Rhodium(III)-Catalyzed [4+2] Annulation via C-H Activation: Synthesis of Multi-Substituted Naphthalenone Sulfoxonium Ylides
Abstract
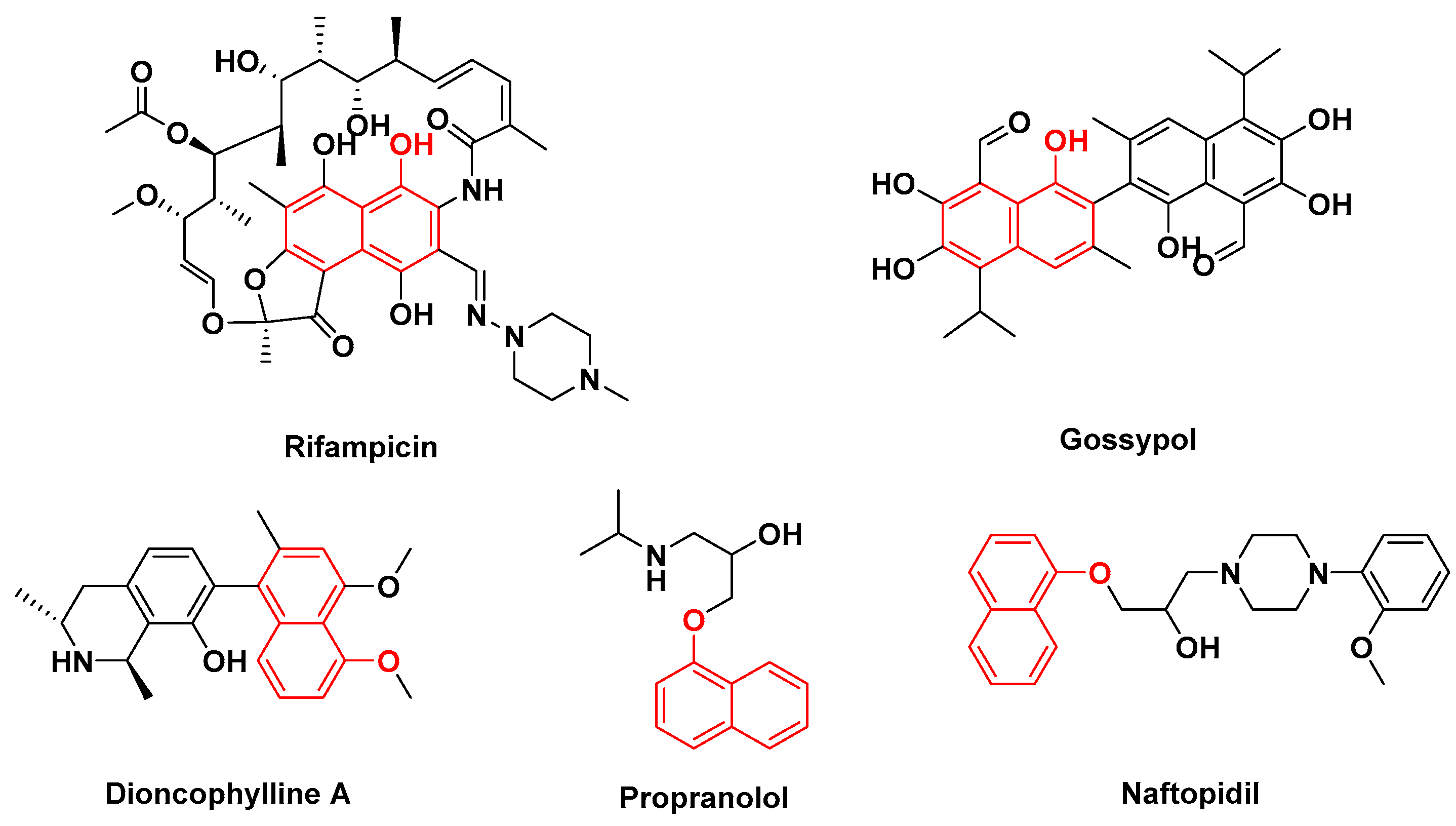
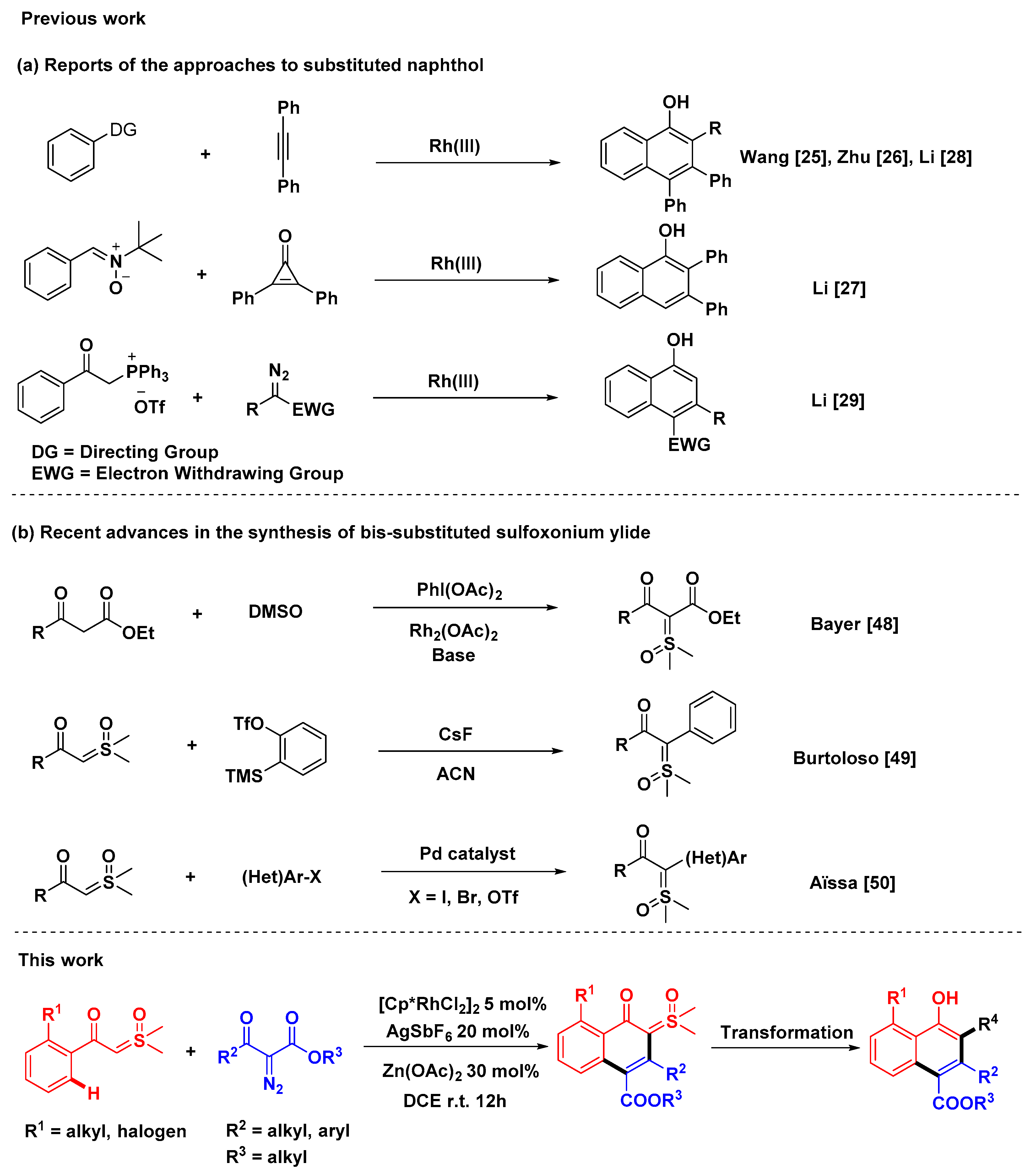
1. Introduction
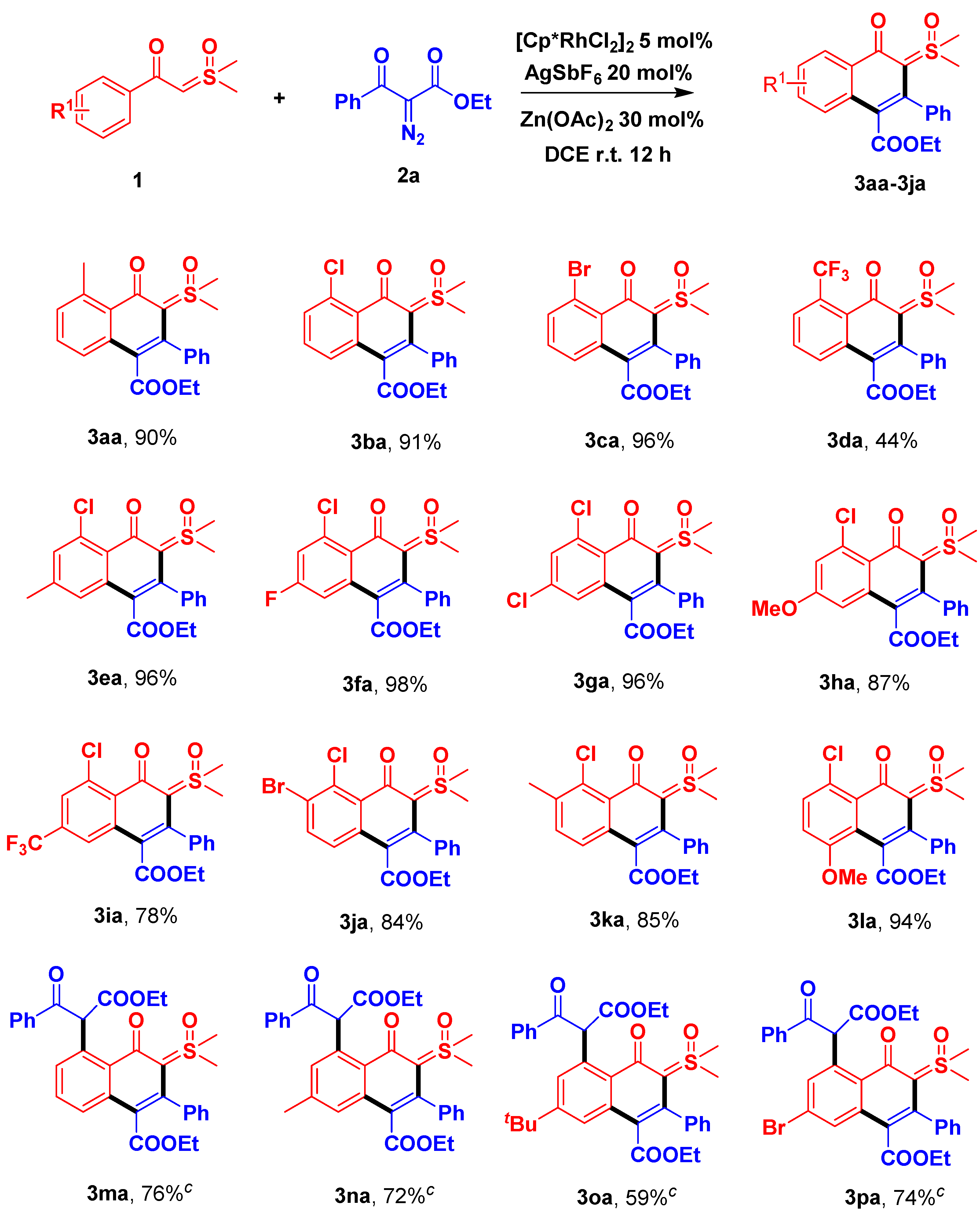
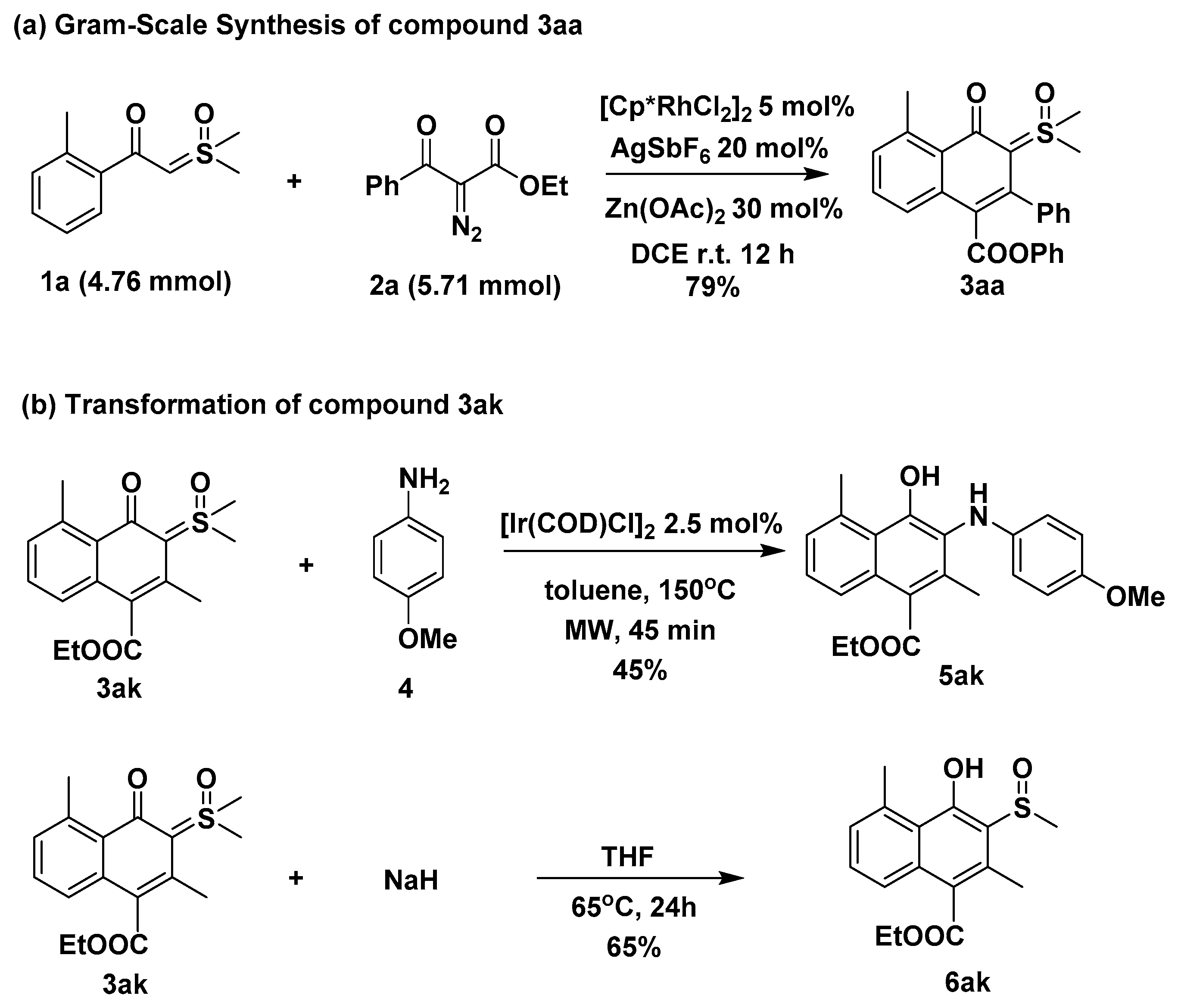
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Experimental Part Method
3.2.1. General Procedure for the Preparation of Sulfoxonium Ylides 1a–1p
3.2.2. General Procedure for the Preparation of α-Diazocarbonyl Compounds 2a–2n
3.2.3. General Procedures for the Products 3aa–3la, 3ab–3an (Compound 3aa as the Example)
3.2.4. General Procedures for the Products 3ma–3pa (Compound 3ma as the Example)
3.2.5. Gram-Scale Synthesis of Compound 3aa
3.2.6. Synthesis of Compound 5ak
3.2.7. Synthesis of Compound 6ak
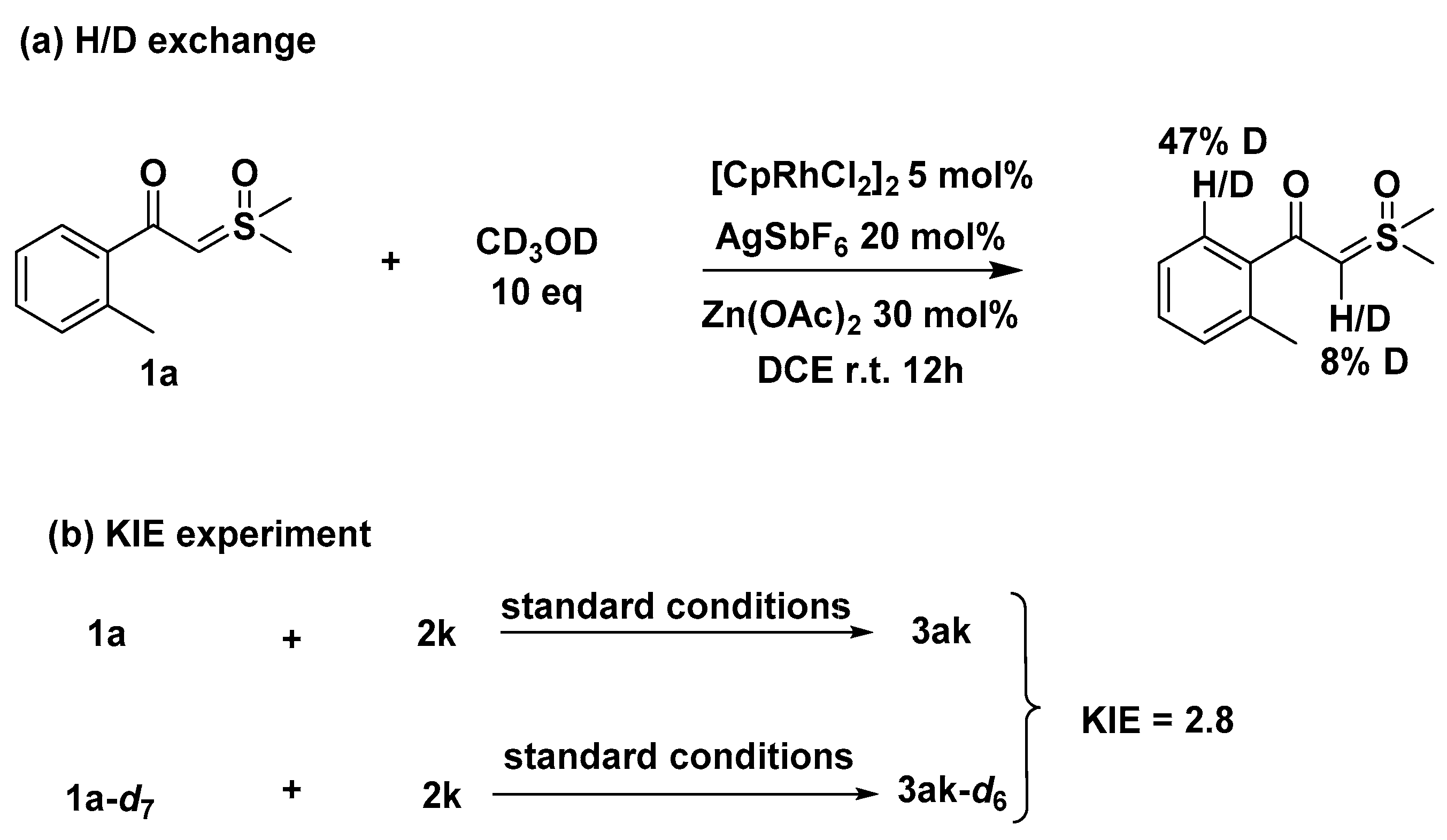
3.2.8. Mechanistic Studies
3.3. Product Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Accession Codes
Funding
Conflicts of Interest
References
- Radner, D.B. Toxicologic and Pharmacologic Aspects of Rifampin. Chest 1973, 64, 213–216. [Google Scholar] [CrossRef]
- Rzepecki, W.M.; Lodziak, A. Pre- and Postoperative Treatment of Chronic Multiresistant Cases of Pulmonary Tuberculosis with Rifampicin. Chemotherapy 1974, 20, 120–124. [Google Scholar] [CrossRef]
- Jean-Baptiste, E.; Blanchemain, N.; Neut, C.; Chai, F.; Maton, M.; Martel, B.; Hildebrand, H.; Haulon, S. Evaluation of the anti-infectious properties of polyester vascular prostheses functionalised with cyclodextrinS. J. Infect. 2014, 68, 116–124. [Google Scholar] [CrossRef]
