Daylight Bactericidal Titania Textiles: A Contribution to Nosocomial Infections Control
Abstract
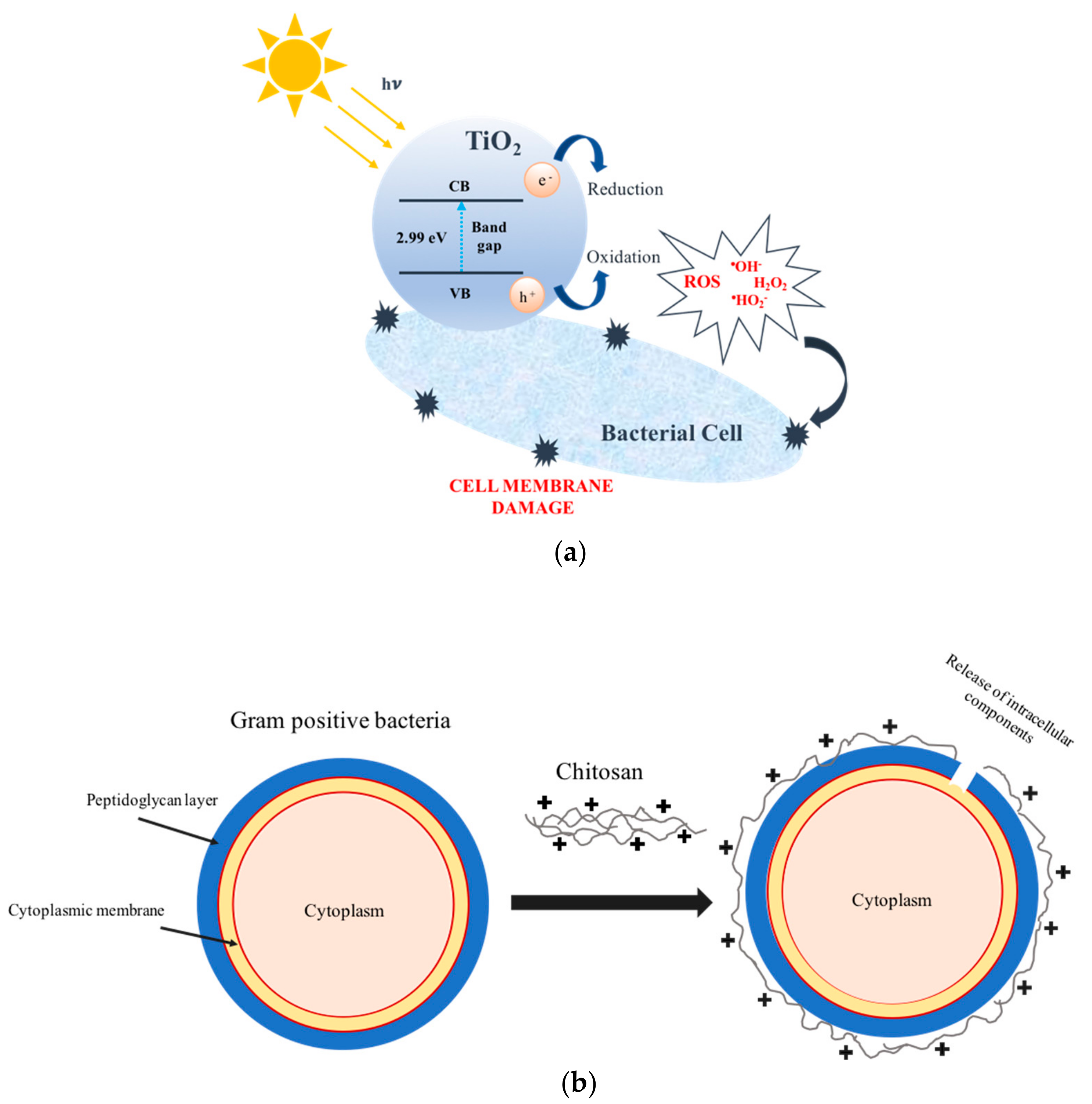
:1. Introduction
2. Materials and Methods
