Abstract
We present two as-synthesized Fe(II)-based molecular catalysts with 1,10-phenanthroline (phen) ligands; Fe(phen)3Cl2 (1) and [Fe(phen)2(CH3CH2OH)Cl]Cl (2), and their robust catalytic properties for the conversion of CO2 to CO in DMF/TEOA (DMF = N,N’-dimethylformamide; TEOA = triethanolamine) solution containing Ru(bpy)32+ and BIH (1,3-dimethyl-2-phenyl-2,3- dihydro-1H-benzo-[d]-imidazole). High turnover numbers (TONs) of 19,376 were achieved with turnover frequencies (TOFs) of 3.07 s−1 for complex 1 (1.5 × 10−7 M). A quantum efficiency of 0.38% was observed after 5 h irradiated by 450 nm monochromatic light. The generation rate of CO2 and H2 were tuned by optimizing the experimental conditions, resulting in a high CO selectivity of 90%. The remarkable contribution of the photosensitizer to the total TONCO was found being 19.2% (as shown by tests under similar conditions without catalysts) when BIH was employed as a sacrificial electron donor. The product selectivity in complex 2 reached 95%, and the corresponding TONCO and TOFCO were 33,167 and 4.61 s−1 in the same concentration with complex 1 used as catalyst; respectively. This work provides guidance for future designs of simple, highly efficient and selective molecular catalytic systems that facilitate carbon-neutral solar-to-fuel conversion processes
1. Introduction
Solar-light-driven reduction of CO2 into energy-rich carbon-based products has attracted wide attention, with specific importance attached to the development of highly efficient catalysts and the establishing new photocataytic systems [1,2]. However, activation and conversion of CO2 into desirable fuel or feedstock chemicals driven by visible light remains a significant challenge [3,4,5,6,7]. One way to promote the transformations is to employ heterogeneous [8,9,10] or homogeneous catalyst [11,12,13]. In 1986, Lehn and co-workers reported the photocatalytic reduction of CO2 with Co polypyridine complex as the catalyst in a homogeneous system [14]. Whereafter, a series of Ru(II)-Re(I) binuclear complexes linked by bridging ligands were then studied by Ishitani’s group, and the TON of photocatalytic reduction of CO2 to CO was increased from previously reported 170 to 232 [15,16]. Nevertheless, it is economic and popular to utilize the noble-metal-free complexes as catalysts rather than noble metal. In the regard, homogeneous catalytic systems with excellent potential in visible-light-driven reduction of CO2 to CO [17,18,19] were developed using Fe [9,20,21,22], Co [23,24,25], Ni [26,27,28], Cu [29,30,31] and Mn [32,33,34] complexes as catalysts.
A stunning homogeneous molecular system containing nickel N-heterocyclic carbene-isoquinoline complex employed as catalyst, iridium complex as photosensitizer (PS) towards photocatalytic transformation of CO2 to CO was successfully established [35]. This system exhibited a high photocatalytic performance of CO2 to CO with TONs up to 98,000 and a TOF reaching 3.9 s−1 for a very low concentration of the catalyst, implying high catalytic sensitivity to the CO2 reduction reaction. Recently, Lau and co-workers reported another highly efficient and selective system that consists of Ru(bpy)32+, Fe(II) or Co(II) quaterpyridine complex in the presence of two electron donors; BIH, the higher TONCO than 3000 with up to 95% selectivity was observed for the iron catalyst [36]. Due to rare characteristics of Ru and Ir, the homogeneous system involving earth-abundant metal complexes for photocatalytic reduction of CO2 was then constructed by Ishitani’s group using Cu(I) Cu(dmp)(P)2+ (dmp = 2,9-dimethyl-1,10-phenanthroline; P = phosphine ligand) as the PS and [Fe(dmp)2(NCS)2]2+ as the catalyst [37]. The TONCO of 273 and quantum yield of 6.7% were observed with selectivity of the CO up to 70.5%. Among molecular catalysts for the CO2-to-CO conversion, a macrocyclic amine cryptate dinuclear cobalt complex has been shown to be robust, using Ru(phen)32+ as the PS, the TON reached 16,896 with a quantum efficiency of 0.04% [38].
