Abstract
Lignin is an important biological polymer in plants that is necessary for plant secondary cell wall ontogenesis. The laccase (LAC) gene family catalyzes lignification and has been suggested to play a vital role in the plant kingdom. In this study, we identified 45 LAC genes from the Brassica napus genome (BnLACs), 25 LAC genes from the Brassica rapa genome (BrLACs) and 8 LAC genes from the Brassica oleracea genome (BoLACs). These LAC genes could be divided into five groups in a cladogram and members in same group had similar structures and conserved motifs. All BnLACs contained hormone- and stress- related elements determined by cis-element analysis. The expression of BnLACs was relatively higher in the root, seed coat and stem than in other tissues. Furthermore, BnLAC4 and its predicted downstream genes showed earlier expression in the silique pericarps of short silique lines than long silique lines. Three miRNAs (miR397a, miR397b and miR6034) target 11 BnLACs were also predicted. The expression changes of BnLACs under series of stresses were further investigated by RNA sequencing (RNA-seq) and quantitative real-time polymerase chain reaction (qRT-PCR). The study will give a deeper understanding of the LAC gene family evolution and functions in B. napus.
1. Introduction
B. napus originated from either the Mediterranean or Northern Europe and was formed by chromosome doubling after an interspecific natural cross between B. rapa (AA, 2n = 20) and B. oleracea (CC, 2n = 18) [1]. Rapeseed oil was once considered as a bad food choice because the seeds contain erucic acid and cholesterol, but with breeding selection and industrial improvement, B. napus has nowadays become the third largest source of vegetable oil. Unfortunately, B. napus is susceptible to various biotic and abiotic stresses, such as drought, heat, low temperature and fungi infection.
Lignin widely existed and composed of three monomers: coniferyl (G), sinapyl (S), and p-coumaryl (H) alcohols. Lignin in plants is involved in the formation of cell walls and together with cellulose increases cellular hardness. Studies have proved that lignin is related to drought stress [2] and a high content can improve the resistance to lodging and Sclerotinia sclerotiorum (S. sclerotiorum) [3,4]. Laccases are widely distributed with obvious functional differences in plants and fungi [5,6]. It can degrade lignin in Pleurotus ostreatus [7] and is expressed in lignifying cells in many plant species [8,9]. LACs are also named multicopper enzymes and supposed to catalyze lignin formation by polymerizing monolignols in plants [10].
To date, LACs have been characterized in many species. Lacquer tree contains LAC in the resin ducts and secreted resin [11]. In cotton, an ex-planta phytoremediation system was built based on the overexpression of LACs [12]. Forty-four, 46 and 84 LACs were identified from Gossypium arboretum (G. arboretum), Gossypium raimondii (G. raimondii) and Gossypium hirsutum (G. hirsutum), respectively [13]. A total of 27 laccase candidates (SbLAC1-SbLAC27) were identified in Sorghum bicolor [14]. LACs are continually being detected in other species. An acidic LAC gene was found through cDNA cloning in sycamore maple and tobacco [15,16]. Five different LAC-encoding cDNA sequences were identified from ryegrass, with four from the stem and one from the meristematic tissue [17]. Acer pseudoplatanus has been found to produce and excrete LAC under cell culture [18,19]. In Populus euramericana, five distinct LACs were found in xylem tissue [20].
Many studies have indicated the relationship between LAC and lignification. In loblolly pine, LAC was purified from different xylem and shown to coincide with lignin formation in time and place. In A. thaliana, gene structure and molecular analysis of the laccase-like multicopper oxidase (LMCO) gene family noted that LAC genes (AtLACs), AtLAC4, AtLAC7, AtLAC8 and AtLAC15 were mainly expressed in the seed coat, root, pollen grains and cell walls, respectively, and all these tissues present high lignification [21,22]. In maize (Zea mays), the ZmLAC2, ZmLAC3, ZmLAC4, and ZmLAC5 coincided with the tissues undergoing lignification [23]. Northern blot analysis indicated that five LACs (LAC1, LAC2, LAC3, LAC90 and LAC110) in poplar were highly expressed in stems, although their sequences vary greatly [20]. SofLAC was reported as a new LAC gene and proven to participate in lignification in sugarcane [24]. In B. napus and Brachypodium distachyon LAC has been shown to affect the accumulation of lignin [25,26]. In addition to oxidative lignin polymerization, LAC can also protect plants from biotic stresses and abiotic stresses such as the toxic phytoalexins and tannins in the host environment. In another study about maize, ZmLAC3 was induced by wound, whereas ZmLAC2 and ZmLAC5 were repressed and ZmLAC4 gene expression was unaffected [23]. The OsChI1 gene encodes a putative LAC precursor protein in rice (Oryza sativa), overexpression of OsChI1 in A. thaliana improved plants drought and salt tolerance [27]. Compared with the numerous reports about LAC gene in other species, few reports are available for B. napus especially at the genome level and the influence of stress on BnLACs [28,29].
In our study, we identified LAC gene family in B. napus and characterized them by gene structure, motif and cis-element analysis. The expression patterns of all BnLACs were identified by RNA -seq and some of them were analyzed under different stresses by RNA-seq or qRT-PCR. The research not only uncovers the evolutionary relationship of LAC gene family but also provides information about LAC respond to biotic and abiotic stresses.
2. Results
2.1. Characterization of the 45 BnLACs
Basic Local Alignment Search Tool Protein (BLASTp) was performed and confirmed 45 BnLACs in the B. napus genome by using 17 AtLACs protein sequences as queries (Table 1). Except for AtLAC2, AtLAC8 and AtLAC16, the remaining AtLACs had more than one homologous gene in the B. napu genome. AtLAC3, AtLAC4, AtLAC5, AtLAC11, AtLAC12 and AtLAC17 had four homologs genes were the most. The genomic sequences lengths of BnLACs had a wide range from 1937 (BnLAC13-1) to 7114 bp (BnLAC5-4). The average MW is 63.02 kDa. The pI values of these proteins varied from 6.10 (BnLAC9-1) to 9.74 (BnLAC14-2). Subcellular localization predicted results showed all the 45 proteins are located in secretory except for BnLAC11-4, which is predicted in mitochondrion. Thirty-nine BnLACs are accurately, unevenly mapped on the 12 B. napus chromosomes and no tandem duplication. The remaining six BnLACs are located on the unmapped scaffolds in the Ann_random and Cnn_ random genome.

Table 1.
Characterization of BnLACs identified in B. napus genome.
Chromosome C04 has the most LACs (eight) and A06, C08 only have one LAC gene (Figure 1). To further infer the phylogenetic mechanisms of BnLACs, a comparative syntenic map of B. napus associated with A. thaliana was constructed. Thirteen BnLACs show syntenic relationship with those in A. thaliana and focus on chromosome 02 and chromosome 05 (Figure 2).
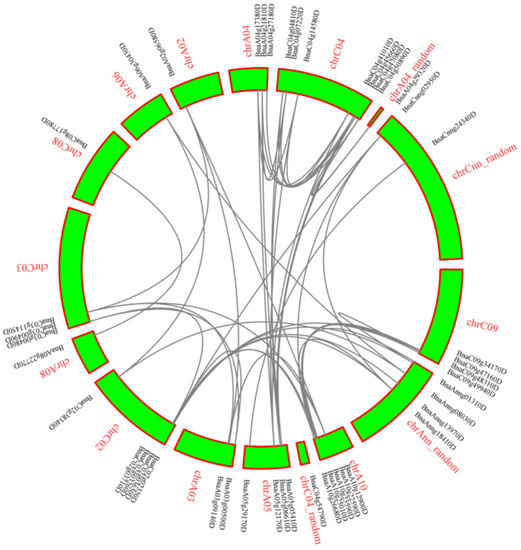
Figure 1.