- Lin, T.S.; Schinazi, R.; Griffith, B.P.; August, E.M.; Eriksson, B.H.F.; Zheng, D.K.; Prusoff, W.H. Selective inhibition of human immunodeficiency virus type 1 replication by the (-) but not the (+) enantiomer of gossypol. Antimicrob. Agents Chemother 1989, 33, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.I.; Willemsen, J.J. The synthesis of (S)-(+)-gossypol via an asymmetric Ullmann coupling. Chem. Commun. 1997, 1573–1574. [Google Scholar] [CrossRef]
- Shelley, M.D.; Hartley, L.; Groundwater, P.W.; Fish, R.G. Structure-activity studies on gossypol in tumor cell lines. Anti-Cancer Drug 2000, 11, 209–216. [Google Scholar] [CrossRef]
- Bringmann, G.; Gramatzki, S.; Grimm, C.; Proksch, P. Feeding deterrency and growth retarding activity of the naphthylisoquinoline alkaloid dioncophylline A against Spodoptera littoralis. Phytochemistry 1992, 31, 3821–3825. [Google Scholar] [CrossRef]
- Bringmann, G.; Saeb, W.; Koppler, D.; Francois, G. Jozimine A (‘dimeric’ dioncophylline A), a non-natural michellamine analog with high antimalarial activity. Tetrahedron 1996, 52, 13409–13418. [Google Scholar] [CrossRef]
- Bringmann, G.; Holenz, J.; Wiesen, B.; Nugroho, B.W.; Proksch, P. Dioncophylline A as a Growth-Retarding Agent against the Herbivorous Insect Spodoptera littoralis: Structure−Activity Relationships. J. Nat. Prod. 1997, 60, 342–347. [Google Scholar] [CrossRef]
- Black, J.W.; Duncan, W.A.M.; Shanks, R.G. Comparison of some properties of pronethalol and propranolol. Brit. J. Pharmacol. 1965, 25, 577–591. [Google Scholar] [CrossRef]
- Kornfeld, E.C. Chapter 6. Antihypertensive Agents. Annu. Rep. Med. Chem. 1966, 59–66. [Google Scholar]
- Crowther, A.F.; Smith, L.H. Beta-Adrenergic blocking agents. II. Propranolol and related 3-amino-1-naphthoxy-2-propanols. J. Med. Chem. 1968, 11, 1009–1013. [Google Scholar] [CrossRef]
- Schwender, C.F.; Farber, S.; Blaum, C.; Shavel, J. Derivatives of 3,4-dihydro-1(2H)-naphthalenone as beta.-adrenergic blocking agents. 1. Bunolol and related analogs. J. Med. Chem. 1970, 13, 684–688. [Google Scholar] [CrossRef]