Herein, we report a highly efficient and selective homogeneous visible-light-driven catalytic system for CO2-to-CO conversion. This includes as-synthesized Fe-based complexes employed as the catalysts, Ru(II) complex as the PS and BIH as the electron donor in DMF/TEOA (Scheme 1). An investigation into catalytic efficiency and selectivity between CO and H2 was performed for various concentrations of catalyst, PS and BIH, and different ratios of DMF/TEOA. The corresponding mechanism was also suggested.
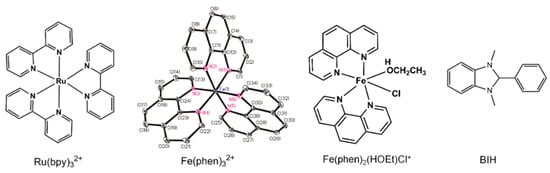
Scheme 1.
Structures of the photosensitizer, catalysts, and BIH.
2. Results and Discussion
2.1. Characterization of Catalysts and Electrochemical Property
Fe(phen)3Cl2 (1), [Fe(phen)2(CH3CH2OH)Cl]Cl (2) and BIH [39] were prepared according to a modification of published procedures. All of these compounds were characterized by MS, 1H NMR spectroscopy and elemental analysis (see the section of materials and methods, Figure S1 in the Supplementary Materials). The crystal structure of Fe(phen)3Cl2 was determined by single-crystal X-ray diffraction analysis (Figure S2 and Table S1). The cyclic voltagrammetry of Fe(phen)3Cl2 was recorded in a DMF solution containing 0.1 M nBu4NPF6 under an Ar atmosphere at ambient temperature (Figure S3). Figure S3 shows three distinct redox waves at −1.05, −1.21 and −1.44 V vs. NHE, with these tentatively attributed to FeII(phen)3/FeI(phen)3, FeI(phen)3/Fe0(phen)3 and Fe0(phen)3/Fe0(phen)2(phen∙−), respectively [40]. After CO2 bubbling to the system, two irreversible reduction waves underwent slight negatively shifts from −1.05 and −1.21 V to −1.08 and −1.32 V, respectively. There was a remarkable current enhancement for the latter, consistent with the electrocatalytic CO2 reduction (Figure S3) [41,42].
2.2. Catalyst-Concentration Dependence of Phtocatalytic CO2-to-CO Conversion for Fe(phen)3Cl2 (1)
The photocatalytic reduction systems were assembled using Ru(bpy)32+, complex 1 and BIH in a CO2-saturated DMF/TEOA solution. The produced gas products were identified using gas chromatography (GC), with Ar as carrier gas. The generating rates of CO and H2 were determined as a function of catalyst concentration. The results in Figure 1 clearly show that the production of CO and H2 was enhanced with increasing catalyst concentrations, and reaction system was accompanied by a H2-generating reaction that becomes less important at higher catalyst concentrations. In comparison to results for a lower catalyst concentration (3.0 × 10−8 M) where 4.25 µmol CO was produced with a selectivity of 68% after 2 h of visible-light irradiation, and a higher catalyst concentration of 3.0 × 10−5 M evolved 61.21 µmol CO at a selectivity of 92% (Table 1). Furthermore, 96.23 µmol of CO and a selectivity of 94% were achieved when the concentration of Fe(phen)3Cl2 reached 1.5 × 10−4 M, which clearly show that concentration of catalyst plays significant role to selectivity of CO2-to-CO conversion. However, in the absence of the Fe(II) complex, small amounts of CO and H2 were detected, with the H2-generation rate being superior to that of CO2 reduction [43,44,45], indicating a significant contribution by the PS to the CO-generating rate and the effect on product selectivity (Table 1, entry 4) [46].