Interchromosomal relationships of BnLACs and gray lines indicate duplicated LAC gene pairs. The chromosome number and gene ID are indicated with red and black, respectively. The figure was generated by MCScanX with the default parameters [30].
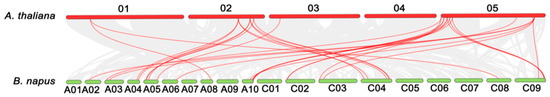
Figure 2.
Synteny analysis of LACs between A. thaliana and B. napus. Gray lines in the background indicate all the collinear blocks, red lines highlight the syntenic LAC gene pairs. The figure was constructed by TBtools 0.66444553 (https://github.com/CJ-Chen/TBtools).
2.2. Phylogenetic Analysis of LACs in A. thaliana, B. napus, B. rapa and B. oleracea
To study the evolutionary relationship of LACs in A. thaliana, B. napus, B. rapa and B. oleracea, a cladogram containing 45 BnLACs, 25 BrLACs, eight BoLACs and 17 AtLACs was constructed and divided into five groups with well-supported bootstrap values (Figure 3). Groups I, II, III, IV and V had 35, 26, 5, 15 and 14 members, respectively. Forty-five BnLACs were unevenly divided into five groups, the most being in Group I which contained 16 BnLACs the least being in Group III that only contained one. According to the bootstrap value in the tree, genes in same group were closely related but in different groups were far apart. Genes divided into the same group are thought to have similar functions and number of LACs in B. oleracea was far less than in B. rapa and B. napus, however, every group contained at least one BoLAC, which is essential to keep complete gene function of LAC in B. oleracea.
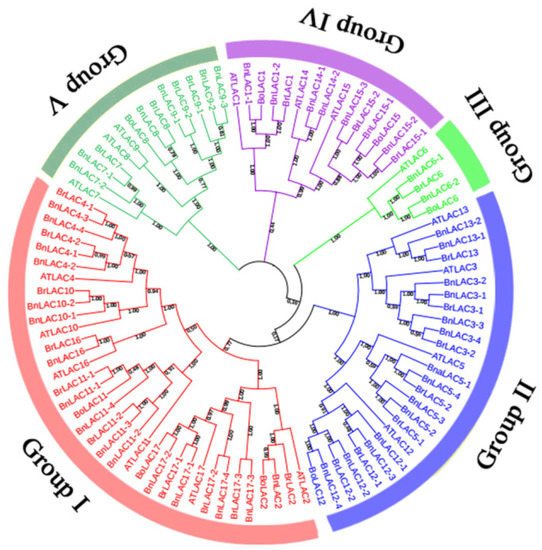
Figure 3.
The cladogram of LAC proteins from A. thaliana (17), B. napus (45), B. rapa (25) and B. oleracea (8). The cladogram was constructed by the MEGA 7.0 software [31] using the neighbour-joining option with 1000 bootstrap replicates and pairwise deletion. Distinct colour segment represents different groups, bootstrap value are shown near nodes.
2.3. Gene Structure and Conservative Domain Analysis of BnLACs and AtLACs
As shown in the cladogram, members in the same group had highly similar gene structures, and the number of exons in the 45 LACs ranged from 4 to 7 (Figure 4). Compared with introns, exons were more stable in length. For example, BnLAC5-4 had a separate longer intron but other members in the Group II didn’t contain one. BnLACs homologous with AtLAC4, AtLAC7, AtLAC8 and AtLAC9 showed diversity with other BnLACs because of one or two long introns existed. For exploring more characteristics about LACs, introns number of LACs from A. thaliana, S. bicolor, G. arboretum, G. raimondii and G. hirsutum were compared. In A. thaliana, there was no LAC gene had more six introns. Most LACs in S. bicolor have one to three introns less than other species with five introns (Figure 5).
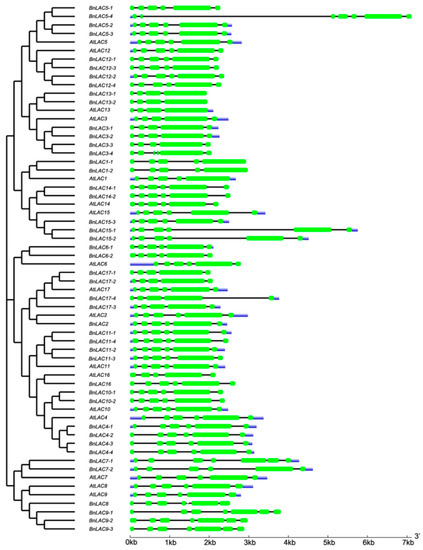
Figure 4.
Exon-intron structures of BnLACs and AtLACs with a cladogram. The result generated by GSDS 2.0 (http://gsds.cbi.pku.edu.cn/). The green boxes, black lines and blue box indicate exons, introns, untranslated region, respectively.
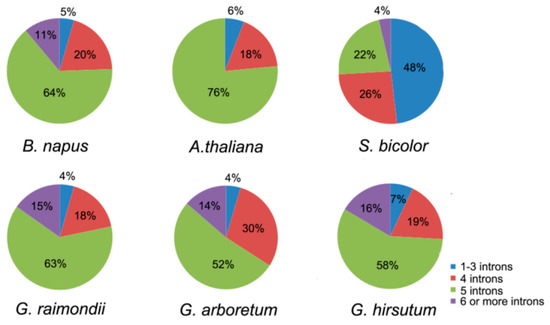
Figure 5.
Number of introns in A. thaliana, S. bicolor, G. arboretum, G. raimondii and G. hirsutum. The figure was constructed by GraphPad Prism 6 (Graphpad Software Inc., La Jolla, CA, USA, www.graphpad.com).
Full-length protein sequences of 17 AtLACs and 45 BnLACs were analyzed to identify their conserved motifs and further understand their functions (Figure 6). The length of the 20 motifs ranged from 6 to 50 amino acids and motif sequences are provided in Table S1. AtLAC4, BnLAC8 had the fewest motifs (eight), BnLAC11-4 had the most motifs (twenty). In contrast to others LACs, AtLAC2 and BnLAC11-4 had an extra motif 18, BnLAC3-3; BnLAC3-4 and BnLAC7-2 lose the motif 2. LACs divided into the same groups also had different motifs, BnLAC5-4 and AtLAC16 lost the motif 6 compared with other genes in their corresponding group. AtLAC8, AtLAC9 and their homologous gene in B. napus genome lacked the motif 5 when compared with others in Group V. Motif 1 and motif 3 are highly conserved and can be found in all the protein sequences of 62 LACs.
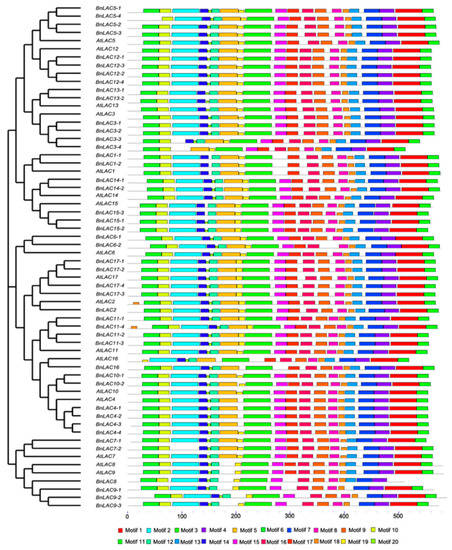
Figure 6.
Conserved motif analysis of BnLACs and AtLACs proteins presented with a cladogram. Conserved motifs were generated by MEME 5.0.4 (http://meme-suite.org/tools/meme) and boxes of different colors represent motifs 1-20. Motif sequences are provided in Table S1.
