Trimethyl Chitosan/Siloxane-Hybrid Coated Fe3O4 Nanoparticles for the Uptake of Sulfamethoxazole from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
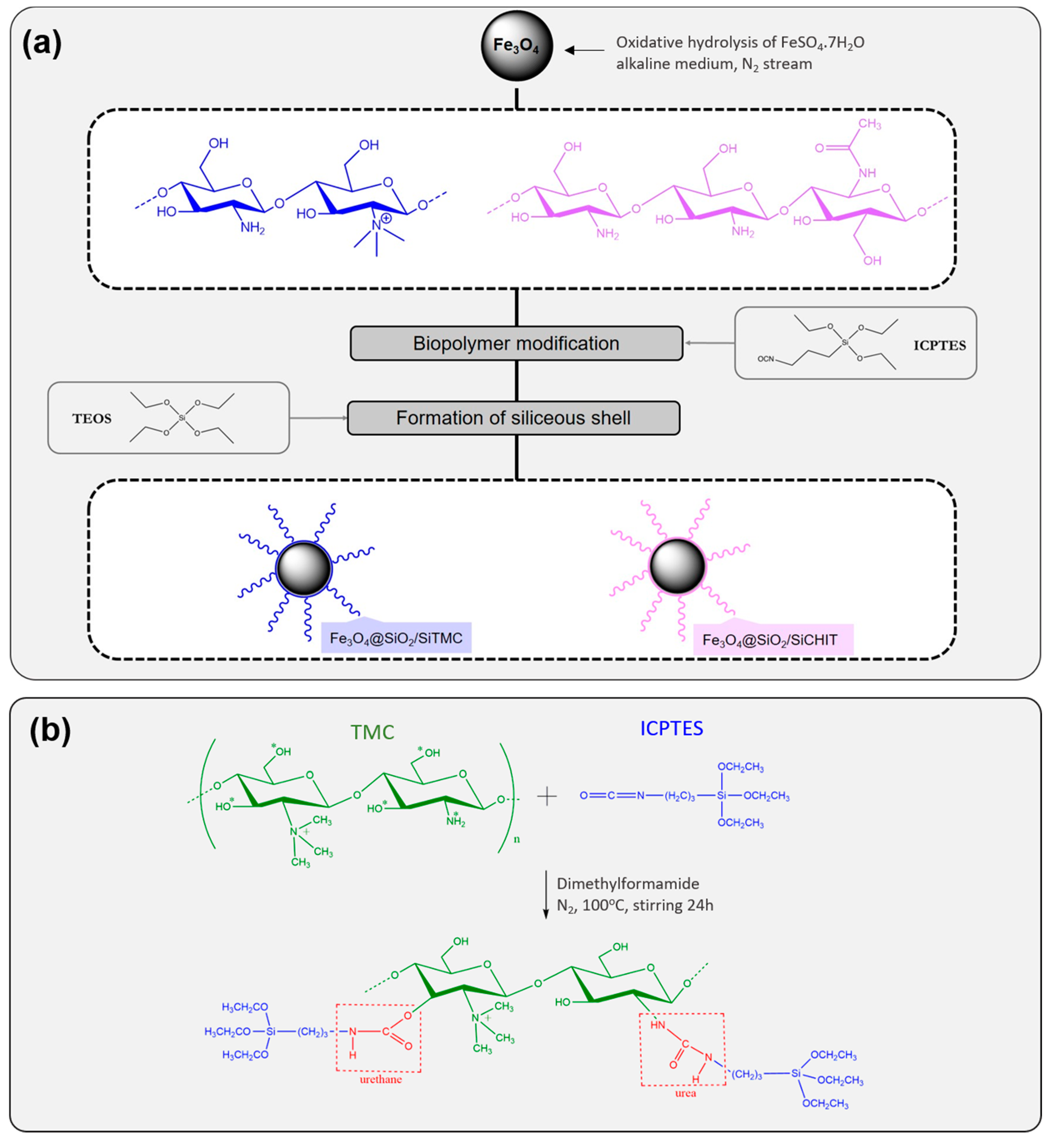
2.2. Synthesis of the Magnetic Core
2.3. Coating of the Magnetic Nanoparticles
2.4. Characterization of the Materials
2.5. Uptake of Sulfamethoxazole from Aqueous Solutions
3. Results and Discussion
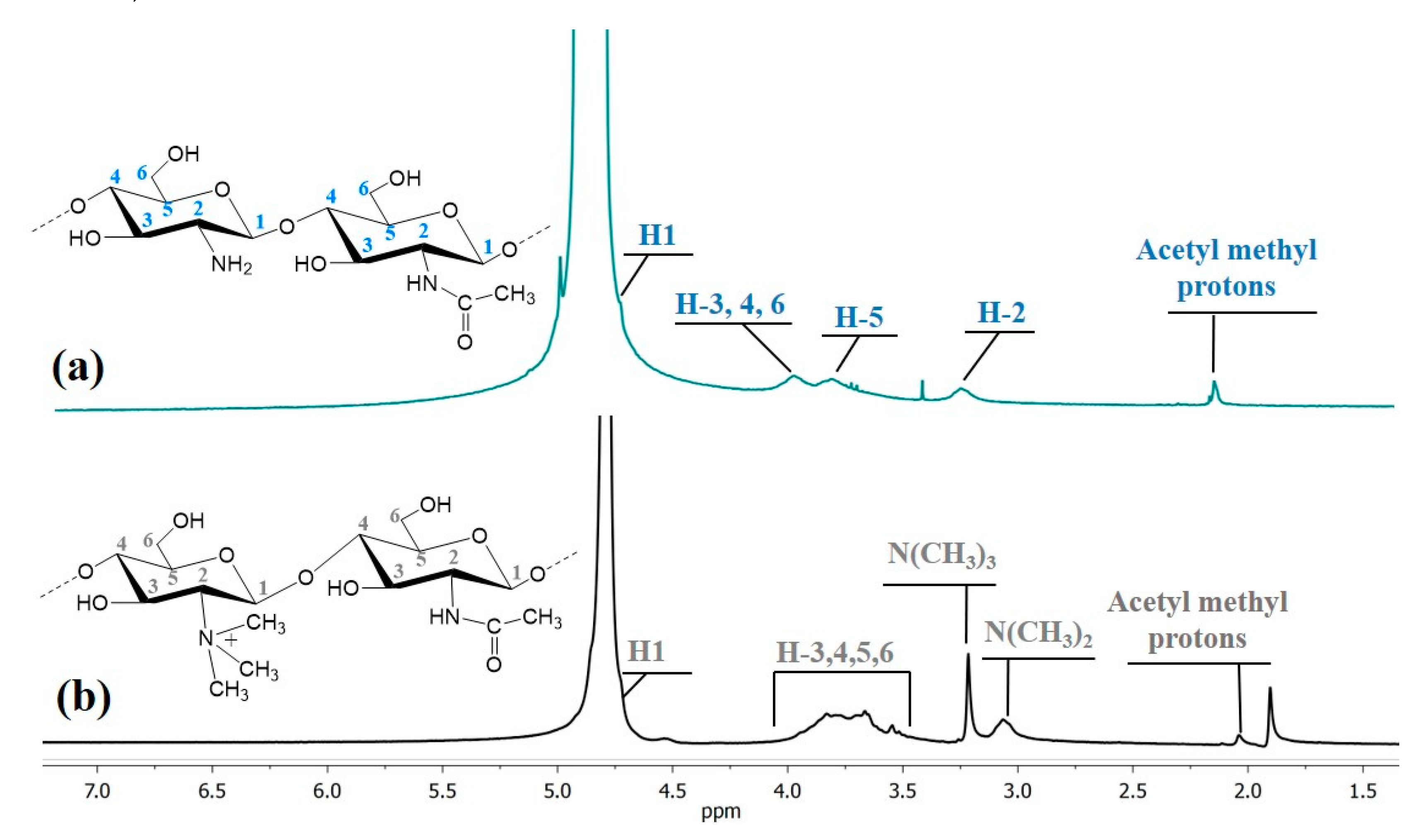
3.1. Characterization of Chitosan Polymers
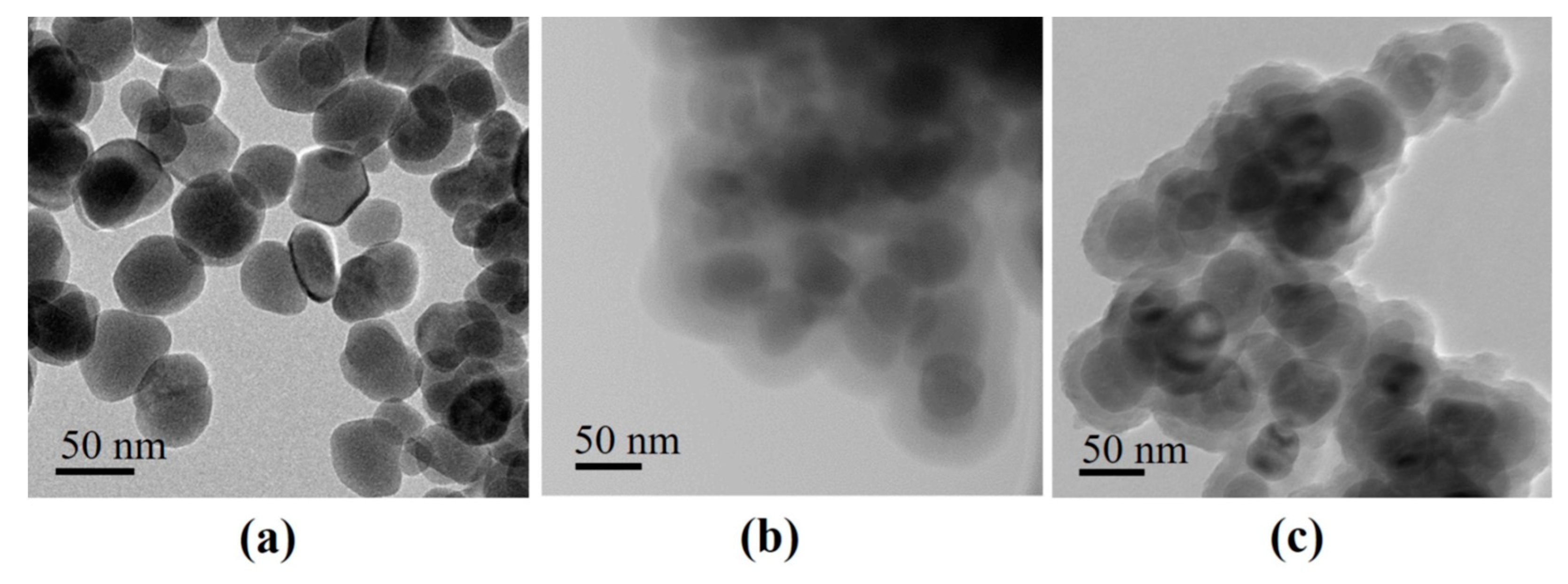
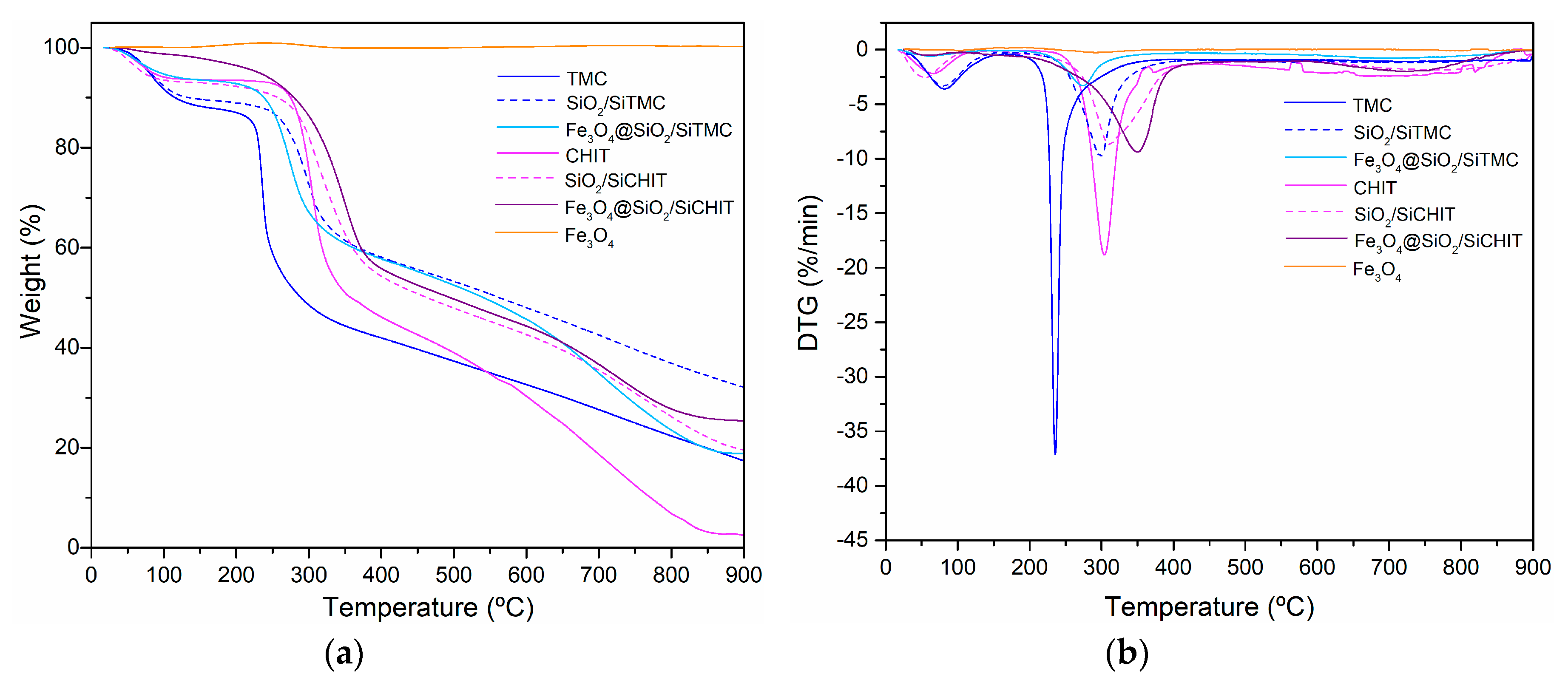
3.2. Characterization of Fe3O4 Coated Particles
3.3. Uptake of Sulfamethoxazole
3.1.1. Effect of Initial pH
3.1.2. Kinetic Studies and Effect of Initial SMX Concentration
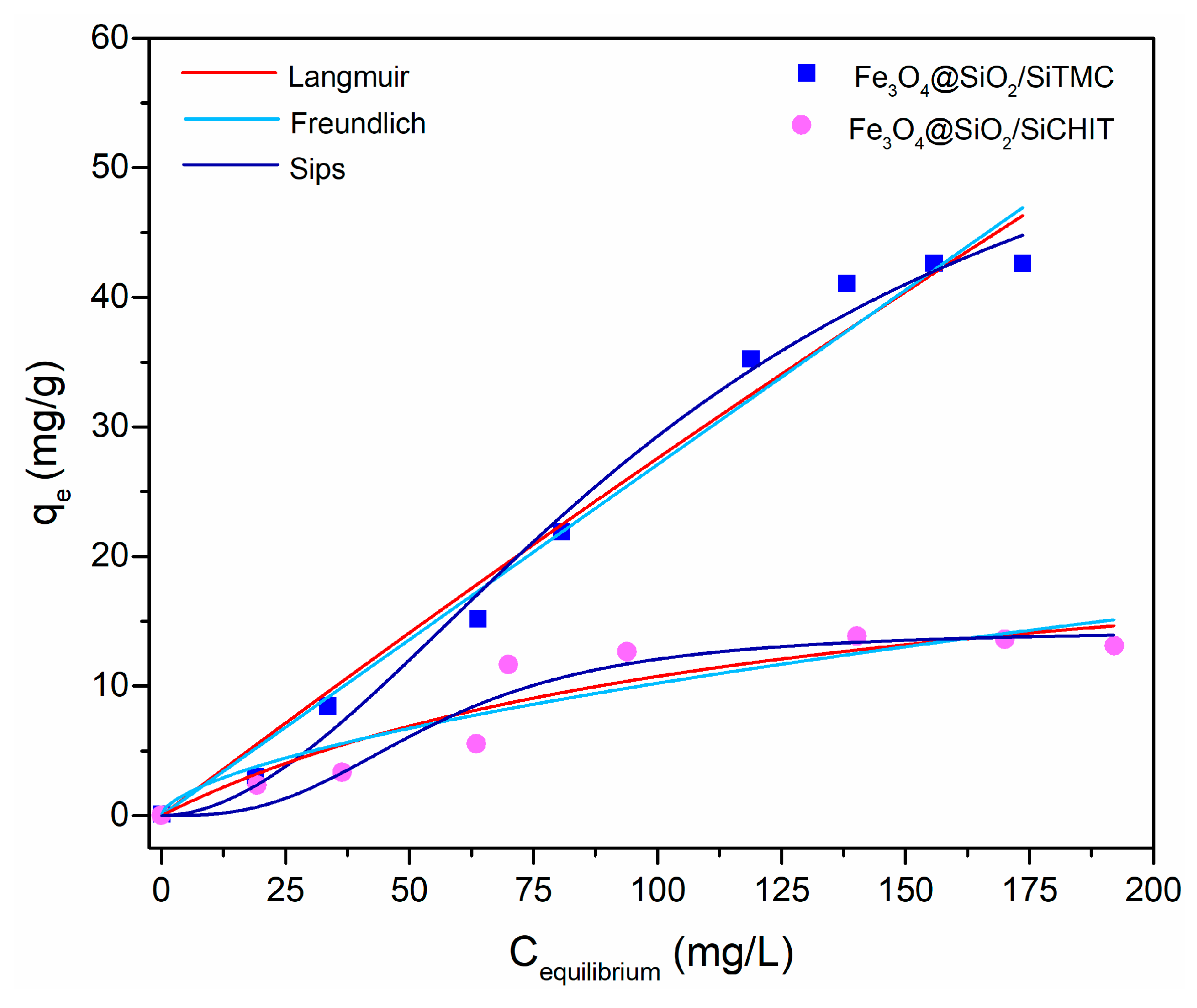
3.1.3. Equilibrium Isotherm Experiments
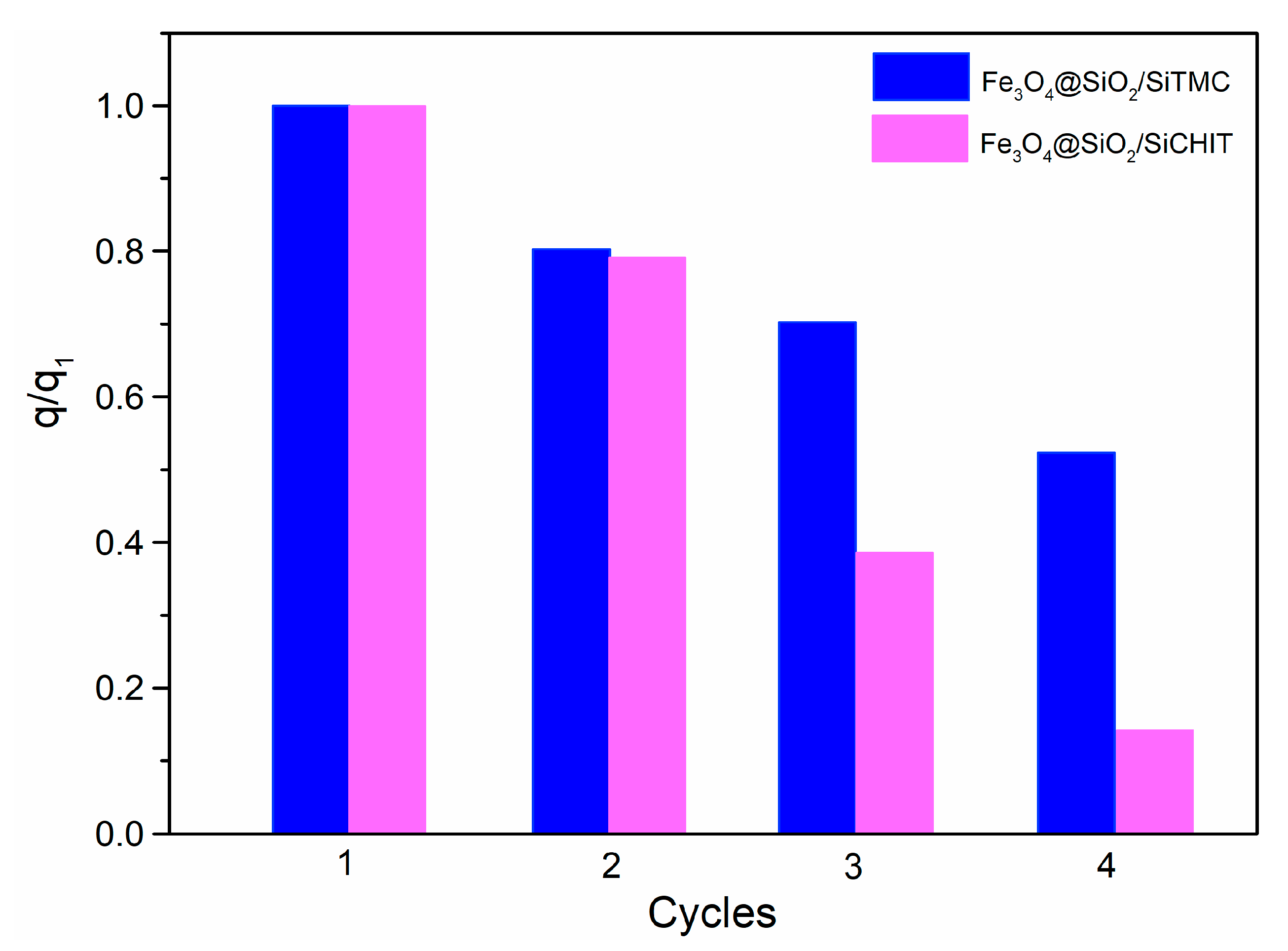
3.1.4. Sorbent Stability, Regeneration and Reuse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radović, T.; Grujić, S.; Petković, A.; Dimkić, M.; Laušević, M. Determination of pharmaceuticals and pesticides in river sediments and corresponding surface and ground water in the Danube River and tributaries in Serbia. Environ. Monit. Assess. 2015, 187, 4092. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef]
- Loos, R.; Locoro, G.; Comero, S.; Contini, S.; Schwesig, D.; Werres, F.; Balsaa, P.; Gans, O.; Weiss, S.; Blaha, L.; et al. Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res. 2010, 44, 4115–4126. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.-F.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef]
- Straub, J.O. Aquatic environmental risk assessment for human use of the old antibiotic sulfamethoxazole in Europe. Environ. Toxicol. Chem. 2016, 35, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Martini, J.; Orge, C.A.; Faria, J.L.; Pereira, M.F.R.; Soares, O.S.G.P. Sulfamethoxazole degradation by combination of advanced oxidation processes. J. Environ. Chem. Eng. 2018, 6, 4054–4060. [Google Scholar] [CrossRef]