- Ikunobu, M.; Kyozo, Y.; Shigeru, K. Pharmacological Profile of the Novel α-Adrenoceptor Antagonist KT-611 (Naftopidil). Jpn. J. Pharmacol. 1991, 55, 391–398. [Google Scholar]
- Farthing, M.J.E.; Alstead, M.; Abrams, S.M.; Haug, G.; Johnston, A.; Hermann, R.; Hermann, G.; Ruus, P.; Molz, K.H.; Turner, P. Pharmacokinetics of naftopidil, a novel anti-hypertensive drug, in patients with hepatic dysfunction. Posgrad. Med. J. 1994, 70, 363–366. [Google Scholar] [CrossRef][Green Version]
- Violetta, C.; Fausto, S.; Oriana, T.; Arnaldo, F. (1,4-Benzothiazinyloxy) alkylpiperazine derivatives as potential antihypertensive agents. Bioorg. Med. Chem. Lett. 2000, 10, 465–468. [Google Scholar]
- de Koning, C.B.; Rousseau, A.L.; van Otterlo, W.A. Modern methods for the synthesis of substituted naphthalenes. Tetrahedron 2003, 59, 7–36. [Google Scholar] [CrossRef]
- Mulrooney, C.A.; Li, X.; DiVirgilio, E.S.; Kozlowski, M.C. General Approach for the Synthesis of Chiral Perylenequinones via Catalytic Enantioselective Oxidative Biaryl Coupling. J. Am. Chem. Soc. 2003, 125, 6856–6857. [Google Scholar] [CrossRef]
- Dohi, T.; Takenaga, N.; Nakae, T.; Toyoda, Y.; Yamasaki, M.; Shiro, M.; Fujioka, H.; Maruyama, A.; Kita, Y. Asymmetric Dearomatizing Spirolactonization of Naphthols Catalyzed by Spirobiindane-Based Chiral Hypervalent Iodine Species. J. Am. Chem. Soc. 2013, 135, 4558–4566. [Google Scholar] [CrossRef]
- Pu, L. Asymmetric Functional Organozinc Additions to Aldehydes Catalyzed by 1,1′-Bi-2-naphthols (BINOLs). Acc. Chem. Res. 2014, 47, 1523–1535. [Google Scholar] [CrossRef]
- Patureau, F.W.; Besset, T.; Kuhl, N.; Glorius, F. Diverse Strategies toward Indenol and Fulvene Derivatives: Rh-Catalyzed C−H Activation of Aryl Ketones Followed by Coupling with Internal Alkynes. J. Am. Chem. Soc. 2011, 133, 2154–2156. [Google Scholar] [CrossRef]