2.1. Materials
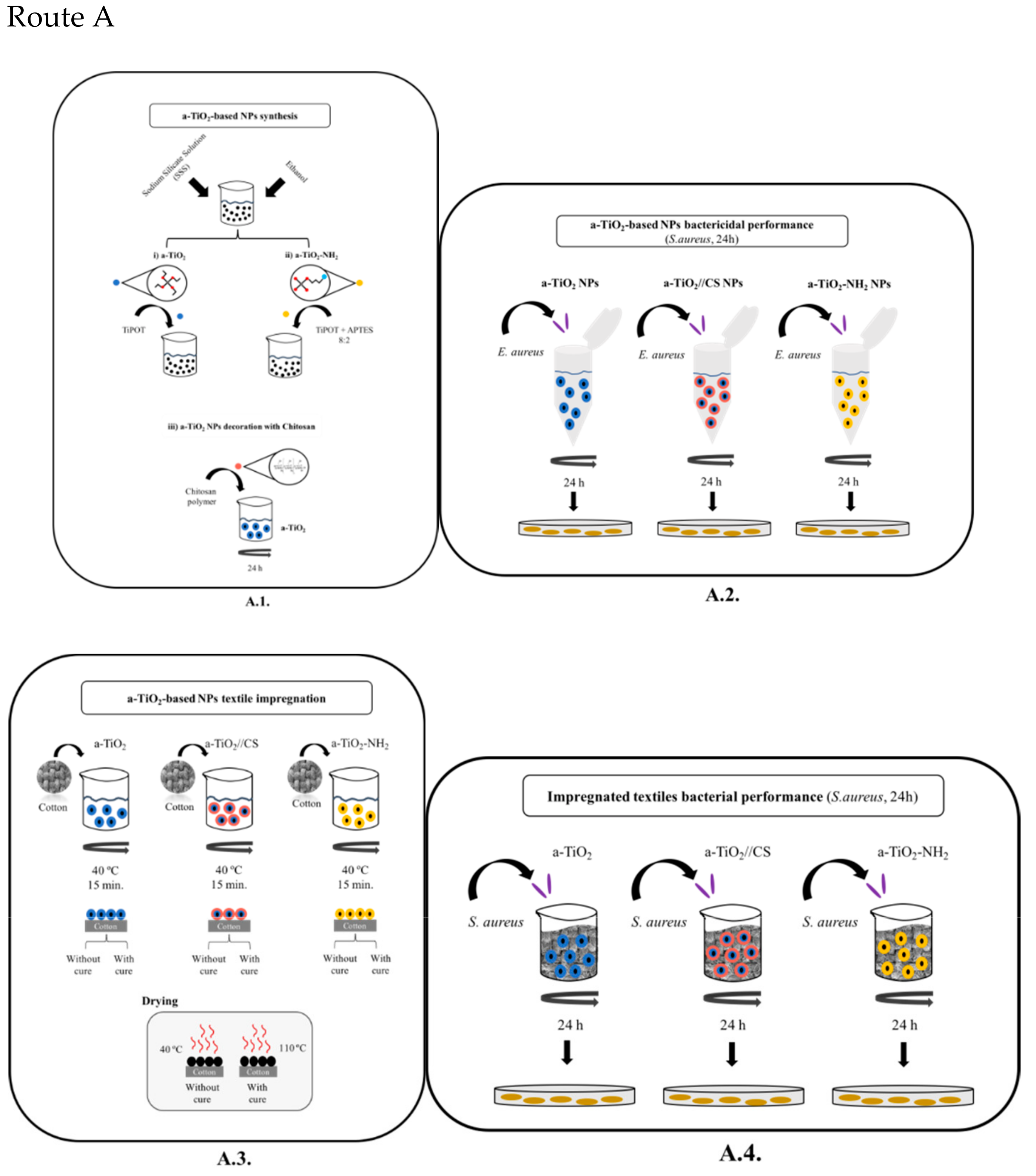
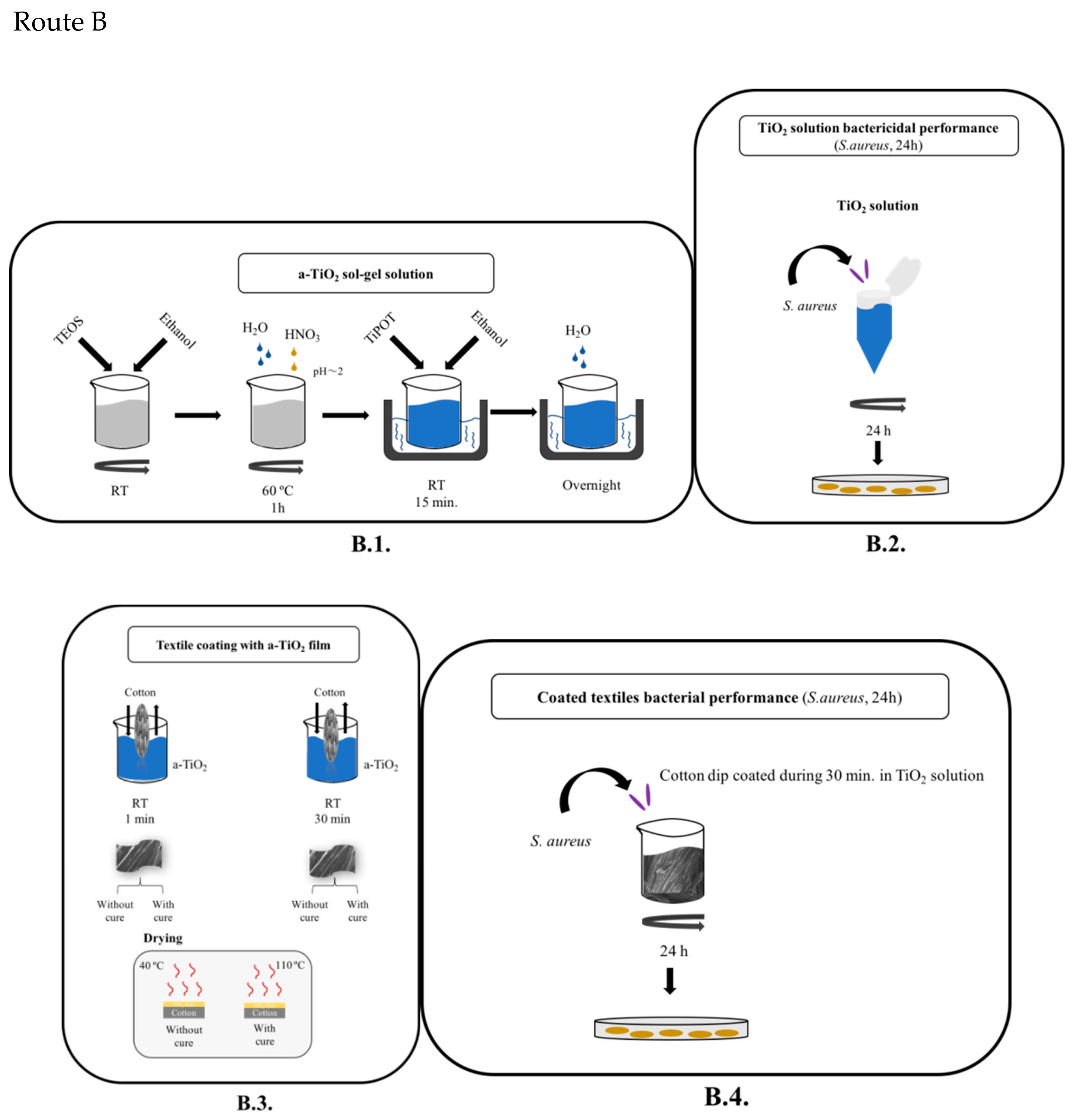
2.2. Experimental Methodology
- Route A (Figure 2, Route A)
- a.