- Falås, P.; Wick, A.; Castronovo, S.; Habermacher, J.; Ternes, T.A.; Joss, A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016, 95, 240–249. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Martella, L.; Massella, M.; Carbonell-Alcaina, C.; Alcaina-Miranda, M.-I.; Iborra-Clar, M.-I. Nanofiltration as tertiary treatment method for removing trace pharmaceutically active compounds in wastewater from wastewater treatment plants. Water Res. 2017, 125, 360–373. [Google Scholar] [CrossRef]
- Shimabuku, K.K.; Kearns, J.P.; Martinez, J.E.; Mahoney, R.B.; Moreno-Vasquez, L.; Summers, R.S. Biochar sorbents for sulfamethoxazole removal from surface water, stormwater, and wastewater effluent. Water Res. 2016, 96, 236–245. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, H.; Zhang, W.; Yang, X.; Zhou, H.; Pan, Z.; Wang, D. Preparation of magnetic polyimide@Mg-Fe layered double hydroxides core-shell composite for effective removal of various organic contaminants from aqueous solution. Chemosphere 2019, 219, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Qiang, Z.; Chang, J.-H.; Ben, W.; Qu, J. Synthesis of carbon-coated magnetic nanocomposite (Fe3O4@C) and its application for sulfonamide antibiotics removal from water. J. Environ. Sci. 2014, 26, 962–969. [Google Scholar] [CrossRef]
- Song, Q.; Wang, H.; Yang, B.; Wang, F.; Sun, X. A novel adsorbent of Ag-FMWCNTs for the removal of SMX from aqueous solution. RSC Adv. 2016, 6, 75855–75861. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Akpotu, S.O.; Azeez, M.A.; Ndungu, P.; Moodley, B. Theoretical and experimental adsorption studies of sulfamethoxazole and ketoprofen on synthesized ionic liquids modified CNTs. Ecotoxicol. Environ. Saf. 2018, 161, 542–552. [Google Scholar] [CrossRef]