- Ackermann, L. Carboxylate-Assisted Ruthenium-Catalyzed Alkyne Annulations by C–H/Het–H Bond Functionalizations. Acc. Chem. Res. 2014, 47, 281–295. [Google Scholar] [CrossRef]
- Zhu, R.; Farmer, M.E.; Chen, Y.; Yu, J. A Simple and Versatile Amide Directing Group for C−H Functionalizations. Angew. Chem. Int. Ed. 2016, 55, 10578–10599. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, S.; Lan, Y.; Wan, B.; Li, X. Rhodium-Catalyzed C–H Activation of Phenacyl Ammonium Salts Assisted by an Oxidizing C–N Bond: A Combination of Experimental and Theoretical Studies. J. Am. Chem. Soc. 2015, 137, 1623–1631. [Google Scholar] [CrossRef]
- Tan, X.; Liu, B.; Li, X.; Li, B.; Xu, S.; Song, H.; Wang, B. Rhodium-Catalyzed Cascade Oxidative Annulation Leading to Substituted Naphtho[1,8-bc]pyrans by Sequential Cleavage of C(sp2)–H/C(sp3)–H and C(sp2)–H/O–H Bonds. J. Am. Chem. Soc. 2012, 134, 16163–16166. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, J.; Wang, L.; Song, C.; Chen, K.; Zhu, J. Enaminones as Synthons for a Directed C−H Functionalization: RhIII-Catalyzed Synthesis of Naphthalenes. Angew. Chem. Int. Ed. 2016, 55, 9384–9388. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Zhou, X.; Kong, L.; Li, X. Rhodium(III)-Catalyzed Synthesis of Naphthols via C–H Activation of Sulfoxonium Ylides. Org. Lett. 2017, 19, 4307–4310. [Google Scholar] [CrossRef]
- Xie, F.; Yu, S.; Qi, Z.; Li, X. Nitrone Directing Groups in Rhodium(III)-Catalyzed C−H Activation of Arenes: 1,3-Dipoles versus Traceless Directing Groups. Angew. Chem. Int. Ed. 2016, 55, 15351–15355. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Yang, X.; Xie, F.; Li, X. Divergent Access to 1-Naphthols and Isocoumarins via Rh(III)-Catalyzed C–H Activation Assisted by Phosphonium Ylide. Org. Lett. 2017, 19, 3410. [Google Scholar] [CrossRef]
