 1.2 Hz, 1H).
13C-NMR (101 MHz, CDCl
3) δ 156.17 (C, d,
J = 16.0 Hz), 149.24 (CH), 147.96 (C), 137.13 (CH, d,
J = 12.2 Hz), 129.72 (CH, d,
J = 15.5 Hz), 128.37 (C), 127.74 (CH), 126.91 (CH), 124.18 (CH), 122.02 (CH), 119.09 (CH). ASAP-MS (MeCN) R
t = 0.59 min [M + H]
+ = 207.1.
(2-Aminothiophen-3-yl)(phenyl)methanone (
1ae): Starting material
1ae was prepared following the procedure of Mallia et al. [
31] as tan needles in 99% yield by column chromatography using hexane/ethyl acetate (1:1).
1H-NMR (400 MHz, DMSO-
d6) δ 8.37 (s, 2H), 7.64–7.55 (m, 2H), 7.55–7.41 (m, 3H), 6.74 (d,
J = 5.9 Hz, 1H), 6.27 (d,
J = 5.9 Hz, 1H).
13C-NMR (101 MHz, DMSO-
d6) δ 189.62 (C), 167.83 (C), 141.38 (C), 130.92 (CH), 128.68 (CH), 128.14 (CH), 127.06 (CH), 113.37 (C), 106.66 (CH). LC-MS (MeCN) R
t = 2.20 min [M + H]
+ = 204.2. HRMS C
11H
10NOS calc. 204.0483, found 204.0484, (Δ = 0.5 ppm).
Phenyl(thiophen-3-yl)methanone (
2ae): [
32] Eluent: hexane/ethyl acetate (1:1) as a tan coloured oil in 66% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 8.21 (dd,
J = 2.8, 1.3 Hz, 1H), 7.83–7.77 (m, 2H), 7.71 (dd,
J = 5.0, 2.8 Hz, 1H), 7.69–7.62 (m, 1H), 7.59–7.50 (m, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 189.57 (C), 140.87 (C), 138.56 (C), 135.73 (CH), 132.91 (CH), 129.50 (CH), 129.05 (CH), 128.50 (CH), 128.05 (CH). LC-MS (MeCN) R
t = 2.41 min [M + H]
+ = 189.2. HRMS C
11H
9OS calc. 189.0374, found 189.0377, (Δ = 1.6 ppm).
(2-Aminothiophen-3-yl)(4-methoxyphenyl)methanone (
1af): Starting material
1xx was prepared following the procedure of Mallia et al. [
31] as tan needles in 99% yield by column chromatography using hexane/ethyl acetate (1:1).
1H-NMR (400 MHz, DMSO-
d6) δ 8.25 (s, 2H), 7.60 (d,
J = 8.25 Hz, 2H), 7.02 (d,
J = 8.25 Hz, 2H), 6.81 (d,
J = 5.9 Hz, 1H), 6.27 (d,
J = 5.9 Hz, 1H), 3.82 (s, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 188.72 (C), 167.35 (C), 161.61 (C), 133.73 (C), 130.38 (CH), 127.16 (CH), 113.93 (CH), 113.40 (C), 106.43 (CH), 55.78 (CH
3). LC-MS (MeCN) R
t = 2.20 min [M + H]
+ = 234.2. HRMS C
12H
12NO
2S calc. 234.0589, found 234.0594, (Δ = 2.1 ppm).
(4-Methoxyphenyl)(thiophen-3-yl)methanone (
2af): [
33] Eluent: hexane/ethyl acetate (4:1). Yellow crystals, 69% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 8.18 (dd,
J = 2.8, 1.3 Hz, 1H), 7.86–7.77 (m, 2H), 7.70 (dd,
J = 5.1, 2.8 Hz, 1H), 7.50 (dd,
J = 5.1, 1.3 Hz, 1H), 7.14–7.02 (m, 2H), 3.86 (s, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 188.26 (C), 163.27 (C), 141.15 (C), 134.32 (CH), 132.01 (CH), 130.92 (C), 128.63 (CH), 127.74 (CH), 114.35 (CH), 55.99 (CH
3). LC-MS (MeCN) R
t = 2.41 min [M + H]
+ = 219.4 HRMS C
12H
10O
2S calc. 219.0480, found 219.0486, (Δ = 2.7 ppm).