- USEPA. Reregistration Eligibility Decision for Trimethoxysilyl Quaternary Ammonium Chloride Compounds. Report EPA 739- R-07-007; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Huang, Y.; Keller, A.A. Magnetic Nanoparticle Adsorbents for Emerging Organic Contaminants. ACS Sustain. Chem. Eng. 2013, 1, 731–736. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-Field Magnetic Separation of Monodisperse Fe3O4 Nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; De Cuyper, M. Assessing iron oxide nanoparticle toxicity in vitro: Current status and future prospects. Nanomedicine 2010, 5, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.N.; Polshettiwar, V. Facile and Sustainable Synthesis of Shaped Iron Oxide Nanoparticles: Effect of Iron Precursor Salts on the Shapes of Iron Oxides. Sci. Rep. 2015, 5, 9733. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Niu, H.; Zhang, X.; Meng, Z.; Cai, Y. Strong adsorption of chlorotetracycline on magnetite nanoparticles. J. Hazard. Mater. 2011, 192, 1088–1093. [Google Scholar] [CrossRef]
- Soares, S.F.; Fernandes, T.; Trindade, T.; Daniel-da-Silva, A.L. Surface engineered magnetic biosorbents for water treatment. In Green Adsorbents for Pollutant Removal. Environmental Chemistry for a Sustainable World; Crini, G., Lichtfouse, E., Eds.; Springer: Basel, Switzerland, 2018; pp. 301–342. ISBN 978-3-319-92111-2. [Google Scholar]
- Soares, S.F.; Simoes, T.R.; Trindade, T.; Daniel-da-Silva, A.L. Highly Efficient Removal of Dye from Water Using Magnetic Carrageenan/Silica Hybrid Nano-adsorbents. Water. Air. Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Soares, S.F.; Fernandes, T.; Sacramento, M.; Trindade, T.; Daniel-da-Silva, A.L. Magnetic quaternary chitosan hybrid nanoparticles for the efficient uptake of diclofenac from water. Carbohydr. Polym. 2019, 203, 35–44. [Google Scholar] [CrossRef]
- Fernandes, T.; Soares, S.; Trindade, T.; Daniel-da-Silva, A. Magnetic Hybrid Nanosorbents for the Uptake of Paraquat from Water. Nanomaterials 2017, 7, 68. [Google Scholar] [CrossRef]
- Soares, S.F.; Simões, T.R.; António, M.; Trindade, T.; Daniel-da-Silva, A.L. Hybrid nanoadsorbents for the magnetically assisted removal of metoprolol from water. Chem. Eng. J. 2016, 302, 560–569. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Y.; Yang, Z.; Yang, W.; Wu, J.; Dong, C. Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem. Eng. J. 2016, 304, 325–334. [Google Scholar] [CrossRef]