- Barday, M.; Janot, C.; Halcovitch, N.R.; Muir, J.; Aissa, C. Cross-Coupling of α-Carbonyl Sulfoxonium Ylides with C−H Bonds. Angew. Chem. Int. Ed. 2017, 56, 13117–13121. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Vaitla, J. Synthesis Sulfoxonium Ylide Derived Metal Carbenoids in Organic. Synthesis 2018, 51, 612–628. [Google Scholar]
- Wu, X.; Sun, S.; Yu, J.; Cheng, J. Recent Applications of α-Carbonyl Sulfoxonium Ylides in Rhodium- and Iridium-Catalyzed C–H Functionalizations. Synlett. 2018, 30, 21–29. [Google Scholar]
- Mangion, I.K.; Ruck, R.T.; Rivera, N.; Huffman, M.A.; Shevlin, M. A Concise Synthesis of a β-Lactamase Inhibitor. Org. Lett. 2011, 13, 5480–5483. [Google Scholar] [CrossRef]
- Molinaro, C.; Bulger, P.G.; Lee, E.E.; Kosjek, B.; Lau, S.; Gauvreau, D.; Howard, M.E.; Wallace, D.J.; O’Shea, P.D. CRTH2 Antagonist MK-7246: A Synthetic Evolution from Discovery through Development. J. Org. Chem. 2012, 77, 2299–2309. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, X.; Zheng, G.; Li, X. Sulfoxonium Ylides as a Carbene Precursor in Rh(III)-Catalyzed C–H Acylmethylation of Arenes. Org. Lett. 2017, 19, 5256–5259. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cui, S. Rh(III)-Catalyzed C–H Activation/Cyclization of Benzamides and Diazonaphthalen-2(1H)-ones for Synthesis of Lactones. Org. Lett. 2017, 19, 4002–4005. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, B.; Li, X. Facile construction of hydrogenated azepino[3,2,1-hi]indoles by Rh(III)-catalyzed C–H activation/[5+2] annulation of N-cyanoacetylindolines with sulfoxonium ylides. Org. Chem. Front. 2018, 5, 3263–3266. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiong, H.; Sun, S.; Yu, J.; Cheng, J. Rh(III)-Catalyzed dual C–H functionalization of 3-(1H-indol-3-yl)-3-oxopropanenitriles with sulfoxonium ylides or diazo compounds toward polysubstituted carbazoles. Org. Biomol. Chem. 2018, 16, 8715–8718. [Google Scholar] [CrossRef]