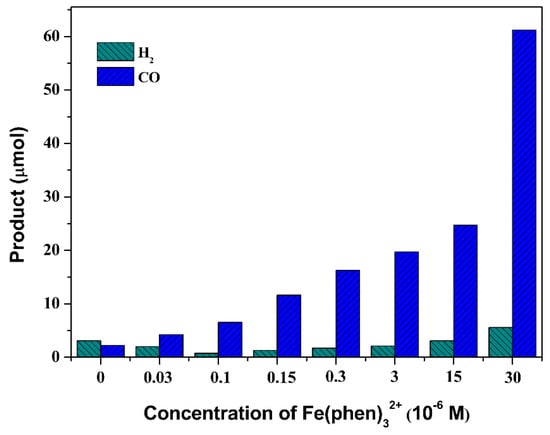
Figure 1.
Catalyst-concentration dependence of CO and H2 production in 4 mL CO2-saturated DMF/TEOA solution (v/v, 7:1) containing 6.7 × 10−4 M Ru(bpy)32+ and 0.022 M BIH after bright LED visible-light irradiation for 2 h at 298 K.

Table 1.
Controlled experimental results from Fe(phen)3Cl2 (1) for photocatalytic conversion of CO2 to CO.
2.3. The Dependence of the Photocatalytic Reduction Rates on PS for Catalyst (1)
To evaluate the catalytic capacity of the catalysts towards CO2 reduction and accompanied H2 production, the dependence of the photocatalytic reduction rates on PS concentration was conducted at a fixed concentration of Fe(phen)3Cl2 and various irradiation time. Figure S4 demonstrates that higher conversion efficiencies of CO2-to-CO were achieved after 6 h irradiation of the reaction system containing Fe(phen)3Cl2 (1.5 × 10−7 M) and BIH (0.022 M) with various PS concentrations. Increasing the amount of PS from 1.7 × 10−4 to 6.7 × 10−4 M significantly enhanced CO2 conversion and H2 evolution (Figure S5), with produced amounts of CO and H2 increasing from 1.64 and 1.74 µmol to 16.63 and 3.45 µmol, respectively (Figures S4 and S5). However, the CO selectivity of 83% (irradiation time for 6 h), when compared with that for a solution at the same concentration of PS and catalyst (Table 1, entry 2), clearly showed that the main-product selectivity decreased for longer irradiation times, suggesting a change of catalytic active species during photochemical reaction. This probably results from the contribution of generated RuI(bpy)3+ species during photocatalytic reaction to CO2-to-CO conversion and H2 evolution.
2.4. Optimization of Reaction Conditions for Photocatalysis
Furthermore, optimized conditions for product selectivity were considered in a CO2-saturated DMF/TEOA solution (v/v, 2.5:1) containing fixed amounts of PS and Fe(phen)3Cl2 at various BIH concentrations. As shown in Figure S6, the amount and selectivity of CO production strongly depends on the concentration of BIH and enhanced with increasing BIH concentration, reaching a limiting value at a scope of 0.022 M of BIH. Subsequent addition of BIH led to a negative relationship of photocatalytic efficiency, and H2 even become dominant. Previous studies revealed that metal nanoparticles such as Fe [47] and Ni [48] may possibly be involved in the homogenous photocatalytic reduction reaction. Thus, considering that BIH has a stronger reducing ability (Eox1/2 = 0.33 V vs. SCE), the possibility of in situ formation of Fe or Ru nanoparticles was assessed by a dynamic light scattering experiment [39,49]. The analysis confirmed that nanosized particles were not found during the photocatalytic reaction (Figure S7). The influence of BIH concentration on the photocatalytic efficiency and product selectivity possibly originates from its intense reduction and proton transfer ability, changing the relative proportion of some active components in the system during the photochemical reaction. Additionally, we tentatively contributed the changes to involving effective one-electron transfer from BIH to the excited state of PS and subsequent electron transfer from BI• to the related species such as Ru(II)*, Ru(I) or Fe(II) catalyst with proton loss from BIH•+ (see mechanistic interpretation). Compared with catalytic activity of Fe-based complex for CO2-to-CO conversion, the accumulation of generated Ru(I) species as catalyst in the case of high concentration of BIH is disadvantageous for reduction efficiency of CO2 in the investigated system [49]; a detailed study is still in progress. Further controlled experiments showed that no CO and H2 were generated when any of the following components were absent: light, CO2 or TEOA (Table 1).