2.4. Diverse cis Regulatory Elements and miRNAs are Predicted
To investigate what condition could influence BnLACs expression, 1500 bp upstream of the initiation codons were analysed for cis-elements. Eleven kinds of elements including light responsive, six hormone-related, four stress-related elements were searched (Table S2). All the BnLACs are light and hormone responsive. Twenty-six BnLACs have a gibberellin element, 25 BnLACs have an ethylene element, which are the two most elements. Seven members contained a wound element and thirty BnLACs were influenced under drought stress. Fourteen members had low temperature elements and 34 members may have been influenced by heat stress.
Eleven BnLACs were predicted to have their expression regulated by miRNAs (Table S3, expectation number under 3.0 were selected). All the predicted BnLACs were regulated by miR397a and miR397b; BnLAC4-1 and BnLAC4-2 were also regulated by miR6034. As the expectation number showed, BnLAC17-3 was the most likely targeted gene by miR397a and miR397b.
2.5. Expression Pattern Analysis of BnLACs
To investigate the expression patterns of 45 BnLACs, a heatmap was built based on the RNA-seq (BioProject ID PRJNA358784) using 32 different tissues and stages of B. napus as samples (Table S4, Figure 7). Results suggested that BnLACs have different expression patterns across tissues and stages. Strong expression occurred in highly lignified tissues such as roots and stems. BnLAC15-1 and BnLAC15-2 had the highest expression in the seeds and seed coats. BnLAC4-1, BnLAC4-2, BnLAC4-3 and BnALC4-4 had high expression in silique pericarps. BnLAC5-2, BnLAC5-3, BnLAC15-1, BnLAC15-2 and BnLAC15-3 highly expressed in seed coats. No BnLACs highly expressed in leaves. BnLAC9-1 and BnLAC14-2 rarely expressed in any tissue and stage. These results showed BnLACs functioned differently and some members are redundant.
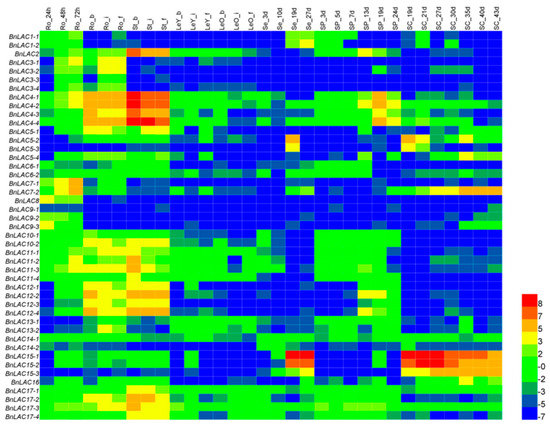
Figure 7.
RNA-seq of BnLACs in B. napus 32 different tissues and stages. Ro, root; St, stem; LeY, young leaf; LeO, old leaf; Se, seed; SP, silique pericarp; SC, seed coat; s, seedling stage; b, bud stage; i, initial flowering stage; and, f, full-bloom stage. The 24, 48, and 72 h means the time that had passed after seed germination. The 3, 10, 27 d and other indicate the number of DAF. The bar on the lower right corner represents the Fragments Per Kilobase of Transcript Per Million Fragments Mapped (FPKM) values and different colors represent different expression levels. Heat map was generated by HemI 1.0 software (http://hemi.biocuckoo.org/faq.php).
2.6. Responses of BnLACs upon Abiotic Stress
As many studies described lignification can response to stresses, RNA-seq and qRT-RCR were used to analyze the expression patterns of several BnLACs under different stresses. RNA-seq showed that under Cd2+ stress, BnLAC14-2, BnLAC15-1 and BnLAC15-2 were up-regulated, BnLAC12-1 and BnLAC12-4 were down-regulated at 24 hours after stress then up-regulated at 72 hours after stressed. BnLAC6-1, BnLAC9-1 and BnLAC15-3 had no change and other BnLACs were down-regulated at the analysed time points. Most BnLACs had low expression after 72 hours of Cd2+ stress and only 7 BnLACs (BnLAC4-1, BnLAC4-2, BnLAC7-2, BnLAC11-2, BnLAC11-3, BnLAC11-4 and BnLAC17-3) still highly expressed (Table S5, Figure 8a). Under NH4+ toxicity, Expression levels of BnLAC6-2, BnLAC13-1 and BnLAC13-2 were low and not influenced by NH4+ toxicity. BnLAC4-1, BnLAC10-2, BnLAC11-1 and BnLAC17-2 were up-regulated at 3 or 12 hours after stress and changed to normal level at 48 hours. Some members responsed NH4+ toxicity until 48 hours after stressed, BnLAC2, BnLAC4-2 and BnLAC14-2 were down-regulated and members such as BnLAC3-2, BnLAC3-3, BnLAC3-4, BnLAC5-2, BnLAC7-2, BnLAC12-2, BnLAC12-4 and BnLAC15-1 show significant upregulation (Table S5, Figure 8b). Under drought and wound stress, expression levels of 14 BnLACs in stems and leaves of three lines (ZS11, 7191 and D2) were analyzed by qRT-RCR.
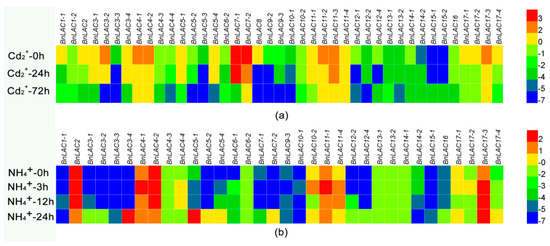
Figure 8.
RNA-seq of BnLACs in B. napus tissues in response to Cd2+ stress (a), NH4+ stress (b). The 0, 3, 12 and other time points in (a,b) denotes the time after Cd2+, NH4+ stress respectively. The bar on the lower right corner represents the FPKM values and different colors represent different expression levels. Heat map was generated by HemI 1.0 software (http://hemi.biocuckoo.org/faq.php).
High expression concentrated at stems, which were consistent with the RNA-seq analysis (Table S6). In leaves of three lines under drought stress, the 14 BnLACs had similar expression pattern (Figure 9). BnLAC2, BnLAC4-1, BnLAC4-2, BnLAC4-4, BnLAC11-3, BnLAC12-1, BnLAC12-2 and BnLAC17-3 have stable expression and close to zero. BnLAC6-1, BnLAC6-2, BnLAC11-2, BnLAC11-4, BnLAC14-1 and BnLAC17-1 have relatively high expression. BnLAC6-1, BnLAC11-2 and BnLAC11-4 were down-regulated, BnLAC6-2 and BnLAC17-1 were slightly up-regulated under drought stress.
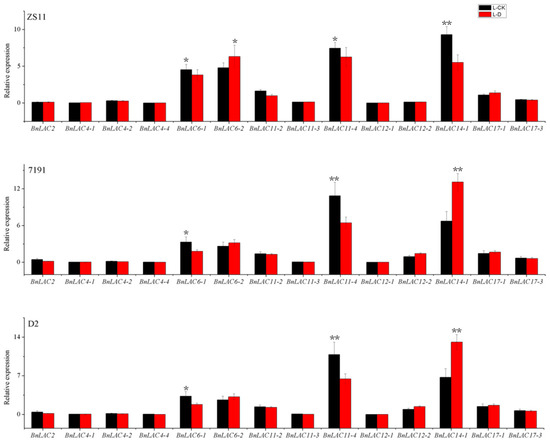
Figure 9.
Quantitative RT-PCR of 14 BnLACs in B. napus leaves in response to drought stress. Expression of Actin and UBC21 were used to normalize the expression level of each LAC gene. Bars represent means ± SEM of three biological replicates. Bars marked with asterisks indicate significant differences (Student’s t-test) to corresponding control samples for the same time point, *P < 0.05, **P < 0.01. The results were presented by OriginPro 8 (OriginLab Corporation, Northampton, MA, USA). L-CK and L-D means no drought treated and drought treated, respectively.