- Sekimoto, T.; Nishihama, S.; Yoshizuka, K.; Maeda, R. Adsorptive removal of sulfamethoxazole with shell-core chitosan immobilized metal ion. Sep. Sci. Technol. 2018, 53, 1116–1123. [Google Scholar] [CrossRef]
- Vieira, E.F.S.; Cestari, A.R.; Oliveira, C.d.S.; de Lima, P.S.; Almeida, L.E. Thermodynamics of pyrimethamine and sulfadiazine binding to a chitosan derivative. Thermochim. Acta 2007, 459, 9–11. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Tan, W.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Preparation and Characterization of Quaternized Chitosan Derivatives and Assessment of Their Antioxidant Activity. Molecules 2018, 23, 516. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, D.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Biomaterials based on N,N,N-trimethyl chitosan fibers in wound dressing applications. Int. J. Biol. Macromol. 2016, 89, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Toledo, L.; Rivas, B.L. Quaternised chitosan in conjunction with ultrafiltration membranes to remove arsenate and chromate ions. Polym. Bull. 2015, 72, 1365–1377. [Google Scholar] [CrossRef]
- Tabriz, A.; Ur Rehman Alvi, M.A.; Khan Niazi, M.B.; Batool, M.; Bhatti, M.F.; Khan, A.L.; Khan, A.U.; Jamil, T.; Ahmad, N.M. Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbohydr. Polym. 2019, 207, 17–25. [Google Scholar] [CrossRef]
- Oliveira-Silva, R.; Pinto da Costa, J.; Vitorino, R.; Daniel-da-Silva, A.L. Magnetic chelating nanoprobes for enrichment and selective recovery of metalloproteases from human saliva. J. Mater. Chem. B 2015, 3, 238–249. [Google Scholar] [CrossRef]
- Soares, S.F.; Rodrigues, M.I.; Trindade, T.; Daniel-da-Silva, A.L. Chitosan-silica hybrid nanosorbents for oil removal from water. Colloid. Surf. A Physicochem. Eng. Asp. 2017, 532, 305–313. [Google Scholar] [CrossRef]