4-(3-chlorophenyl)thiazol-2-amine (
1ag): Prepared following the procedure of Potopnyk et al. [
34]. Recrystallised from acetone to yield a white solid, 90% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 7.85 (t,
J = 2.0 Hz, 1H), 7.72 (dt,
J = 7.3, 1.7 Hz, 1H), 7.56–7.44 (m, 2H), 7.40 (s, 1H).
13C-NMR (101 MHz, DMSO-
d6) δ 170.49 (C), 139.07 (C), 134.19 (C), 131.74 (CH), 131.33 (C), 129.39 (CH), 125.98 (CH), 124.94 (C), 104.93 (CH). LC-MS (MeCN) R
t = 1.25 min [M + H]
+ = 211.0. HRMS C
9H
7ClN
2S calc. 211.0097, found 211.0111, (Δ = 6.6 ppm).
4-(3-chlorophenyl)thiazole (2ag): Isolated as an inseparable mixture of the product and a secondary species in a ratio of 1:0.33 (2ag:by-product) equating to 45% of the desired product.
4-(3-bromophenyl)thiazol-2-amine (
1ah): Prepared following the procedure of Potopnyk et al. [
34]. Recrystallised from acetone to yield a white solid. White crystals, 88% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 9.04 (s, 2H), 7.97 (t,
J = 1.8 Hz, 1H), 7.76 (dt,
J = 7.7, 1.2 Hz, 1H), 7.61 (dt,
J = 7.7, 1.8 Hz, 1H), 7.52–7.29 (m, 2H).
13C-NMR (101 MHz, DMSO-
d6) δ 170.53 (C), 138.41 (C), 132.38 (CH), 131.65 (C), 131.57 (CH), 128.75 (CH), 125.31 (CH), 122.74 (C), 105.01 (CH). LC-MS (MeCN) R
t = 2.06 min [M + H]
+ = 257.4. HRMS C
9H
879BrN
2S calc. 254.9592, found 254.9603, (Δ = 4.3 ppm, mDa 1.1).
4-(3-Bromophenyl)thiazole (2ah): Isolated in 51% using Hexane/EtOAc 1:1. 1H-NMR (400 MHz, CDCl3) δ 8.91 (d, J = 2.0 Hz, 1H), 8.13 (t, J = 1.8 Hz, 1H), 7.88 (ddd, J = 8.0, 1.7, 1.0 Hz, 1H), 7.59 (d, J = 2.0 Hz, 1H), 7.50 (ddd, J = 8.0, 2.0, 1.1 Hz, 1H), 7.35–7.30 (m, 1H).LC-MS Rt 2.64 found 240.1; HRMS found 239.9501 for C9H7NS79Br calc. 239.9483 (7.5 ppm, mDa 1.8).
4-(4-Trifluorophenyl)thiazol-2-amine (
1ai): Prepared following the procedure of Potopnyk et al. [
34]/ Recrystallised from acetone to yield a white solid, 78% yield
1H-NMR (400 MHz, DMSO-
d6) δ 7.99 (m, 2H), 7.82 (m, 2H), 7.46 (s, 1H).
13C-NMR (101 MHz, DMSO-
d6) δ 170.61 (C), 139.09 (C), 133.46 (C), 129.46 (C, q,
J = 32.3 Hz), 126.95 (CH), 126.33 (CH, q,
J = 3.8 Hz), 124.48 (C, q,
J = 272.4 Hz), 106.10 (CH).
19F NMR (376 MHz, DMSO) δ −61.26.