- Novel synthesis of a-TiO2 NPs (room temperature, sol-gel, base-catalyzed, eco-friendly protocol):
- a.1
- pristine a-TiO2 NPs (A.1.)
- a.2
- in situ amine-functionalized amorphous titania (a-TiO2-NH2) NPs (A.1.)
- a.3
- ex situ CS-decorated amorphous titania (a-TiO2//CS) NPs (A.1.)
- b.
- Textile impregnation with a-TiO2 based NPs, to be industrially implemented as a finishing process (A.3.)
- c.
- Daylight bactericidal performance of NPs and impregnated cotton textiles according to ASTM E 2149 and AATCC 100 test methods using S. aureus (ATCC®6538TM) as bacterial indicator strain (A.2., A.4.)
- Route B (Figure 2, Route B)
- d.
- TiO2 (acid-catalyzed) sol-gel solution preparation (B.1.)
- e.
- Textile coating with a-TiO2 film by dip in sol-gel solution, to be industrially implemented as a finishing process (B.3.)
- f.
- Daylight bactericidal performance of films and coated cotton textiles according to ASTM E 2149 and AATCC 100 test methods using S. aureus (ATCC®6538TM) as bacterial indicator strain (B.2., B.4.).
2.2.1. Amorphous Titania Nanoparticles Synthesis
2.2.2. TiO2 Solution Preparation
2.2.3. Textile Impregnation/Coating
- Textile impregnation with amorphous titania-based NPs (a-TiO2, a-TiO2-NH2, and a-TiO2//CS; Figure 2A.3).
- The textile impregnation was performed with 0.5% (in weight) on a cotton textile (according to the Portuguese Standard ISO 105-C06, 1994, see details in the literature [34]). Shortly, 25 mg of a-TiO2 NPs were suspended in 50 mL of distilled water, and were sonicated for 30 min. Then, 5 g of cotton textile (100% cotton) was immersed in the colloidal suspension, under magnetic stirring, at 40 °C for 15 min.
- Textile coating with a titania solution (a-TiO2 film; Figure 2B.3).