- Domard, A.; Gey, C.; Rinaudo, M.; Terrassin, C. 13C and 1H NMR spectroscopy of chitosan and N-trimethyl chloride derivatives. Int. J. Biol. Macromol. 1987, 9, 233–237. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Qiao, C.; Mu, X.; Li, T.; Xu, J.; Shi, L.; Zhang, D. A simple and convenient method to synthesize N-[(2-hydroxyl)-propyl-3-trimethylammonium] chitosan chloride in an ionic liquid. Carbohydr. Polym. 2015, 130, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wan, Y.; Wang, X.; Zha, Q.; Liu, H.; Qiu, Z.; Zhang, S. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf. B Biointerfaces 2012, 91, 168–174. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Ren, J.; Liu, C.; Wang, X.; Wu, J.; Sun, R. Preparation and characterization of new quaternized carboxymethyl chitosan/rectorite nanocomposite. Compos. Sci. Technol. 2010, 70, 1161–1167. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Elella, M.H.A.; Sabaa, M.W. Synthesis, characterization and applications of N-quaternized chitosan/poly(vinyl alcohol) hydrogels. Int. J. Biol. Macromol. 2015, 80, 149–161. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef]
- He, Y.; Fu, Y.; Geng, L.; Zhao, Y.; Lü, C. A facile route to enhance the properties of polymer electrolyte-based organic–inorganic hybrid proton exchange membranes. Solid State Ion. 2015, 283, 1–9. [Google Scholar] [CrossRef]
- Joint Committee for Powder Diffraction Studies, JCPDS, Card No. 19-0629; The International Centre for Diffraction Data (ICDD): Newtown Square, PA, USA.
- Daniel-da-Silva, A.L.; Pinto, F.; Lopes-da-Silva, J.A.; Trindade, T.; Goodfellow, B.J.; Gil, A.M. Rheological behavior of thermoreversible κ-carrageenan/nanosilica gels. J. Colloid Interface Sci. 2008, 320, 575–581. [Google Scholar] [CrossRef]
- Soares, S.F.; Trindade, T.; Daniel-da-Silva, A.L. Carrageenan-Silica Hybrid Nanoparticles Prepared by a Non-Emulsion Method. Eur. J. Inorg. Chem. 2015, 27, 4588–4594. [Google Scholar] [CrossRef]