4-(4-Trifluorophenyl)thiazole (2ai): Isolated in 43% using Hexane/EtOAc 1:1. 1H-NMR (400 MHz, CDCl3) δ 8.94 (d, J = 2.0 Hz, 1H), 8.07 (app. dp, J = 7.7, 0.9 Hz, 2H), 7.77–7.62 (m, 3H). 13C-NMR (101 MHz, CDCl3) δ 154.90 (C), 153.33 (CH), 137.38 (C), 130.08 (C, q, J = 31.8 Hz), 128.80 (C), 126.65 (CH), 125.83 (CH, q, J = 3.8 Hz), 124.13 (C, q, J = 273 Hz), 114.44 (CH). LC-MS Rt 2.71 found 230.1 [M + H] 271.1 [MH + MeCN], HRMS found 230.0253 for C10H7NSF3 calc. 230.0251 (0.9 ppm).
5-Amino-1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carbonitrile (
1aj): Prepared following the procedure of Brand et al. [
35] in 77% yield following recrystallization from ethanol as a red solid.
1H-NMR (400 MHz, DMSO-
d6) δ 7.51 (m, 1H), 7.26 (s, 2H), 7.21–7.10 (m, 2H), 5.40 (d,
J = 1.1 Hz, 2H).
13C-NMR (101 MHz, DMSO-
d6) δ 161.17 (C, dd,
J = 249.3, 8.2 Hz), 148.59 (C), 132.04 (CH, t,
J = 10.7 Hz), 114.02 (C), 112.31 (CH, app. dd,
J = 24.9, 5.8 Hz), 101.34 (C), 37.89 (CH
2).
19F NMR (376 MHz, DMSO-
d6) δ −114.54. ASAP-MS (MeCN) R
t = 0.44 min [M + H]
+ = 236.1. HRMS C
10H
8N
5F
2 calc. 236.0748, found 236.0755, (Δ = 3.0 ppm).
1-(2,6-Difluorobenzyl)-1H-1,2,3-triazole-4-carbonitrile (
2aj): [
36] Recrystallised from ethanol and water as a red solid in 73% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 9.18 (s, 1H), 7.54 (tt,
J = 8.5, 6.7 Hz, 1H), 7.33–7.07 (m, 2H), 6.02 – 5.52 (m, 2H).
13C-NMR (101 MHz, DMSO-
d6) δ 162.1.2 (C, dd,
J = 249.5, 6.7 Hz), 132.94, (CH), 132.62 (CH, t,
J = 10.6 Hz), 120.12 (C), 112.51 (C), 112.46 (CH, m), 110.80 (C, t,
J = 19.1 Hz), 42.33 (CH
2).
19F NMR (376 MHz, DMSO-
d6) δ −114.42. LC-MS (MeCN) R
t = 1.93 min [M + H]
+ = 221.1. HRMS C
10H
7N
4F
2 calc. 221.0639, found 221.0643, (Δ = 1.8 ppm).
Methyl 5-amino-1-(phenylmethyl)-1H-1,2,3-triazole-4-carboxylate (1ak): Eluent: hexane/ethyl acetate (25: 1). Prepared from the following procedure: benzyl bromide (20 mmol) and sodium azide (30 mmol) in acetone (5 mL) and water (2 mL) were stirred with sodium hydroxide (30 mmol) at ambient temperature for 24 h. The resulting mixture was concentrated under reduced pressure and then ethyl acetate (10 mL) and water (10 mL) were added. Following phase separation, the organic phase was concentrated under reduce pressure to give benzyl azide. The benzyl azide and cyanoacetic acid (20 mmol) was dissolved in dimethyl formamide (5 mL) and water (2 mL) and heated at 120 °C for 48 h. To the resultant mixture was added ethyl acetate (10 mL) and water (10 mL). Following phase-separation, the organic phase was concentrated under reduced pressure, to give a brown oil, which was twice purified by column chromatography to give the desired product as a pale powder in 8% yield. 1H-NMR (400 MHz, CDCl3) δ 7.49 (m, 2H), 7.16 (m, 3H), 4.56 (s, 2H), 3.84 (s, 3H). LC-MS (MeOH) Rt = 0.56 min [M + H]+ = 232.2.