- Briefly, the cotton textile was immersed in the TiO2 sol–gel solution (at a constant rate of 2 cm/min) and then pulled up (also at the same rate). An a-TiO2 coating forms, its thickness being determined by the withdrawal rate (being inversely proportional). During the deposition and drainage steps, the solvent (alcohol) and the excess/unreacted reagents (water and/or silica/titania precursors) evaporate.
- The impregnation/coating was performed without cure (woven at 40 °C, for 24 h) and with cure (at 110 °C, for 1h) to enhance the NPs’/films’ adhesion to the cotton textile [34].
2.3. Physical Characterization
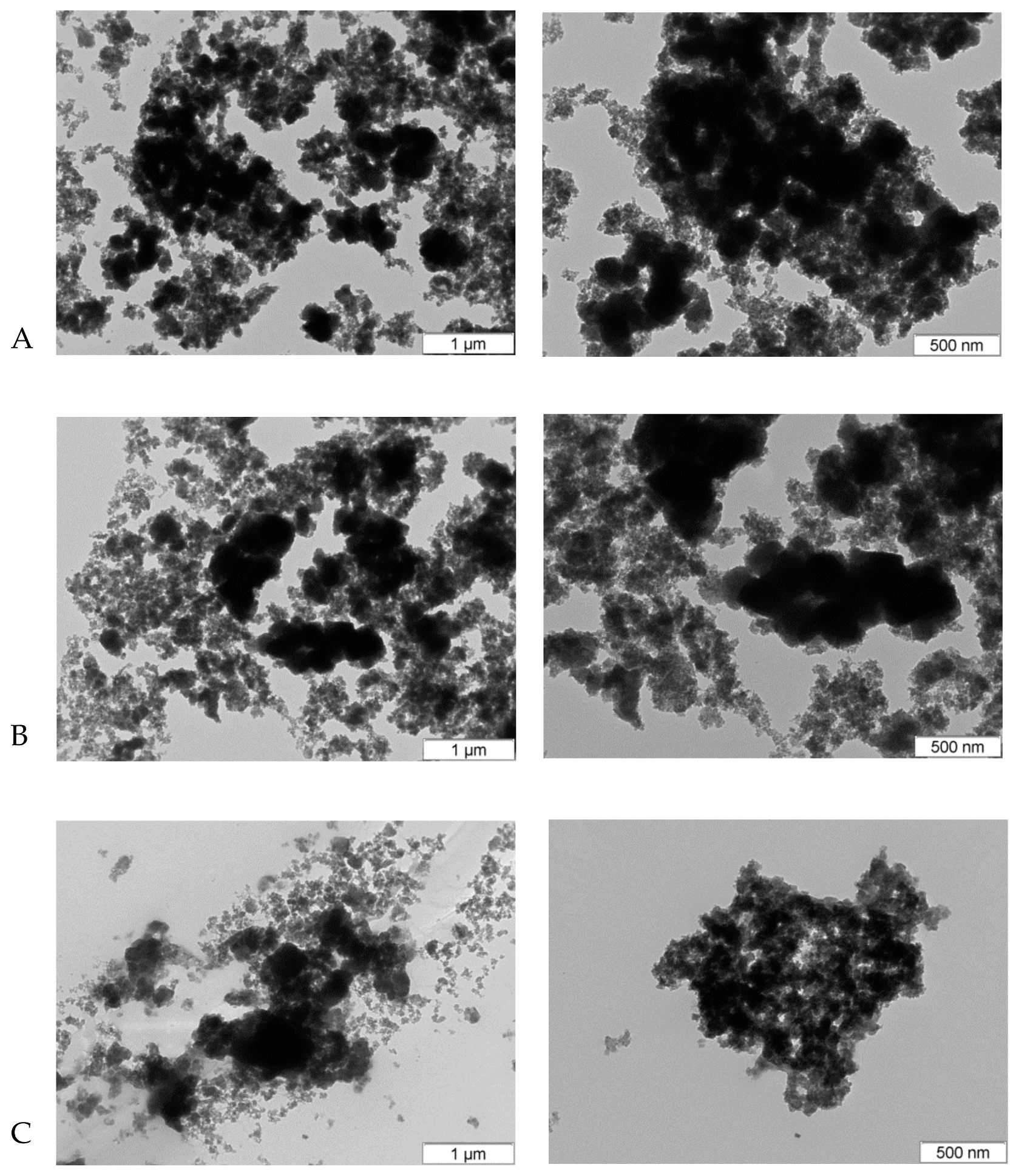
2.3.1. Transmission Electron Microscopy (TEM)
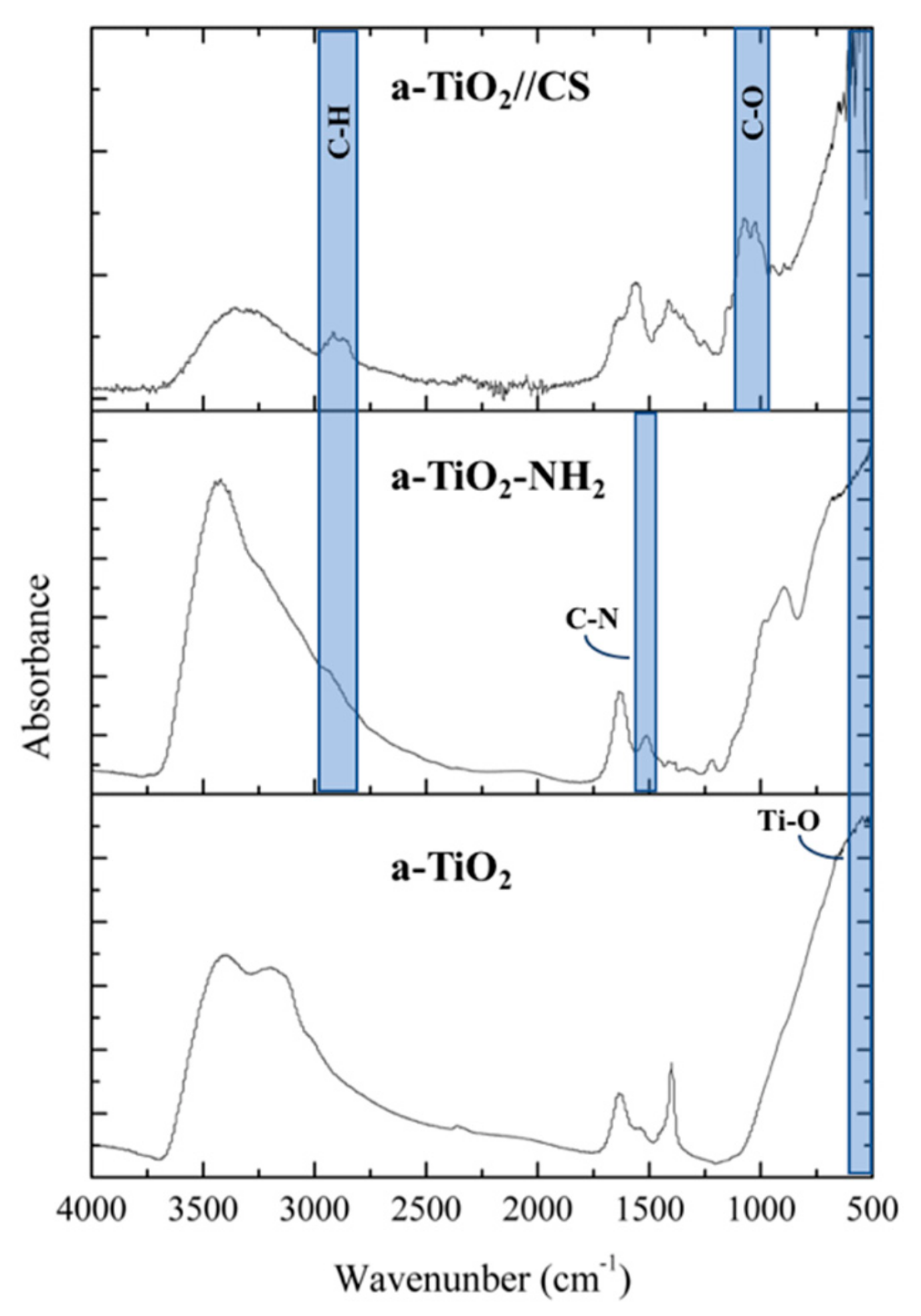
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
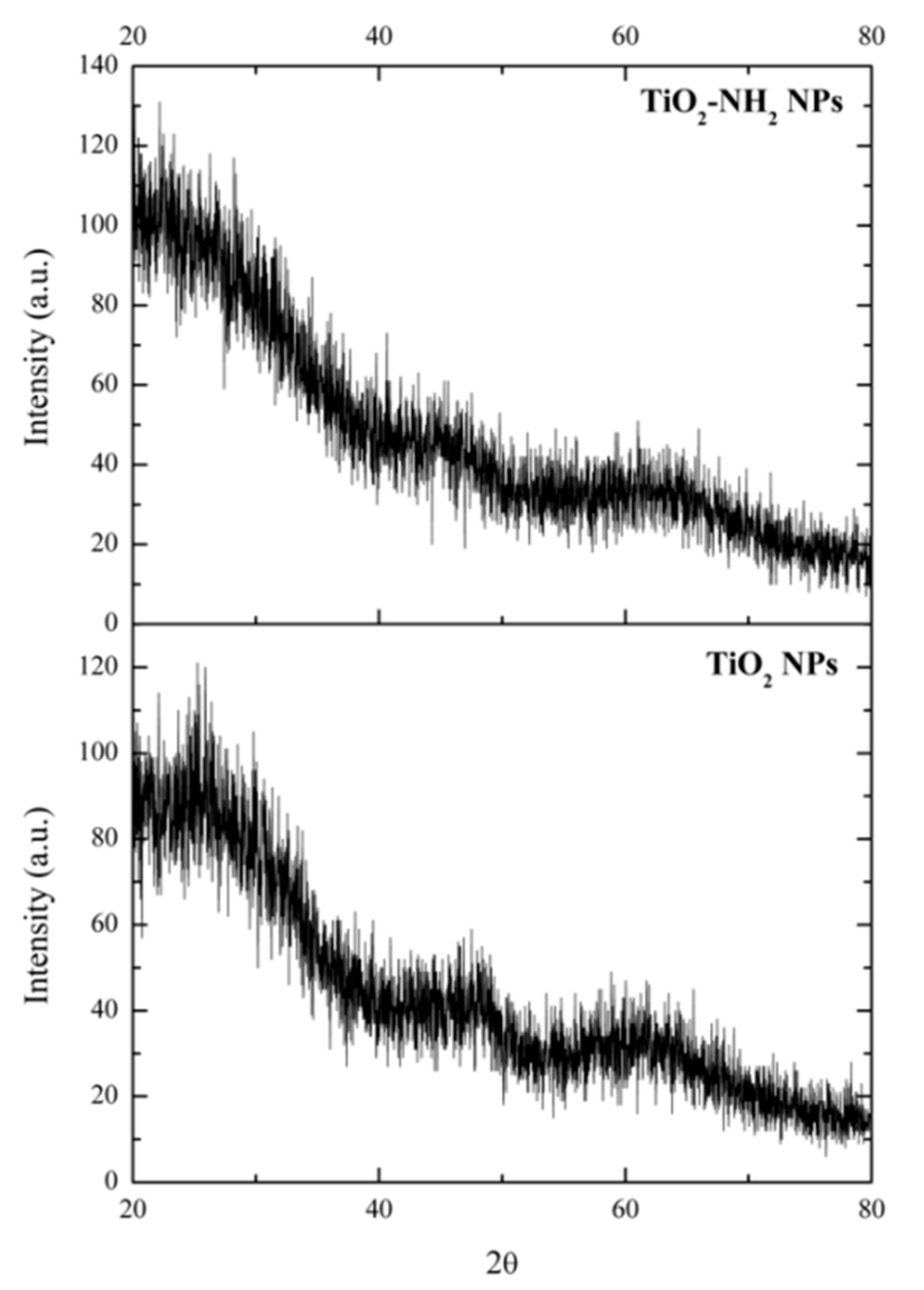
2.3.3. X-ray Powder Diffraction (XRD)
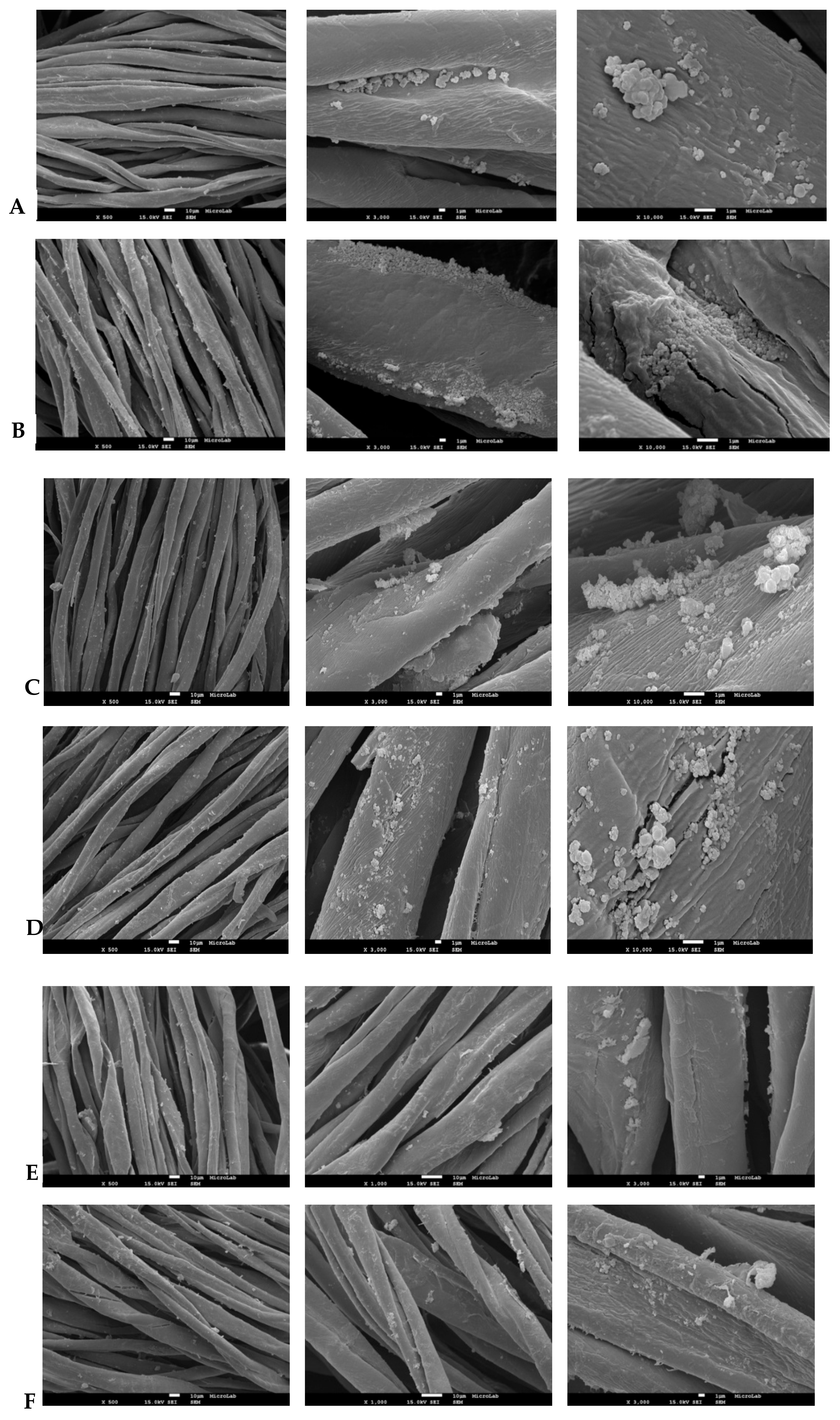
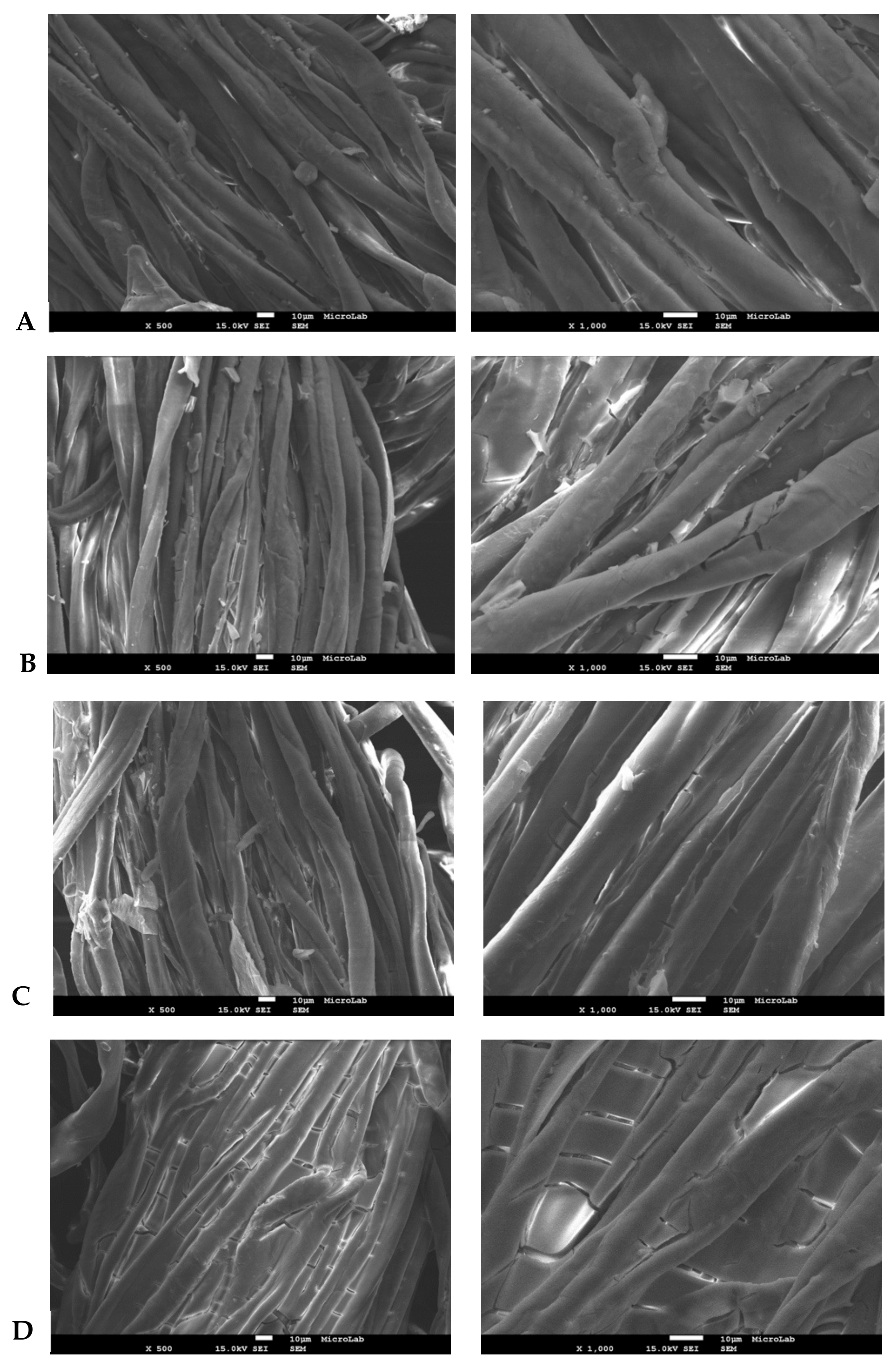
2.3.4. Scanning Electron Microscopy (SEM)
2.4. Daylight Bactericidal Performance Evaluation
3. Results and Discussion
3.1. NPs Characterization
3.2. NPs Textile Impregnation Efficiency
3.3. Film Textile Coating Efficiency
3.4. Daylight Bactericidal Performance of NPs/Films and Impregnated/Coated Textiles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Girard, R.; Perraud, M.; Prüss, A.; Savey, A.; Tikhomirov, E.; Thuriaux, M.; Vanhems, P. Prevention of Hospital-Acquired Infections: A Practical Guide. World Health Organization, 2nd ed.; Ducel, G., Fabry, J., Nicolle, L., Eds.; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- ECDC, European Centre for Disease Prevention and Control, An agency of the European Union, Communicable Disease Threats Reports. Available online: https://ecdc.europa.eu/en/home (accessed on 24 April 2019).