- Zheng, G.; Tian, M.; Xu, Y.; Chen, X.; Li, X. Rhodium(III)-catalyzed annulative coupling between arenes and sulfoxonium ylides via C–H activation. Org. Chem. Front. 2018, 5, 998–1002. [Google Scholar] [CrossRef]
- Halskov, K.S.; Witten, M.R.; Hoang, G.L.; Mercado, B.Q.; Ellman, J.A. Rhodium(III)-Catalyzed Imidoyl C–H Activation for Annulations to Azolopyrimidines. Org. Lett. 2018, 20, 2464–2467. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Xu, Y.; Yang, S.; Liu, B.; Li, X. Construction of (Dihydro)naphtho[1,8-bc]pyrans via Rh(III)-Catalyzed Twofold C–H Activation of Benzoylacetonitriles. Org. Lett. 2018, 20, 2160–2163. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, H.; Sun, S.; Cheng, J. Rhodium-Catalyzed Relay Carbenoid Functionalization of Aromatic C–H Bonds toward Fused Heteroarenes. Org. Lett. 2018, 20, 1396–1399. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, G.; Yang, X.; Li, X. Rhodium(III)-catalyzed chemodivergent annulations between N-methoxybenzamides and sulfoxonium ylides via C–H activation. Chem. Commun. 2018, 54, 670–673. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, S.; Cheng, J. Rh(III)-catalyzed [4+1]-annulation of azobenzenes with α- carbonyl sulfoxonium ylides toward 3-acyl-(2H)-indazoles. Tetrahedron Lett. 2018, 59, 2284–2287. [Google Scholar] [CrossRef]
- Ji, S.; Yan, K.; Li, B.; Wang, B. Cp*Co(III)-Catalyzed C–H Acylmethylation of Arenes by Employing Sulfoxonium Ylides as Carbene Precursors. Org. Lett. 2018, 20, 5981–5984. [Google Scholar] [CrossRef]