Ethyl oxazole-4-carboxylate (2am): 1H-NMR (400 MHz, CDCl3) δ 8.27 (d, J = 1.1 Hz, 1H), 7.94 (d, J = 1.1 Hz, 1H), 4.39 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, CDCl3) δ 160.95 (C), 151.40 (CH), 144.01 (CH), 133.33 (C), 61.31 (CH2), 14.24 (CH3). EI GC polar compounds (MeCN) Rt = 3.20 min M+ = 141.1. HRMS C6H8NO3 calc. 142.050419, found 142.050416 (Δ = 1.7 ppm).
1-Chloro-4-phenoxybenzene (2al): 1H-NMR (400 MHz, CDCl3) δ 7.46–7.35 (m, 1H), 7.27–7.17 (m, 1H), 7.10–6.98 (m, 1H). 13C-NMR (101 MHz, CDCl3) δ 155.04, 146.68, 130.32, 128.47, 126.89, 125.14, 119.40, 118.17. LC-MS (MeOH) Rt = 1.21 min [M + H]+ = 205.0.
6-methyl-[1,2,4]triazolo[1,5-a]pyridin-2-amine (
1an) was prepared according to the general procedure of Verček et al. [
37].
N-Ethoxycarbonyl-
N’-(5-methyl-2-pyridinyl)thiourea:
1H-NMR (400 MHz, DMSO-
d6) δ 12.11 (s, 1H), 11.43 (s, 1H), 8.57 (s, 1H), 8.32–8.02 (m, 1H), 7.69 (ddd,
J = 8.5, 2.4, 0.8 Hz, 1H), 4.22 (q,
J = 7.1 Hz, 2H), 2.28 (s, 3H), 1.26 (t,
J = 7.1 Hz, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 177.57 (C), 153.97, 149.57, 148.54, 138.76, 130.85, 115.48, 62.63 (CH
2), 17.81 (CH
3), 14.57 (CH
3). ESI-LC MeCN (TQD) R
t = 2.20 min, M
+ = 241.3 HRMS C
10H
14N
3O
2S calc. 240.0807, found 240.0807 (Δ = 2.9 ppm).
6-Methyl-[1,2,4]triazolo[1,5-a]pyridin-2-amine (
1an):
1H-NMR (400 MHz, DMSO-
d6) δ 8.37 (s, 1H), 7.25 (d,
J = 1.3 Hz, 2H), 5.90 (s, 2H), 2.25 (d,
J = 1.1 Hz, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 166.32 (C), 149.46 (C), 131.57 (CH), 126.16 (CH), 121.08 (C), 112.25 (CH), 17.60 (CH
3). ESI-LC MeCN (TQD) R
t = 0.70 min, M
+ = 149.6. HRMS C
7H
9N
4 calc. 149.0827, found 149.0830 (Δ = 2.0 ppm).
2,6-Diethyl-5-methylpyrimidin-4-aminium acetate (
1aq): Starting material synthesized following the procedure Baxendale et al. [
38]. Salt formed by addition of 1 equivalent of acetic acid and the material recrystallized from ethanol as white crystals in 60% yield.
1H-NMR (400 MHz, DMSO-
d6) δ 13.37 (s, 1H), 8.79 (s, 1H), 8.19 (s, 1H), 2.72 (m,
J = 7.6 Hz, 4H), 2.03 (s, 3H), 1.24 (t,
J = 7.6 Hz, 3H), 1.16 (t,
J = 7.6 Hz, 3H).
13C-NMR (101 MHz, DMSO-
d6) δ 165.06 (C), 163.58 (C), 155.76 (C), 108.63 (C), 27.83 (CH
2), 23.79 (CH
2), 13.00 (CH
3), 11.69 (CH
3), 10.85 (CH
3). ASAP-MS (MeCN) R
t = 0.34 min M
+ = 166.1. HRMS C
9H
16N
3 calc. 166.1344, found 166.1344, (Δ = 0.0 ppm).