UHPLC-UV/Vis Quantitative Analysis of Hydroxylated and O-prenylated Coumarins in Pomegranate Seed Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. UHPLC Analysis
2.2. Quantification of Auraptene, Umbelliprenin, and Umbelliferone in Pomegranate Seed Extracts
3. Materials and Methods
3.1. Chemicals, Plant Materials, and Extraction Procedures
3.2. UHPLC Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Galli, G.V.; Corbett, Y.; Taramelli, D.; Lucantoni, L.; Habluetzel, A.; Maschi, O.; Caruso, D.; Giavarini, F.; Romeo, S.; et al. Antiplasmodial activity of Punica granatum L. fruit rind. J. Ethnopharmacol. 2009, 125, 279–285. [Google Scholar] [CrossRef]
- Qnais, E.Y.; Elokda, A.S.; Abu Ghalyun, Y.Y.; Abdulla, F.A. Antidiarrheal activity of the aqueous extract of Punica granatum (pomegranate) peels. Pharm. Biol. 2007, 45, 715–720. [Google Scholar] [CrossRef]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Gopinath, H.; Kumar, B.; Duraivel, S.; Aravind, G.; Kumar, K. Medicinal uses of Punica granatum and its health benefits. J. Pharmacogn. Phytochem. 2013, 1, 8–35. [Google Scholar]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Genovese, S. Biomolecular targets of oxyprenylated phenylpropanoids and polyketides (2019). In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.K., Eds.; Springer: Cham, Switzerland, 2019; Volume 108, pp. 143–205. [Google Scholar]
- Hasan, M.; Genovese, S.; Fiorito, S.; Epifano, F.; PWitt-Enderby, P.A. Oxyprenylated phenylpropanoids bind to MT1 melatonin receptors and inhibit breast cancer cell proliferation and migration. J. Nat. Prod. 2017, 80, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Taddeo, V.A.; Genovese, S. Recent acquisitions on oxyprenylated secondary metabolites as anti-inflammatory agents. Eur. J. Med. Chem. 2018, 153, 116–122. [Google Scholar] [CrossRef]
- Epifano, F.; Genovese, S.; Squires, J.E.; Gray, M.A. Nelumal A, the active principle from Ligularia nelumbifolia, is a novel farnesoid X receptor agonist. Bioorg. Med. Chem. Lett. 2012, 22, 3130–3135. [Google Scholar] [CrossRef]
- Genovese, S.; Ashida, H.; Yamashita, Y.; Nakgano, T.; Ikeda, M.; Daishi, S.; Epifano, F.; Taddeo, V.A.; Fiorito, S. The interaction of auraptene and other oxyprenylated phenylpropanoids with glucose transporter type 4. Phytomedicine 2017, 32C, 74–79. [Google Scholar] [CrossRef]