In wounded leaves of line ZS11, the expression of BnLAC2, BnLAC11-2, BnLAC11-3, BnLAC17-1 and BnLAC17-3 rose firstly then returned to normal levels. BnLAC6-1, BnLAC11-4, BnLAC12-2 and BnLAC14-1 had similar expression patterns and showed high expression at 0.5 and 3 hours after wounding. BnLAC4-1, BnLAC4-2, BnLAC4-4, BnLAC6-2 and BnLAC12-1 showed no expression in any samples. In wounded leaves of line 7191, BnLAC4-1, BnLAC4-2, BnLAC4-4, BnLAC11-4 and BnLAC12-1 were up-regulated followed by down-regulation and showed the highest expression at different time points. BnLAC4-1, BnLAC4-2, BnLAC4-4 and BnLAC12-1 showed the highest expression at 1.5 hours after wounding and BnLAC11-4 showed the highest expression at 0.5 hours after wounding. BnLAC6-2, BnLAC12-2 and BnLAC14-1 were down-regulated for all the analyzed time points and BnLAC11-3 had no change after wounding. In wounded leaves of line D2, BnLAC2, BnLAC6-2 BnLAC11-2, BnLAC12-2, BnLAC17-1 and BnLAC17-3 had high expression at two time points, 0.5 and 1.5 hours after wounding, respectively. BnLAC4-1, BnLAC4-2, BnLAC4-4, BnLAC11-3 and BnLAC12-1 nearly have no expression in the control sample and kept stable under wounding stress. Expression levels of BnLAC6-1, BnLAC11-4, and BnLAC14-1 responded to wounds slowly and showed high expression at 3 and 6 hours (Figure 10).
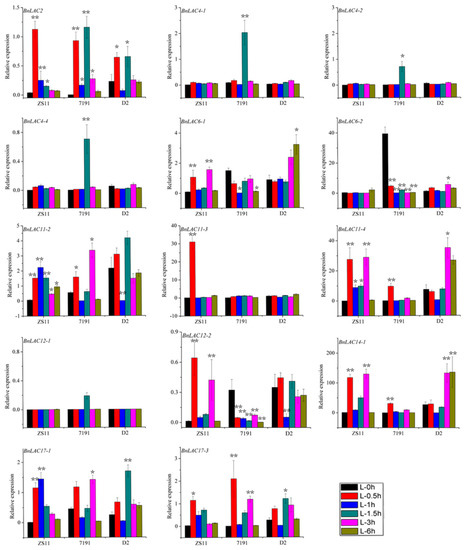
Figure 10.
Quantitative RT-PCR of 14 BnLACs in B. napus leaves in response to wounding stress. Expression of Actin and UBC21 were used to normalize the expression level of each LAC gene at each time point. Bars represent means ± SEM of three biological replicates. Bars marked with asterisks indicate significant differences (Student’s t-test) to corresponding control samples for the same time point, *P < 0.05, **P < 0.01. The results were presented by OriginPro 8. Different color means different time after wound shown as bar at lower right corner.
In wounded stems of line ZS11, BnLAC2, BnLAC4-1, BnLAC4-4, BnLAC11-3, BnLAC11-4 and BnLAC12-1 had the highest expression at 1.5 hours after wounding and changed to zero at 6 hours. BnLAC6-2, BnLAC11-2, BnLAC12-2, BnLAC17-1 and BnLAC17-3 were up-regulated followed by down-regulation. BnLAC6-1 and BnLAC14-1 were down-regulated all the time after wounding. In wounded stems of 7191 line, BnLAC4-1, BnLAC4-4, BnLAC11-2 and BnLAC11-3 were down-regulated followed by up-regulation and showed high expression at 6 hours after wounding. BnLAC12-1, BnLAC12-2 and BnLAC17-1 were down-regulated at all the analyzed time points. BnLAC6-1, BnLAC6-2, BnLAC11-4 and BnLAC14-1 showed similar expression patterns and had their highest expression at 6 hours after wounding. In wounded stems of D2 line, BnLAC2 and BnLAC4-4 had the highest expression at 0.5 hour after wounding. BnLAC4-1, BnLAC4-2, BnLAC11-3, BnLAC12-1, BnLAC17-1 and BnLAC17-3 showed high expression at 0.5 and 1 hours after wounding. Expression levels of BnLAC6-1, BnLAC12-2 and BnLAC14-1 almost no change and were close to zero for the analyzed time points. Both BnLAC11-2 and BnLAC11-4 had the highest expression at 1 hour and lowest expression at 1.5 hours after wounding (Figure 11).
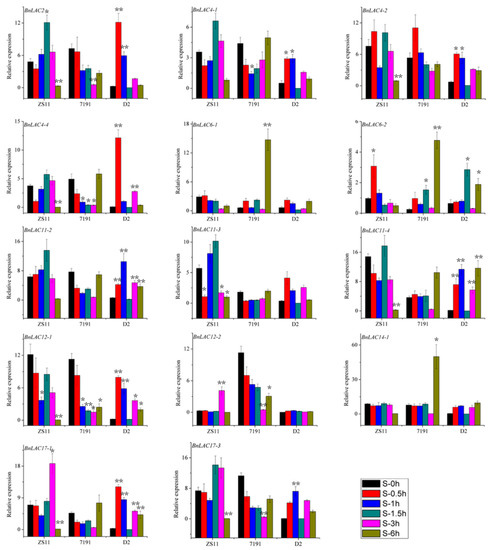
Figure 11.
Quantitative RT-PCR of 14 BnLACs in B. napus stems in response to wounding stress. Expression of Actin and UBC21 were used to normalize the expression level of each LAC gene at each time point. Bars represent means ± SEM of three biological replicates. Bars marked with asterisks indicate significant differences (Student’s t-test) to corresponding control samples for the same time point, *P < 0.05, **P < 0.01. The results were presented by OriginPro 8. Different color means different time after wound shown as bar at lower right corner.
2.7. BnLAC4 and its Predicted Downstream Genes are Differentially Expressed in the Silique Pericarp between Long and Short Silique Lines
In STRING platform, CTL2, IRX3, CESA4, IRX1, LAC17, GAUT12, IRX6, PGSIP1, GLP10 and FLA11 were predicted to interact with protein AtLAC4. A total 70 homologous genes were identified in the B. napus genome and expression levels of them in silique pericarps of long and short siliques lines were showed by a heatmap (Table S7, Figure 12). Most of the identified genes showed higher expression in silique pericarps of short silique lines on the 16th Days After Flower (DAF) and almost equal expression in two kinds of silique pericarps on the 25th DAF. On the contrary, many genes showed a higher expression in long silique lines on the 35th DAF. Those results showed BnLAC4 and its’ predicted downstream genes expressed earlier in silique pericarp of short siliques lines.
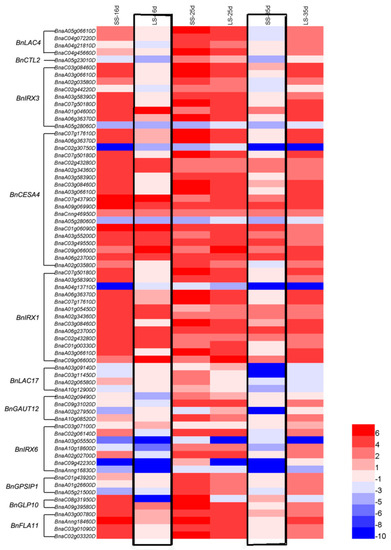
Figure 12.