- World Health Organization (WHO). Global Antimicrobial Resistance Surveillance System: Manual for Early Implementation; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Arora, H.; Doty, C.; Yuan, Y.; Boyle, J.; Petras, K.; Rabatic, B.; Paunesku, T.; Woloschak, G. Nanomaterials for the Life Sciences. In Nanocomposites; Kumar, C.S.S.R., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; Volume 8, p. 1. [Google Scholar]
- FDA U.S. Food & Drug Administration. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed on 1 April 2018).
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-Cleaning Applications of TiO2 by Photo-Induced Hydrophilicity and Photocatalysis. Appl. Catal. B 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Pereira, J.C.; Matos, J.; Vasconcelos, H.C. Antimicrobial and Photocatalytic Application of Amorphous Titania: A Paradigma Change. Molecules 2018, 23, 1677. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.G.; Allegri, M.; Chiu, M.; Costa, A.L.; Blosi, M.; Ortelli, S.; Bussolati, O.; Bergamaschi, E. Lipopolysaccharide Adsorbed to the Bio-Corona of TiO2 Nanoparticles Powerfully Activates Selected Pro-Inflammatory Transduction Pathways. Front. Immunol. 2017, 8, 866. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, Z.; Mirzaee, M.; Ejtehadi, M.M.; Mokhtari, M. The Bactericidal Effect of Simultaneous Titanium Oxide on Common Hospital Bacteria. Environ. Monit. Assess. 2017, 189, 342. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A Review of Solar and Visible Light Active TiO2 Photocatalysis for Treating Bacteria, Cyanotoxins and Contaminants of Emerging Concern. Mater. Sci. Semicond. Proc. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Leyland, N.S.; Podporska-Carrol, J.; Browne, J.; Hinder, S.J.; Quilty, B.; Pillai, S.C. Highly Efficient F, Cu Doped TiO2 Anti-Bacterial Visible Light Active Photocatalytic Coatings to Combat Hospital-Acquired Infections. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Valentin, C.D.