- Genovese, S.; Foreman, J.E.; Borland, M.G.; Epifano, F.; Gonzalez, F.J.; Curini, M.; Peters, J.M. A natural propenoic acid derivative activates peroxisome proliferator-activated receptor-β/δ (PPARβ/δ). Life Sci. 2010, 86, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Jungbauer, A. Pomegranate a fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef]
- Khaiebishak, Y.; Payahoo, L.; Alivand, M.; Hamishekar, H.; Mobasseri, M.; Ebrahimzadeh, V.; Alipour, M.; Alipour, B. Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: A randomized controlled clinical trial. J. Cell. Physiol. 2019. In press. [Google Scholar] [CrossRef]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-γ pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef]
- Lansky, E.; Harrison, G.; Froom, P.; Jiang, W. Pomegranate (Punica granatum) pure chemical show possible synergistic inhibition of human PC-3 prostate cancer cell invasion across Matrigel. Invest. New Drugs 2005, 23, 121–122. [Google Scholar] [CrossRef]
- Gill, N.S.; Dhawan, S.; Jain, A.; Arora, R.; Bali, M. Antioxidant and anti-ulcerogenic activity of wild Punica granatum ethanolic seed extract. Res. J. Med. Plant. 2012, 6, 47–55. [Google Scholar] [CrossRef]
- Hussein, A.A.; Barberena, I.; Capson, T.; Kursar, T.; Coley, P.D.; Solis, P.N.; Gupta, M.P. New cytotoxic naphthopyrane derivatives from Adenaria floribunda. J. Nat. Prod. 2004, 67, 451–453. [Google Scholar] [CrossRef]
- Fiorito, S.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Taddeo, V.A.; Genovese, S. Novel biologically active principles from spinach, goji and quinoa. Food Chem. 2019, 276, 262–265. [Google Scholar] [CrossRef]
- Genovese, S.; Taddeo, V.A.; Epifano, F.; Fiorito, S.; Bize, C.; Rives, A.; de Medina, P. Characterization of the degradation profile of umbelliprenin, a bioactive prenylated coumarin of a Ferulago species. J. Nat. Prod. 2017, 80, 2424–2431. [Google Scholar] [CrossRef]
- Ferrone, V.; Genovese, S.; Carlucci, M.; Tiecco, M.; Germani, R.; Preziuso, F.; Epifano, F.; Carlucci, G.; Taddeo, V.A. A green deep eutectic solvent dispersive liquid-liquid micro-extraction (DES-DLLME) for the UHPLC-PDA determination of oxyprenylated phenylpropanoids in olive, soy, peanuts, corn, and sunflower oil. Food Chem. 2018, 245, 578–585. [Google Scholar] [CrossRef]
- Scotti, L.; Genovese, S.; Bucciarelli, T.; Martini, F.; Epifano, F.; Fiorito, S.; Preziuso, F.; Taddeo, V.A. Analysis of biologically active oxyprenylated phenylpropanoids in tea tree oil using selective solid-phase extraction with UHPLC–PDA detection. J. Pharm. Biomed. Anal. 2018, 154, 174–179. [Google Scholar] [CrossRef]