RNA-seq of BnLAC4 BnCTL2, BnIRX3, BnCESA4, BnIRX1, BnLAC17, BnGAUT12, BnIRX6, BnPGSIP1, BnGLP10 and BnFLA11 genes in six kinds of B. napus silique pericarps. LS, long silique lines; SS, short silique lines. The 16, 25, and 35 d means DAF. The bar on the lower right corner represents the FPKM values and different colors represent different expression levels. Heat map was generated by HemI 1.0 software (http://hemi.biocuckoo.org/faq.php). Lower expression between long and short silique lines at 16th and 35th DAF were indicated by black box.
3. Discussion
B. napus is the third largest source of vegetable oil worldwide and plays an important role in national economies and food industries. The rapeseed yield decreases frequently because of lodging and other biotic and abiotic stresses. Many studies have shown that lignin aids in the resistance to fungi, stress and the LAC acts as an enzyme related to lignification in plants. Previous studies on the LAC gene family have been performed, but no related reports on the analysis of this family in B. napus exist until now. We identified and analyzed the LAC gene family with the aim of providing a reference at the genome level and deeper understanding of lignification.
3.1. Loss Events Occurred in the LAC gene family Along with the Evolution
Loss events occur frequently during evolution because of hybridization and chromosome doubling [32]. As a result of whole-genome triplication (WGT), the genes in A. thaliana should have three homologs in B. rapa and B. oleracea. In the study, only AtLAC1 had three orthologous genes in B. rapa and no AtLACs corresponding to three orthologous genes were found in B. oleracea. Some AtLACs even have no orthologous genes in B. rapa and B. oleracea like AtLAC14 in B. rapa, AtLAC3 and AtLAC4 in B. oleracea. Furthermore, B. napus formed by natural hybridization and polyploidization of B. rapa and B. oleracea, but no one AtLAC corresponding to six BnLACs was seen in this study and the most is four. Hence, the conclusion can be drawn that not only during the whole-genome triplication but also the formation of B. napus, gene loss events existed in LAC gene family universally.
Twenty four BoLACs were identified in B. oleracea, but only eight of them contained four essential conserved domains as described in the Materials and Methods section. Compared with B. rapa that contained 25 BrLACs, faster or broader gene loss happened in B. oleracea.
3.2. Regulation of BnLAC Genes
Cis-element analysis showed the expression of BnLACs was regulated at transcriptional level (Table S2). Research has shown LAC genes were also regulated at the post-tanscriptional level or through post-translational modifications [14]. G-box can be found in some BnLACs and related to light-response and salt tolerance in rice flag leaf by combinating with bZIP, bHLH, and NAC TFs [33,34,35]. The AtLAC4 gene has been confirmed to be up-regulated after MYB58 binds to AC elements [36]. Our results show diverse cis-elements in promoters of BnLACs, 11 kinds of promoters were selected for analysis including light responsive element, six hormone-related elements and four stress-related elements (Table S2). These findings revealed that BnLACs are also regulated by a series of factors. Related studies have reported that Ptr-miR397a is a negative regulator of LACs in Populus trichocarpa [37]. Several LACs are targeted by miR408, miR397, and miR857 in A. thaliana when Cu is absent [38]. Ptr-miR397a and Os-miR397 are involved in negative regulation of PtrLACs and OsLACs [38,39]. Seven SbLACs have also been predicted to be sbi-miRNA targets [14]. In the study, 11 BnLACs were predicted to be regulated by miR397a, miR397b and miR6034 (Table S3). All the 11 predicted BnLACs were regulated by miR397a and miR397b, and BnLAC4-1 and BnLAC4-2 were also regulated by miR6034. Research about miR6034 is very few and the process it participates in is not clear. Results have proved BnLAC17-3 was the most likely targeted gene by miR397a and miR397b in our study and in A. thaliana [40]. The findings of the study and combined with previous researches suggest LAC genes are truly regulated by miRNAs, and miRNA397 likely plays a very important role in the regulatory network.
3.3. Expression Patterns and Response to Stress
Abundant expression focuses on the roots, stems, and seed coats, whereas the expression in leaves, petals, pistils, and stamens are very low. The expression coincides with lignification in different parts of the plant. BnLAC13-1 and BnLAC13-2 showed different expression pattern though contain same cis-elements (Table S2, Figure 7). The reason would be a network containing other factors exists and regulates expression patterns of BnLACs such as miRNA and epigenetic modifications [41]. Some members, such as BnLAC9-1 and BnLAC14-1, were never highly expressed in any tissues or stages. It might be that their function was not required in biological processes or was only induced. by certain environmental factors, similar genes can also be found in cotton and Sorghum bicolor [13,14].
Many studies have illustrated that LACs are influenced by different kinds of stress and our study also proved that. OsChL1 as a putative laccase precursor, its expression was increased under drought stress and overexpressed the gene in A. thaliana can increase drought and salt tolerance [27]. In the study, six out of 14 BnLACs were influenced by drought stress. BnLAC6-2, BnLAC11-2 and BnLAC11-4 were up-regulated, BnLAC6-1 and BnLAC11-4 were down-regulated. BnLAC14-1 was up-regulated in line 7191 and D2 but down-regulated in line ZS11. Metal ions would influence the expression of LACs and miRNAs directly or indirectly. In Citrus, the expression of LAC7 was up-regulated by boron toxicity [42]. miR397 has been confirmed as a regulatory factor of LACs and its expression were influenced by Cd2+ [43]. In our study, most BnLACs were down-regulated after Cd2+ treatment and only four BnLACs showed upregulation. Another study in our lab has indicated NH4+ enrichment treatment would increase the lignin content in stem and root. Consistent with phenotype, the expression of most BnLACs were up-regulated at different time after NH4+ enrichment treatment by RNA-seq. According to the results of the promoter analysis, some members like BnLAC5-1, BnLAC6-1, BnLAC6-2 contain cis element about wound and a study has been reported LACs were influenced by wound [23]. qRT-RCR results showed the expression of the selected 14 BnLACs in leaves and sterms changed intricately after wounded. We also found gene in different lines responded to stress differently, BnLAC11-4 was up-regulated in lines 7191 and D2 but down-regulated in line ZS11. Further works are needed to find out the relation between wound healing and LACs.
3.4. BnLAC4 and its Downstream Genes May Participate in Silique Elongation in B. napus
Studies have reported that miR397 (both miR397a and b) regulate lignin content and yield traits in Rice and Populus trichocarpa via modulating LACs [39]. In A. thaliana, overexpression of miR397b-resistant AtLAC4 results in an increased silique length and decreased lignin content [40]. In our research, homologous genes of AtLAC4, AtCTL2, AtIRX3, AtCESA4, AtIRX1, AtLAC17, AtGAUT12, AtIRX6, AtPGSIP1, AtGLP10 and AtFLA11 in B. napus show earlier expression in silique pericarp of short silique lines than long silique lines. Some of those genes predicted like AtIRX1 and AtIRX6 are related to secondary cell wall biosynthesis. Combined with the reports, it could be that the period of BnLAC4 and its downstream genes expression may regulate silique length in B. napus.
4. Materials and Methods
4.1. Plant Materials and Stress Treatment
Inbred line Zhongshuang11 (ZS11), 7191, and D2 were sown in humus and grown to the four-leaf-stage. Half of the plants in each line were transplanted for drought stress and the remaining were left for the control. After 25 days without irrigation, the lines in the drought stress treatment showed a wilted phenotype and young leaves from control and stressed lines were frozen immediately in liquid nitrogen and stored at −80 °C.
Leaves and stems were wounded at the bolt stage. Leaves were wounded by a plastic comb-like brush, which was 8.5 cm long and had 42 spikes with a diameter of 1 mm that were equally arrange. Every leaf received three rows of wounds on each side of the midrib and parallel with it; the total number of punctures in each leaf was 252. Stems were wounded to a centimeter depth by scalpel blades. Samples were harvested around the cut at 0.5, 1, 1.5, 3, and 6 hours after wound [44]. The collected samples were frozen immediately in liquid nitrogen and stored at −80 °C for RNA isolation.