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatlysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial Activity of Zinc and Titanium Dioxide Nanoparticles Against Biofilm-Producing Methicillin-Resistant Staphylococcus Aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Liou, J.-W.; Chang, H.-H. Bactericidal Effects and Mechanisms of Visible Light-Responsive Titanium Dioxide Photocatalysts on Pathogenic Bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium Oxide Antibacterial Surfaces in Biomedical Devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef]
- Huang, Z.; Blake, D.M.; Maness, P.-C.; Wolfrum, E. Bactericidal Mode of Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. A Chem. 2000, 130, 163–170. [Google Scholar] [CrossRef]
- Raafat, D.; Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and Its Antimicrobial Potential—A Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Deng, Z.; Zhen, Z.; Hu, X.; Wu, S.; Xu, Z.; Chu, P. Hollow Chitosan-Silica Nanospheres as pH-Sensitive Targeted Delivery Carriers in Breast Cancer Therapy. Biomaterials 2011, 32, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and Anti-Inflamatory Activity of Chitosan-Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef]
- Shahidi, S.; Wiener, J. Antibacterial Agents in Textile Industry. In Antimicrobial Agents; Bobbarala, V., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Goyal, N.; Rastogi, D.; Jassal, M.; Agrawal, A.K. Chitosan as a Potential Stabilizing Agent for Titania Nanoparticle Dispersions for Preparation of Multifunctional Cotton Fabric. Carbohydr. Polym. 2016, 10, 167–175. [Google Scholar] [CrossRef]
- Kadib, A.; Molvinger, K.; Guimon, C.; Quignard, F.; Brunel, D. Design of Stable Nanoporous Hybrid Chitosan/Titania as Cooperative Bifunctional Catalysts. Chem. Mater. 2008, 20, 2198–2204. [Google Scholar] [CrossRef]