- Chen, C.; Tao, S.; Qiu, X.; Ren, X.; Hu, S. Long-alkane-chain modified N-phthaloyl chitosan membranes with controlled permeability. Carbohydr. Polym. 2013, 91, 269–276. [Google Scholar] [CrossRef]
- Daniel-da-Silva, A.L.; Bordado, J.C.M.; Martín-Martínez, J.M. Moisture curing kinetics of isocyanate ended urethane quasi-prepolymers monitored by IR spectroscopy and DSC. J. Appl. Polym. Sci. 2008, 107, 700–709. [Google Scholar] [CrossRef]
- Silva, S.S.; Ferreira, R.A.S.; Fu, L.; Carlos, L.D.; Mano, J.F.; Reis, R.L.; Rocha, J. Functional nanostructured chitosan–siloxane hybrids. J. Mater. Chem. 2005, 15, 3952–3961. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S. Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr. Polym. 2010, 81, 931–936. [Google Scholar] [CrossRef]
- Hong, P.-Z.; Li, S.-D.; Ou, C.-Y.; Li, C.-P.; Yang, L.; Zhang, C.-H. Thermogravimetric analysis of chitosan. J. Appl. Polym. Sci. 2007, 105, 547–551. [Google Scholar] [CrossRef]
- Carp, O.; Patron, L.; Culita, D.C.; Budrugeac, P.; Feder, M.; Diamandescu, L. Thermal analysis of two types of dextran-coated magnetite. J. Therm. Anal. Calorim. 2010, 101, 181–187. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Zhang, L.; Zhan, H.; Levit, M.V. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb(II). Carbohydr. Polym. 2014, 101, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.-W.; Chen, E.-C.; Wu, T.-M. Thermal Stability and Magnetic Properties of Polyvinylidene Fluoride/Magnetite Nanocomposites. Materials 2015, 8, 4553–4564. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. The pH-dependent surface charging and points of zero charge. J. Colloid Interface Sci. 2011, 353, 1–15. [Google Scholar] [CrossRef]
- Plaza, R.C.; Arias, J.L.; Espín, M.; Jiménez, M.L.; Delgado, A.V. Aging Effects in the Electrokinetics of Colloidal Iron Oxides. J. Colloid Interface Sci. 2002, 245, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Riggio, C.; Calatayud, M.P.; Hoskins, C.; Pinkernelle, J.; Sanz, B.; Torres, T.E.; Ibarra, M.R.; Wang, L.; Keilhoff, G.; Goya, G.F.; et al. Poly-l-lysine-coated magnetic nanoparticles as intracellular actuators for neural guidance. Int. J. Nanomed. 