The seeds were grown at 22 °C with a light intensity of 200 mol/m2/s and a photoperiod of 16 hours for 7 days in hydroponic culture. Subsequently, the plants were collected after exposure to 1 mM Cd2+ (CdCl2) at 0, 24, and 72 h immediately frozen in liquid nitrogen for RNA sequencing.
Seeds of the B. napus line ZS11 were surface-sterilized with 1.2% sodium hypochlorite and germinated in a chamber room (16 hours light 15000Lx/8 hours dark at temperature 25 °C) with Hoagland solution. At four-leaf-stage, a portion of the seedlings were cultivated with modified Hoagland solution (0 mM NO3−, 10 mM NH4+, pH 6.0; other ions were not changed) for NH4+ toxicity treatment. After 3, 12 and 48 hours, the third and fourth true leaves were immediately frozen in liquid nitrogen and stored at −80 °C for RNA sequencing.
Long and short silique lines were selected from a recombinant inbred line (RIL) population constructed from a cross between GH06 (female parent) and P174 (male parent). Lines were planted in open field and grew under normal condition, silique pericarps of two kinds lines were collected respectively at 16th, 25th and 35th DAF and frozen in liquid nitrogen for RNA sequencing.
4.2. Characterisation of the LAC Gene Family
To date, 17 LACs have been reported in A. thaliana [35]. BLASTp was performed in the B. napus, B. rapa and B. oleracea genome using the AtLACs protein sequences as queries and sequences with E-value less than 1 × 10-20 were selected [45]. Some repeated sequences were manually deleted according to the E-value. All the remaining genes were checked by InterProScan (http://www.ebi.ac.uk/interpro) [46], and the sequences with four essential conserved domains of multicopper oxidase type 1 (IPR001117), multicopper oxidase type 2 (IPR011706), multicopper oxidase type 3 (IPR011707) and laccase (IPR017761) were deemed as candidate LACs [13]. Another BLASTp was performed in A. thaliana genome using candidate BnLACs protein sequences as queries and hold those genes that corresponded to AtLACs. LACs identified from B. napus, B. rapa and B. oleracea were named according to the orthologous sequence in A. thaliana. Information about putative sequences were searched in the date bases of BRAD (http://brassicadb.org/) and B. napus Genome Browser (http://www.genoscope.cns.fr/brassicanapus/). The chromosomal locations were shown by MapChart software [47], and the number of amino acids, isoelectric point (pI) and molecular weight (MW) of the protein sequences were searched using the ExPASy website (http://web.expasy.org/). The subcellular localization pattern of LAC genes were predicted using the web-based tool TargetP1.1 server (http://www.cbs.dtu.dk/services/TargetP/) [48]. Multiple Collinearity Scan toolkit (MCScanX) was adopted to analyze the gene duplication events with the default parameters [30]. The synteny relationship of the LACs in B. napus and A. thaliana were constructed using the Dual Systeny Plotter software (https://github.com/CJ-Chen/TBtools).
4.3. Evolutionary relationship of the LAC Genes Family in A. thaliana, B. napus, B. rapa, and B. oleracea
A cladogram containing the sequences identified from the four species was built using MEGA 7.0 software [31], with 1000 bootstrap replicates performed, and it was then modified by iTOL (http://itol.embl.de/) and Photoshop CS 5 to further visualize evolutionary relationship.
4.4. Gene Structure and Conserved Motif Analysis
The cDNA sequences, genomic sequences and full-length protein sequences of AtLACs and BnLACs were obtained from the A. thaliana genome (http://www.arabidopsis.org/) and B. napus Genome Browser (http://www.genoscope.cns.fr/brassicanapus/) respectively. Gene structures were analysed by Gene Structure Display Server (GSDS2.0, http://gsds.cbi.pku.edu.cn/) [49], conserved motifs were tested by and Multiple EM for Motif Elicitation version 5.0.4 (MEME, http://meme-suite.org/tools/meme) with a limit of 20 motifs and any number of repetitions deemed as motif sites [50].
4.5. Cis-Elements Analysis and Prediction of miRNA Target BnLACs
One thousand and five hundred base pairs (bp) upstream of the initiation codons (ATG) were searched in the B. napus Genome Browser and cis-elements were analysed using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The genome sequences of the 45 BnLACs were submitted to the psRNATarget Server (http://plantgrn.noble.org/psRNATarget/) with default parameters to predicte the miRNAs with a target site on BnLACs. MiRNAs from the B. napus genome were selected and expectation number under 3 were selected [51].
4.6. Expression Patterns Analysis of B. napus LAC Genes
The expression patterns of BnLACs were based on RNA-seq, using data from the BioProject ID PRJNA358784. The data included the expression in different tissues in different stages of the B. napus cultivar ZS11. The clean reads were aligned to the B. napus reference genome and these sequence data and corresponding gene annotation files were downloaded from the genome website (http://www.genoscope.cns.fr/ brassicanapus). The BWA and Bowtie softwares were used to map the reads to a reference genome and the reference genes, respectively [52]. The alignment results were visualized by IGV (Integrative Genomics Viewer) and genes expression levels were quantified on the basis of their FPKM values using Cufflinks with default parameters. For RNA-seq data about Cd2+ and NH4+ stresses, HISAT2 was used to map the reads to a reference genome and genes [53]. The alignment results were also visualized by IGV and the level of each gene expression was measured as FPKM by StringTie [54]. The heatmaps were built to represent the expression level of the BnLACs using HemI 1.0 software (http://hemi.biocuckoo.org/faq.php) [55].
4.7. RNA Extraction, Reverse Transcription and qRT-PCR
Total RNA was extracted using the EZ-10 DNAaway RNA Mini-prep Kit (Sangon Biotech, Shanghai, China). NanoDrop 2000 (Thermo Fisher Scientific, Worcester, MA, USA) and electrophoresis were used to measure concentrations and RNA integrity. Complementary DNA was obtained using the iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) and diluted 15 times with distilled deionized water for qRT-PCR. The composition of qRT-PCR contained 2 µL of 15-fold diluted cDNA solution, 10 µL of SYBR® Green Supermix (Bio-Rad), 0.4 µL of 10 mM forward and reverse primers and 7.2 µL of distilled deionized water. Primers were designed on Primer Premier Software (version 5.0) (Table S8) [56] and qRT-PCR was performed on a CFX96 Real-time System (Bio-Rad) with the following conditions: 98 °C for 30 s, then 40 cycles of 98 °C for 15 s, 55 °C for 30 s, and an increase from 65–95 °C at increments of 0.5°C every 0.05 s. Three biological replicates and three technical replications were used for qRT-PCR. According to the 2−ΔΔCt method using Actin7 and UBC21 as internal controls, the gene expression levels were determined and displayed by OriginPro 8 (OriginLab Corporation, Northampton, MA, USA).
4.8. Proteins Interaction with AtLAC4 and Identified their Homologous Genes in the B. napus genome
MiR397b regulated both lignin content and silique length via modulating AtLAC4 has been identified [39]. To understand whether the similar interaction exit in B. napus, the protein sequences of AtLAC4 was obtained from the A. thaliana genome (https://www.arabidopsis.org/) and used to predict the interacting proteins in STRING platform (https://string-db.org/?tdsourcetag=s_pctim_aiomsg). Homologous genes of predicted protein sequences were searched in the B. napus genome as the method of identifying BnLACs.