- Al-Sagheer, F.A.; Merchant, S. Visco-Elastic Properties of Chitosan–Titania Nano-Composites. Carbohydr. Polym. 2011, 85, 356–362. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, J.; Liu, X.; Huband, Q.; Huang, J. Antibacterial Hybrid Materials Fabricated Bynanocoating of Microfibril Bundles of Cellulosesubstance with Titania/Chitosan/Silver-Nanoparticle Composite Films. J. Mater. Chem. B 2013, 1, 3477. [Google Scholar] [CrossRef]
- Kavitha, K.; Prabhu, M.; Rajendran, V.; Manivasankan, P.; Prabu, P.; Jayakumar, T. Optimization of Nano-Titania and Titania–Chitosan Nanocomposite to Enhance Biocompatibility. Curr. Nanosci. 2013, 9. [Google Scholar] [CrossRef]
- Seco, A.M.; Gonçalves, M.C.; Almeida, R.M. Densification of Hybrid Silica–Titania Sol–Gel Films Studied by Ellipsometry and FTIR. Mater. Sci. Eng. B 2000, 76, 193–199. [Google Scholar] [CrossRef]
- Matos, J.C.; Avelar, I.; Martins, B.; Gonçalves, M.C. Silica Vectors for Smart Textiles. Carbohydr. Polym. 2017, 120, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C. Nanomaterials. In Materials for Construction and Civil Engineering: Science, Processing, and Design; Gonçalves, M.C., Margarido, F., Eds.; Springer: New York, NY, USA, 2015; pp. 629–677. [Google Scholar]
- Murashkevich, A.N.; Lavitskaya, A.S.; Barannikova, T.I.; Zharskii, I.M. Infrared Absorption Pectra and Structure of TiO2-SiO2 Composites. J. Appl. Spectrosc. 2008, 75, 730. [Google Scholar] [CrossRef]
- Mugundan, S.; Rajamannan, B.; Viruthagiri, G.; Shanmugam, N.; Gobi, R.; Praveen, P. Synthesis and Characterization of Undoped and Cobalt-Doped TiO2 Nanoparticles Via Sol–Gel Technique. Appl. Nanosci. 2015, 5, 449–456. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Luminescence Characteristics of Cobalt Doped TiO2 Nanoparticles. J. Lumin. 2012, 132, 178–184. [Google Scholar] [CrossRef]
- Lacey, K.A.; Geoghegan, J.A.; McLoughlin, R.M. The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens. Pathogens 2016, 5, 22. [Google Scholar] [CrossRef]