As discussed above (Figure S6), the optimized concentration of BIH was 0.022 M. A typical example of the products as a function of irradiation time for a CO2-saturated solution with and without catalyst was given in Figure 2. Upon bright white LED irradiation, little CO and H2 were produced in the initial 15 min. We assumed that during this period, the phen ligand attached to the central Fe underwent photoinduced partial dissociation and/or a TEOA-substitution reaction to generate active-site-bearing catalytic species [50]. Figure 2 clearly demonstrates changes in the relative amounts of products for the competing pathways with time. Following the initial period, a higher CO-generating rate was observed. A TON of 19,367 was achieved under subsequent 105 min of light irradiation, with a TOF of 3.07 s−1 and a CO selectivity that reached 90% (Table 1, entry 2). Under the same conditions, the quantum yield of the photocatalytic reduction of CO2 to CO was measured as 0.38% after 5 h of irradiation with 450 nm monochromatic light (Xe lamp, 15 A). The inset in Figure 2 demonstrates that although the contribution of PS to the total catalytic efficiency was less important (2.23 µmol of CO, 3.09 µmol of H2), in the parallel catalytic reduction processes without Fe(phen)3Cl2 the Ru(II) complex exhibited a relatively high catalytic activity for H2 generation. The amount of H2 generated for the catalytic system compared with those in the system containing catalyst suggested that Fe(phen)3Cl2 exhibited higher selectivity to CO than to H2. Besides, the dependence of the calculated TONCO on the catalyst concentration was not linear because of the sluggish kinetics involving multiple electrons and protons. High TONs of up to 35,417 after 2 h of irradiation were observed for Fe(phen)3Cl2 concentration as low as 3.0 × 10−8 M, which is consistent with previous results [35,36,51].

Figure 2.
Evolution of CO (blue) and H2 (dark cyan), and TONCO during bright LED visible-light irradiation in 4 mL CO2-saturated DMF/TEOA solution (v/v, 7:1) containing 6.7 × 10−4 M Ru(bpy)32+, 0.022 M BIH and 1.5 × 10−7 M Fe(phen)32+. Inset: without Fe(phen)32+.
2.5. Comparison of Catalytic Efficiencies of CO2-to-CO Conversion for Catalysts 1 and 2 and Isotopic Labeling Experiment
The catalytic activity of [Fe(phen)2(CH3CH2OH)Cl]Cl (2) was then evaluated under the same conditions. A comparison of the CO-evolution over 2 h of irradiation demonstrated that catalytic activity of complex 2 for the transformation of CO2 to CO was greater than that of complex 1 (Figure 3). An unexpected high TON of 33,167 was achieved with a TOF of 4.61 s−1 for 1.5 × 10−7 M of catalyst 2. When the catalyst concentration was increased to 3.0 × 10−5 M, 119.86 µmol of CO was generated, almost twice the number of times than that produced by catalyst 1 (61.21 µmol), with a CO selectivity of over 85% (Table S2). A comparison of TONCO for catalytic performance of CO2 reduction between catalyst 2 and Fe(II) quaterpyridine complex of previous report in different solvents [36], TONCO values of 9,988 for the former (3.0 × 10−5 M, Table S2) and 1,879 for the latter (5.0 × 10−5 M) in the presence of Ru(bpy)32+ and BIH, demonstrates that catalyst 2 used in the work exhibits higher catalytic activity for CO2 reduction. Additionally, we found that the contribution of PS to CO2-to-CO conversion is remarkable in the absence of Fe(II) complex catalyst when BIH is used as an electron donor, and the effect is enhanced with increasing concentration of BIH and the change of DMF/TEOA ratio (inset in Figure 2 and Figure S8). In order to identify the origin of the produced CO, an isotopic labeling experiment was conducted using 13CO2 as the substrate on a GC-MS system under identical catalysis conditions [52,53]. As shown in Figure S9, the peak with m/z = 29 could be assigned to the reduced product of 13CO2 and the m/z value of 45 was the original 13CO2. These results confirmed that the CO evolution originated from the photocatalytic reduction of CO2 and precluded possible degradation of organics used.