5. Conclusions
A total of 45 putative BnLACs were identified in the B. napus genome and unevenly mapped on the 12 B. napus chromosomes with no tandem duplication. BnLACs were divided into five groups in the cladogram and members in same group had similar structures and motifs. BnLACs had high expression in lignified tissues such as roots, stems and seed coats. After high concentration of NH4+ toxicity, most BnLACs were up-regulated and lignin more and faster deposited. Expression of many BnLACs were close to zero in leaves and uninfluenced by drought stress. Some BnLACs were down- regulated and individual gene showed different responses in different lines. Many members were intricately influenced by wounding stress, and significantly regulated members may take part in the healing process. By RNA-seq between long and short silique lines, we forecasted that earlier lignification may be a reason for the short siliques. The results in the study give a chance to further study the functions of BnLACs in lignification and the interaction with other biological processes.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/10/1985/s1, Table S1: Motif sequences of conserved motif analysis, Table S2: Putative cis-elements in 45 BnLACs promoters, Table S3: List of BnLACs with putative miRNA target sites, Table S4: Expression levels of the BnLACs in different tissues and stages of B. napus. The values represent the FPKM values, Table S5: Expression levels of the BnLACs under Cd2+, NH4+ enrichment treatment. The values represent the FPKM values, Table S6: Expression levels of 14 selected BnLACs in the L(leaf) and S(stem) of the three lines under drought and wound stress, Table S7: Expression levels of BnLAC4, BnCTL2, BnIRX3, BnCESA4, BnIRX1, BnLAC17, BnGAUT12, BnIRX6, BnPGSIP1, BnGLP10 and BnFLA11 genes in 6 kinds of silique pericarps. The values represent the FPKM values, Table S8: Primers used for qRT-PCR analysis.
Author Contributions
L.L., H.W. and J.L. designed the experiments. X.P., F.D., Y.L., T.W. and H.J. performed the experiments. X.P., T.W., N.L., X.X. and K.L. analyzed the data. X.P. wrote the paper.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2016YFD0100202), National Natural Science Foundation of China (31771830, 31701335), the Fundamental Research Funds for Central Universities (XDJK2017A009) and the “111” Project (B12006).
Conflicts of Interest
The authors declared no conflict of interest.
References
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B. Early Allopolyploid Evolution in the Post-Neolithic Brassica Napus Oilseed Genome. Science 2014, 6199, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Mourasobczak, J.; Souza, U.; Mazzafera, P. Drought Stress and Changes in the Lignin Content and Composition in Eucalyptus. BMC Proc. 2011, 7, 103. [Google Scholar]
- Peng, D.; Chen, X.; Yin, Y.; Lu, K.; Yang, W.; Tang, Y.; Wang, Z. Lodging Resistance of Winter Wheat (Triticum aestivum L.): Lignin Accumulation and Its Related Enzymes Activities Due to the Application of Paclobutrazol or Gibberellin Acid. Field Crops Res. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Cruickshank, A.W.; Cooper, M.; Ryley, M.J.; Cruickshank, A.W.; Cooper, M.; Ryley, M.J. Peanut Resistance to Sclerotinia Minor and S. Sclerotiorum. Aust. J. Agric. Res. 2002, 10, 1105–1110. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E. Polyphenol Oxidase in Plants. Phytochemistry 1979, 18, 193–195. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 7, 2563. [Google Scholar] [CrossRef]
- Cohen, R.; Persky, L.; Hadar, Y. Biotechnological Applications and Potential of Wood-Degrading Mushrooms of the Genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 5, 582–594. [Google Scholar] [CrossRef]
- Bao, W.; O’malley, D.M.; Whetten, R.; Sederoff, R.R. A Laccase Associated with Lignification in Loblolly Pine Xylem. Science 1993, 5108, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Sterjiades, R.; Dean, J.F.; Eriksson, K.E. Laccase from Sycamore Maple (Acer pseudoplatanus) Polymerizes Monolignols. Plant Physiol. 1992, 3, 1162–1168. [Google Scholar]
- Liang, M.; Haroldsen, V.; Cai, X.; Wu, Y. Expression of a Putative Laccase Gene, Zmlac1, in Maize Primary Roots under Stress. Plant Cell Environ. 2006, 5, 746. [Google Scholar] [CrossRef]
- Hüttermann, A.; Mai, C.; Kharazipour, A. Modification of Lignin for the Production of New Compounded Materials. Appl. Microbiol. Biotechnol. 2001, 4, 387–394. [Google Scholar] [CrossRef]
- Wang, G.D.; Li, Q.J.; Luo, B.; Chen, X.Y. Ex Planta Phytoremediation of Trichlorophenol and Phenolic Allelochemicals Via an Engineered Secretory Laccase. Nat. Biotechnol. 2004, 7, 893. [Google Scholar] [CrossRef]
- Balasubramanian, V.K.; Rai, K.M.; Thu, S.W.; Mei, M.H.; Mendu, V. Genome-Wide Identification of Multifunctional Laccase Gene Family in Cotton (Gossypium spp.); Expression and Biochemical Analysis During Fiber Development. Sci. Rep. 2016, 6, 34309. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Jia, W.; Fan, P.; Bao, H.; Li, S.; Li, Y. Genome-Wide Identification of Sorghum Bicolor Laccases Reveals Potential Targets for Lignin Modification. Front. Plant Sci. 2017, 8, 714. [Google Scholar] [CrossRef]
- Lafayette, P.R.; Eriksson, K.E.; Dean, J.F. Nucleotide Sequence of a Cdna Clone Encoding an Acidic Laccase from Sycamore Maple (Acer pseudoplatanus L.). Plant Physiol. 1995, 2, 667–668. [Google Scholar] [CrossRef]
- Kiefermeyer, M.C.; Gomord, V.; O’Connell, A.; Halpin, C.; Faye, L. Cloning and Sequence Analysis of Laccase-Encoding Cdna Clones from Tobacco. Gene 1996, 1–2, 205–207. [Google Scholar] [CrossRef]
- Gavnholt, B.; Larsen, K.; Rasmussen, S.K. Isolation and Characterisation of Laccase Cdnas from Meristematic and Stem Tissues of Ryegrass (Lolium Perenne). Plant Sci. 2002, 6, 873–885. [Google Scholar] [CrossRef]
- Bligny, R.; Gaillard, J.; Douce, R. Excretion of Laccase by Sycamore (Acer pseudoplatanus L.) Cells. Effects of a Copper Deficiency. Biochemical Journal. 1983, 2, 583–588. [Google Scholar]
- Tezuka, K.; Hayashi, M.; Ishihara, H.; Onozaki, K.; Nishimura, M.; Takahashi, N. Occurrence of Heterogeneity of N-Linked Oligosaccharides Attached to Sycamore (Acer pseudoplatanus L.) Laccase after Excretion. Biochem. Mol. Biol. Int. 1993, 3, 395–402. [Google Scholar]
- Ranocha, P.; Mcdougall, G.; Hawkins, S.; Sterjiades, R.; Borderies, G.; Stewart, D.; Cabanesmacheteau, M.; Boudet, A.M.; Goffner, D. Biochemical Characterization, Molecular Cloning and Expression of Laccases—A Divergent Gene Family—in Poplar. FEBS J. 1999, 259, 485–495. [Google Scholar] [CrossRef]
- Turlapati, P.V.; Kim, K.W.; Davin, L.B.; Lewis, N.G. The Laccase Multigene Family in Arabidopsis Thaliana: Towards Addressing the Mystery of Their Gene Function(S). Planta 2011, 3, 439–470. [Google Scholar] [CrossRef] [PubMed]