- Cui, X.F.; Ban, Z.H.; Tian, W.F.; Hu, F.P.; Zhou, X.Q.; Ma, H.J.; Zhan, Z.Z.; Huang, G.S. Ruthenium-catalyzed synthesis of indole derivatives from N-aryl-2-aminopyridines and alpha-carbonyl sulfoxonium ylides. Org. Biomol. Chem. 2019, 17, 240–243. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, J.; He, G.; Li, H.; Yang, Q.; Wang, R.; Zhou, Y.; Liu, H. Ruthenium(II)-catalyzed selective C–H bond activation of imidamides and coupling with sulfoxonium ylides: An efficient approach for the synthesis of highly functional 3-ketoindoles. Org. Chem. Front. 2019, 6, 1183–1188. [Google Scholar] [CrossRef]
- Vaitla, J.; Hopmann, K.H.; Bayer, A. Rhodium-Catalyzed Synthesis of Sulfur Ylides via in Situ Generated Iodonium Ylides. Org. Lett. 2017, 19, 6688–6691. [Google Scholar] [CrossRef]
- Talero, A.G.; Martins, B.S.; Burtoloso, A.C.B. Coupling of Sulfoxonium Ylides with Arynes: A Direct Synthesis of Pro-Chiral Aryl Ketosulfoxonium Ylides and Its Application in the Preparation of α-Aryl Ketones. Org. Lett. 2018, 20, 7206–7211. [Google Scholar] [CrossRef]
- Janot, C.; Palamini, P.; Dobson, B.C.; Muir, J.; Aissa, C. Palladium-Catalyzed Synthesis of Bis-Substituted Sulfoxonium Ylides. Org. Lett. 2018, 21, 296–299. [Google Scholar] [CrossRef]
- Jia, Q.; Kong, L.; Li, X. Cobalt(III)-catalyzed C–H amidation of weakly coordinating sulfoxonium ylides and α-benzoylketene dithioacetals. Org. Chem. Front. 2019, 6, 741–745. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Zhang, X.; Fan, X. Rh(III)-Catalyzed Cascade Reactions of Sulfoxonium Ylides with α-Diazocarbonyl Compounds: An Access to Highly Functionalized Naphthalenones. Org. Lett. 2019, 21, 2541–2545. [Google Scholar] [CrossRef]
- Alhamadsheh, M.M.; Waters, N.C.; Sachdeva, S.; Lee, P.; Reynolds, K.A. Synthesis and biological evaluation of novel sulfonyl-naphthalene-1,4-diols as FabH inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 6405–6420. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Entry | Catalyst | Additive | Ag Salt | Solvent | Yield b |