2012, 3155. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Niu, F.; Peng, N.; Su, Y.; Yang, Y. Synthesis, characterization, and application of Fe3O4@SiO2-NH2 nanoparticles. RSC Adv. 2015, 5, 18128–18136. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Degradation of sulfamethoxazole by ionizing radiation: Kinetics and implications of additives. Sci. Total Environ. 2019, 668, 67–73. [Google Scholar] [CrossRef]
- Ling, C.; Li, X.; Zhang, Z.; Liu, F.; Deng, Y.; Zhang, X.; Li, A.; He, L.; Xing, B. High Adsorption of Sulfamethoxazole by an Amine-Modified Polystyrene–Divinylbenzene Resin and Its Mechanistic Insight. Environ. Sci. Technol. 2016, 50, 10015–10023. [Google Scholar] [CrossRef]
- Lozano-Morales, V.; Gardi, I.; Nir, S.; Undabeytia, T. Removal of pharmaceuticals from water by clay-cationic starch sorbents. J. Clean. Prod. 2018, 190, 703–711. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, B.; Wu, M.; Wang, B.; Zhang, H.; Peng, H.; Wu, D.; Ning, P. Adsorption of sulfamethoxazole on functionalized carbon nanotubes as affected by cations and anions. Environ. Pollut. 2011, 159, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pan, B.; Zhang, H.; Ning, P.; Xing, B. Contribution of Different Sulfamethoxazole Species to Their Overall Adsorption on Functionalized Carbon Nanotubes. Environ. Sci. Technol. 2010, 44, 3806–3811. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.M.L.; Rendueles, M.; Díaz, M. Sulfamethoxazole Removal from Synthetic Solutions by Ion Exchange using a Strong Anionic Resin in Fixed Bed. Solvent Extr. Ion Exch. 2013, 31, 763–781. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe; Handlingar, Ed.; Springer: Berlin, Germany, 1907; Volume 2, ISBN 0368-6590. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.Z. Concerning adsorption in solutions. Z. Phys. Chem. 1906, 57, 444–448. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Fe3O4@SiO2/SiTMC and Fe3O4@SiO2/SiCHIT are available from the authors. |