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Al Othman, Z.A.; Alam, M.M.; Naushad, M.; Khan, M.F. Inorganic Nanoparticles and Nanomaterials Based on Titanium (Ti): Applications in Medicine. Mater. Sci. Forum 2013, 754, 21–87. [Google Scholar] [CrossRef]
- Arain, R.A.; Khatri, Z.; Memon, M.H.; Kim, I.S. Antibacterial Property and Characterization of Cotton Fabric Treated with Chitosan/AgCl-TiO2 Colloid. Carbohydr. Polym. 2013, 96, 326–331. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]

| Acronym | Precursors | VTiPOT:VPrec(μL) | Catalyst |
|---|---|---|---|
| a-TiO2 NPs | TiPOT | 500 | Hydrothermal water pH ≈ 11 |
| a-TiO2-NH2 NPs | TiPOT:APTES 8:2 | TiPOT: 417 APTES: 83 | Hydrothermal water pH ≈ 11 |
| a-TiO2 film | TiPOT | 830 | Nitric acid pH ≈ 2 |
| Acronym | Decoration | NPs:Decoration amount | |
| a-TiO2/CS NPs | Chitosan | 10 mg NPs + 25 mg CS | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, J.C.; Oliveira, C.; Gonçalves, M.C. Daylight Bactericidal Titania Textiles: A Contribution to Nosocomial Infections Control. Molecules 2019, 24, 1891. https://doi.org/10.3390/molecules24101891
Matos JC, Oliveira C, Gonçalves MC. Daylight Bactericidal Titania Textiles: A Contribution to Nosocomial Infections Control. Molecules. 2019; 24(10):1891. https://doi.org/10.3390/molecules24101891
Chicago/Turabian StyleMatos, Joana C., Cláudia Oliveira, and M. Clara Gonçalves. 2019. "Daylight Bactericidal Titania Textiles: A Contribution to Nosocomial Infections Control" Molecules 24, no. 10: 1891. https://doi.org/10.3390/molecules24101891
APA StyleMatos, J. C., Oliveira, C., & Gonçalves, M. C. (2019). Daylight Bactericidal Titania Textiles: A Contribution to Nosocomial Infections Control. Molecules, 24(10), 1891. https://doi.org/10.3390/molecules24101891