|---|---|---|---|---|---|
| 1 | [RuCl2(p-cymene)]2 | - | AgSbF6 | DCE d | N.R. f |
| 2 | Cp*C°COI2 | - | AgSbF6 | DCE | N.R. |
| 3 | [Cp*IrCl2]2 c | - | AgSbF6 | DCE | N.R. |
| 4 | [Cp*RhCl2]2 | - | AgSbF6 | DCE | 65% |
| 5 | [Cp*RhCl2]2 | PivOH | AgSbF6 | DCE | 77% |
| 6 | [Cp*RhCl2]2 | CsOAc | AgSbF6 | DCE | N.R. |
| 7 | [Cp*RhCl2]2 | Zn(OTf)2 | AgSbF6 | DCE | N.R. |
| 8 | [Cp*RhCl2]2 | Cu(OAc)2 | AgSbF6 | DCE | 65% |
| 9 | [Cp*RhCl2]2 | Zn(OAc)2 | AgSbF6 | DCE | 95% (90% g) |
| 10 | [Cp*RhCl2]2 | Zn(OAc)2 | AgSbF6 | DCE | 48% |
| 11 | [Cp*RhCl2]2 | Zn(OAc)2 | AgSbF6 | TFE e | 85% |
| 12 | [Cp*RhCl2]2 | Zn(OAc)2 | AgSbF6 | MeOH | 14% |
| 13 | [Cp*RhCl2]2 | Zn(OAc)2 | AgSbF6 | MeCN | trace |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Han, X.; Zhang, R.; Liu, H.; Wang, J. Rhodium(III)-Catalyzed [4+2] Annulation via C-H Activation: Synthesis of Multi-Substituted Naphthalenone Sulfoxonium Ylides. Molecules 2019, 24, 1884. https://doi.org/10.3390/molecules24101884
Song X, Han X, Zhang R, Liu H, Wang J. Rhodium(III)-Catalyzed [4+2] Annulation via C-H Activation: Synthesis of Multi-Substituted Naphthalenone Sulfoxonium Ylides. Molecules. 2019; 24(10):1884. https://doi.org/10.3390/molecules24101884
Chicago/Turabian StyleSong, Xiaohan, Xu Han, Rui Zhang, Hong Liu, and Jiang Wang. 2019. "Rhodium(III)-Catalyzed [4+2] Annulation via C-H Activation: Synthesis of Multi-Substituted Naphthalenone Sulfoxonium Ylides" Molecules 24, no. 10: 1884. https://doi.org/10.3390/molecules24101884
APA StyleSong, X., Han, X., Zhang, R., Liu, H., & Wang, J. (2019). Rhodium(III)-Catalyzed [4+2] Annulation via C-H Activation: Synthesis of Multi-Substituted Naphthalenone Sulfoxonium Ylides. Molecules, 24(10), 1884. https://doi.org/10.3390/molecules24101884