| Sample | C (%) 1 | H (%) 1 | N (%) 1 | D (nm) 2 | SBET (m2/g) 3 | VP (cm3/g) |
|---|---|---|---|---|---|---|
| Fe3O4 | 0.06 | 0.16 | 0.01 | 54 ± 9 | 13.6 | 0.027 |
| Fe3O4@SiO2/SiTMC | 28.6 | 5.3 | 5.1 | 98 ± 11 | 7.03 | 0.007 |
| Fe3O4@SiO2/SiCHIT | 28.5 | 5.8 | 5.3 | 68 ± 11 | 7.74 | 0.007 |
| Isotherm | Model Parameters | Goodness of Fit | |||
|---|---|---|---|---|---|
| Langmuir | qL (mg/g) | KL (L/mg) | R2 | χ2 | |
| Fe3O4@SiO2/SiTMC | 598.0 | 0.0004 | 0.9796 | 7.123 | |
| Fe3O4@SiO2/SiCHIT | 24.1 | 0.0080 | 0.8799 | 4.221 | |
| Freundlich | KF (mg(1−1/n)·L(1/n)·g−1) | n | R2 | χ2 | |
| Fe3O4@SiO2/SiTMC | 0.3582 | 1.3241 | 0.9788 | 7.417 | |
| Fe3O4@SiO2/SiCHIT | 0.1105 | 1.247 | 0.7225 | 9.759 | |
| Sips | qS (mg/g) | Ks (mg/L)−1/βS | βS | R2 | χ2 |
| Fe3O4@SiO2/SiTMC | 63.1 | 0.0001 | 0.5306 | 0.9911 | 3.614 |
| Fe3O4@SiO2/SiCHIT | 14.32 | 0.00001 | 0.3500 | 0.9259 | 3.057 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, S.F.; Fernandes, T.; Trindade, T.; Daniel-da-Silva, A.L. Trimethyl Chitosan/Siloxane-Hybrid Coated Fe3O4 Nanoparticles for the Uptake of Sulfamethoxazole from Water. Molecules 2019, 24, 1958. https://doi.org/10.3390/molecules24101958
Soares SF, Fernandes T, Trindade T, Daniel-da-Silva AL. Trimethyl Chitosan/Siloxane-Hybrid Coated Fe3O4 Nanoparticles for the Uptake of Sulfamethoxazole from Water. Molecules. 2019; 24(10):1958. https://doi.org/10.3390/molecules24101958
Chicago/Turabian StyleSoares, Sofia F., Tiago Fernandes, Tito Trindade, and Ana L. Daniel-da-Silva. 2019. "Trimethyl Chitosan/Siloxane-Hybrid Coated Fe3O4 Nanoparticles for the Uptake of Sulfamethoxazole from Water" Molecules 24, no. 10: 1958. https://doi.org/10.3390/molecules24101958
APA StyleSoares, S. F., Fernandes, T., Trindade, T., & Daniel-da-Silva, A. L. (2019). Trimethyl Chitosan/Siloxane-Hybrid Coated Fe3O4 Nanoparticles for the Uptake of Sulfamethoxazole from Water. Molecules, 24(10), 1958. https://doi.org/10.3390/molecules24101958