- Genovese, S.; Fiorito, S.; Locatelli, M.; Carlucci, G.; Epifano, F. Analysis of biologically active oxyprenylated ferulic acid derivatives in Citrus fruits. Plant Food Hum. Nutr. 2014, 69, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Zhu, T.; Cai, B. Development and validation of an UHPLC-QqQ-MS technique for simultaneous determination of ten bioactive components in Fangji Huangqi Tang. J. Anal. Meth. Chem. 2016, 2016, 1435106. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of pomegranate seeds are available from the authors. |
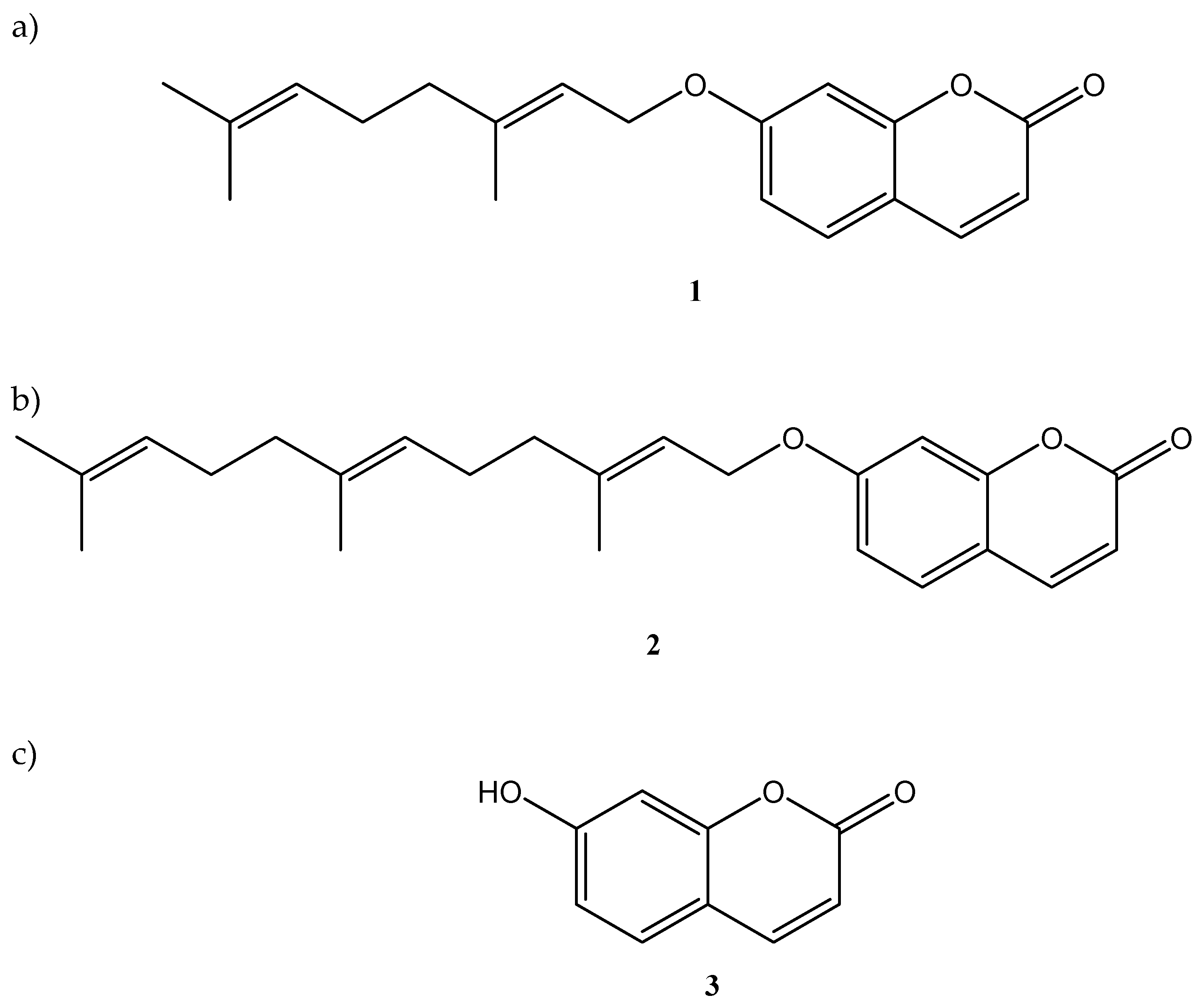

| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Retention time (min) a,b | 11.41 (0.4%) | 13.56 (0.4%) | 7.94 (0.3%) |
| Peak symmetry (10%) | 1.05 | 1.08 | 1.17 |
| Compound | Slope | Intercept | r2 | Recovery (%) |
|---|---|---|---|---|
| 1 | 181,298 (167,944–194,709) | 25,055 (−145,287–195,411) | 0.9991 | 99.8 |
| 2 | 46,555 (42,578–50,521) | 10,802 (−39,690–61,300) | 0.9986 | 100.3 |
| 3 | 23,565 (21,967–25,163) | 7985 (−18,712–34,520) | 0.9989 | 96.4 |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Precision | |||
| Intra-day (n = 6) | 3.7–6.7 | 1.5–4.1 | 2.3–4.8 |
| Inter-day (n = 6) | 4.3–7.6 | 0.8–5.4 | 1.2–3.1 |
| Accuracy | |||
| Intra-day (n = 6) | 1.4–3.4 | 0.8–2.7 | 1.5–3.6 |
| Inter-day (n = 6) | 1.5–3.6 | 1.3–2.6 | 1.1–2.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorito, S.; Ianni, F.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Genovese, S. UHPLC-UV/Vis Quantitative Analysis of Hydroxylated and O-prenylated Coumarins in Pomegranate Seed Extracts. Molecules 2019, 24, 1963. https://doi.org/10.3390/molecules24101963
Fiorito S, Ianni F, Preziuso F, Epifano F, Scotti L, Bucciarelli T, Genovese S. UHPLC-UV/Vis Quantitative Analysis of Hydroxylated and O-prenylated Coumarins in Pomegranate Seed Extracts. Molecules. 2019; 24(10):1963. https://doi.org/10.3390/molecules24101963
Chicago/Turabian StyleFiorito, Serena, Federica Ianni, Francesca Preziuso, Francesco Epifano, Luca Scotti, Tonino Bucciarelli, and Salvatore Genovese. 2019. "UHPLC-UV/Vis Quantitative Analysis of Hydroxylated and O-prenylated Coumarins in Pomegranate Seed Extracts" Molecules 24, no. 10: 1963. https://doi.org/10.3390/molecules24101963
APA StyleFiorito, S., Ianni, F., Preziuso, F., Epifano, F., Scotti, L., Bucciarelli, T., & Genovese, S. (2019). UHPLC-UV/Vis Quantitative Analysis of Hydroxylated and O-prenylated Coumarins in Pomegranate Seed Extracts. Molecules, 24(10), 1963. https://doi.org/10.3390/molecules24101963