- Mccaig, B.C.; Meagher, R.B.; Dean, J.F.D. Gene Structure and Molecular Analysis of the Laccase-Like Multicopper Oxidase (Lmco) Gene Family in Arabidopsis Thaliana. Planta 2005, 5, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Caparrós-Ruiz, D.; Fornalé, S.; Civardi, L.; Puigdomènech, P.; Rigau, J. Isolation and Characterisation of a Family of Laccases in Maize. Plant Sci. 2006, 2, 217–225. [Google Scholar] [CrossRef]
- Cesarino, I.; Araújo, P.; Mayer, J.L.S.; Vicentini, R.; Berthet, S.; Demedts, B.; Vanholme, B.; Boerjan, W.; Mazzafera, P. Expression of Soflac, a New Laccase in Sugarcane, Restores Lignin Content but Not S:G Ratio of Arabidopsis Lac17 Mutant. J. Exp. Bot. 2013, 6, 1769–1781. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, K.; Qu, C.; Liang, Y.; Wang, R.; Chai, Y.; Li, J. Gene Silencing of Bntt10 Family Genes Causes Retarded Pigmentation and Lignin Reduction in the Seed Coat of Brassica Napus. PLoS ONE 2013, 4, e61247. [Google Scholar] [CrossRef]
- Wang, Y.; Le, B.P.; Antelme, S.; Soulhat, C.; Gineau, E.; Dalmais, M.; Bendahmane, A.; Morin, H.; Mouille, G.; Lapierre, C. Laccase 5 Is Required for Lignification of the Brachypodium Distachyon Culm. Plant Physiol. 2015, 1, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Lee, C.; Hwang, S.G.; Park, Y.C.; Lim, H.L.; Jang, C.S. Overexpression of the Oschi1 Gene, Encoding a Putative Laccase Precursor, Increases Tolerance to Drought and Salinity Stress in Transgenic Arabidopsis. Gene 2014, 1, 98–105. [Google Scholar] [CrossRef]
- Sato, Y.; Bao, W.; Sederoff, R.; Whetten, R. Molecular Cloning and Expression of Eight Laccase Cdnas in Loblolly Pine (Pinus taeda). J. Plant Res. 2001, 2, 147–155. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Ross, W.; Bao, W.; Chen, C.; Sederoff, R.R. The Role of Laccase in Lignification. Plant J. 1993, 5, 751–757. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. Mcscanx: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 7, e49. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Paterson, A.H.; Bowers, J.E.; Chapman, B.A. Ancient Polyploidization Predating Divergence of the Cereals, and Its Consequences for Comparative Genomics. Proc. Natl. Acad. Sci. USA 2004, 26, 9903–9908. [Google Scholar] [CrossRef]
- Toledoortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis Basic/Helix-Loop-Helix Transcription Factor Family. Plant Cell 2003, 8, 1749–1770. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, S. Atnap, a Nac Family Transcription Factor, Has an Important Role in Leaf Senescence. Plant J. 2006, 4, 601–612. [Google Scholar] [CrossRef]
- Shen, H.; Cao, K.; Wang, X. Atbzip16 and Atbzip68, Two New Members of Gbfs, Can Interact with Other G Group Bzips in Arabidopsis Thaliana. BMB Rep. 2008, 2, 132–138. [Google Scholar] [CrossRef]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.H. Myb58 and Myb63 Are Transcriptional Activators of the Lignin Biosynthetic Pathway During Secondary Cell Wall Formation in Arabidopsis. Plant Cell 2009, 1, 248–266. [Google Scholar] [CrossRef]
- Lu, S.; Li, Q.; Wei, H.; Chang, M.J.; Tunlayaanukit, S.; Kim, H.; Liu, J.; Song, J.; Sun, Y.H.; Yuan, L. Ptr-Mir397a Is a Negative Regulator of Laccase Genes Affecting Lignin Content in Populus Trichocarpa. Proc. Natl. Acad. Sci. USA 2013, 26, 10848–10853. [Google Scholar] [CrossRef]
- Abdelghany, S.E.; Pilon, M. Microrna-Mediated Systemic Down-Regulation of Copper Protein Expression in Response to Low Copper Availability in Arabidopsis. J. Biol. Chem. 2008, 23, 15932–15945. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yu, Y.; Wang, C.Y.; Li, Z.Y.; Liu, Q.; Xu, J.; Liao, J.Y.; Wang, X.J.; Qu, L.H.; Chen, F. Overexpression of Microrna Osmir397 Improves Rice Yield by Increasing Grain Size and Promoting Panicle Branching. Nat. Biotechnol. 2013, 9, 848. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhang, S.; Yu, Y.; Luo, Y.-C.; Liu, Q.; Ju, C.; Zhang, Y.-C.; Qu, L.-H.; Lucas, W.J.; Wang, X. Mir397b Regulates Both Lignin Content and Seed Number in Arabidopsis Via Modulating a Laccase Involved in Lignin Biosynthesis. Plant Biotechnol. J. 2015, 8, 1132–1142. [Google Scholar] [CrossRef]
- Bottcher, A.; Cesarino, I.; Santos, A.B.; Vicentini, R.; Mayer, J.L.; Vanholme, R.; Morreel, K.; Goeminne, G.; Moura, J.C.; Nobile, P.M. Lignification in Sugarcane: Biochemical Characterization, Gene Discovery, and Expression Analysis in Two Genotypes Contrasting for Lignin Content. Plant Physiol. 2013, 4, 1539. [Google Scholar] [CrossRef]
- Jin, L.F.; Liu, Y.Z.; Yin, X.X.; Peng, S.A. Transcript Analysis of Citrus Mirna397 and Its Target Lac7 Reveals a Possible Role in Response to Boron Toxicity. Acta Physiol. Plant. 2016, 1, 18. [Google Scholar] [CrossRef]
- Shen, C.; Huang, Y.Y.; He, C.T.; Zhou, Q.; Chen, J.X.; Tan, X.; Mubeen, S.; Yuan, J.G.; Yang, Z.Y. Comparative Analysis of Cadmium Responsive Micrornas in Roots of Two Ipomoea Aquatica Forsk. Cultivars with Different Cadmium Accumulation Capacities. Plant Physiol. Biochem. 2016, 111, 329–339. [Google Scholar] [CrossRef]
- Bo, P.; Hopkins, R.; Rask, L.; Meijer, J. Differential Wound Induction of the Myrosinase System in Oilseed Rape (Brassica napus): Contrasting Insect Damage with Mechanical Damage. Plant Sci. 2005, 3, 715–722. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped Blast and Psi-Blast: A New Generation of Protein Database Search. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Mitchell, A.; Chang, H.Y.; Daugherty, L.; Fraser, M.; Hunter, S.; Lopez, R.; Mcanulla, C.; Mcmenamin, C.; Nuka, G.; Pesseat, S. The Interpro Protein Families Database: The Classification Resource after 15 Years. Nucleic Acids Res. 2015, 43, 213–221. [Google Scholar] [CrossRef]
- Voorrips, R.E. Mapchart: Software for the Graphical Presentation of Linkage Maps and Qtls. J. Hered. 2002, 1, 77–78. [Google Scholar] [CrossRef]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; Von Heijne, G. Predicting Subcellular Localization of Proteins Based on Their N-Terminal Amino Acid Sequence. J. Mol. Biol. 2000, 4, 1005–1016. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. Gsds 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 8, 1296. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. Meme Suite: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, P.X. Pssrnaminer: A Plant Short Small Rna Regulatory Cascade Analysis Server. Nucleic Acids Res. 2008, 36, W114–W118. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Daehwan, K.; Langmead, B.; Salzberg, S.L. Hisat: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 4, 357–360. [Google Scholar]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. Stringtie EnablesImproved Reconstruction of a Transcriptome from Rna-Seq Reads. Nat. Biotechnol. 2015, 3, 290–295. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. Hemi: A Toolkit for Illustrating Heatmaps. PLoS ONE 2014, 11, e111988. [Google Scholar] [CrossRef]
- Lalitha, S. Primer Premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).