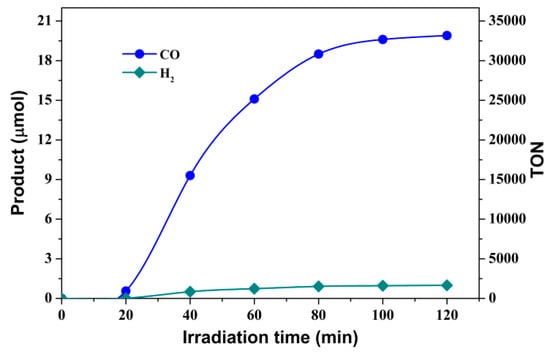
Figure 3.
Visible-light-driven production of CO (blue) and H2 (dark cyan) in 4 mL CO2-saturated DMF/TEOA solution (v/v, 7:1) containing 6.7 × 10−4 M Ru(bpy)32+, 0.022 M BIH and 1.5 × 10−7 M Fe(phen)2(CH3CH2OH)Cl+.
2.6. Detection of Reaction Intermediates and Mechanistic Interpretation
The detection and characterization of stable intermediates during the photoinduced reduction of CO2 are of fundamental importance to the identification of the reaction mechanism leading to CO formation. High resolution mass spectrometry (HRMS) was therefore used to probe active intermediates under the given catalytic conditions. Figure 4 clearly shows a signal at m/z = 663.1272 in the positive mode, which was attributed to the intermediate [Fe(phen)3 + CO2 + Na]+. Nevertheless, we did not observe a signal corresponding to [Fe(phen)2(TEOA) + CO2 + Na]+ (m/z = 632.17). The HRMS results obtained can be considered as clear evidence for the interchange mechanism during CO2 photoreduction. On the basis of these results, mechanism for photocatalytic CO2-to-CO conversion by Fe-based complexes is proposed in Scheme 2. Upon excitation at visible light, the triplet excited state of PS was reductively quenched by BIH to give the one-electron reduced species, then the Fe(II) catalyst was doubly reduced to the active Fe(0) species. In such a case of lower oxidation state, we cannot distinguish whether the dissociation of ligand from the complex is anterior to formation of Fe-CO2 adduct, but the intermediate Fe(phen)3∙∙∙CO2 is observed by HRMS analysis. Thus, we speculate that this is accompanied by the contribution of one Fe-N bond breaking and Fe-CO2 bond making in terms of an interchange of the ligand and CO2, after which photodriven one-electron transfer from RuI(bpy)3+ to the adduct takes place, which needs to be protonated to cleave the C–OH bond, give CO, and regenerate FeI(phen)3+. It is noteworthy that in subsequent catalytic reactions, BI• produced as an intermediate of sacrificial electron donor has strong reducing power (Eox = −2.06 V vs. Fc+/Fc), and donate performance. This is good agreement with experimental observation that in the initial stage there is remarkable induction period for H2 and CO production. This means that the generated BI• not only accelerates the formation of RuI(bpy)3+ but also probably directly transfers electron to Fe(II) catalyst to produce active catalytic species. Further studies on the contributions of PS to H2 and CO evolution are in progress in our laboratory and will bring further insight into the solution of the problem.
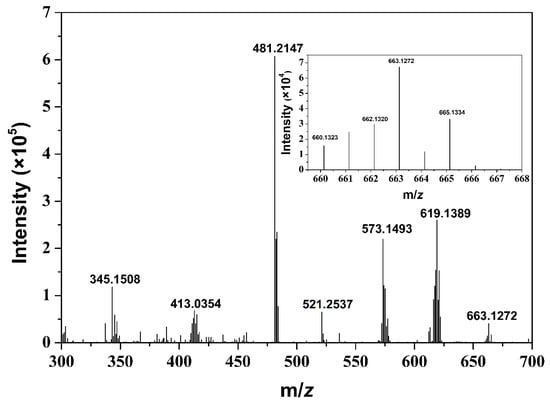
Figure 4.
HRMS spectra of CO2-saturated DMF/TEOA solution (v/v, 7:1) containing 0.67 mM Ru(bpy)32+, 0.022 M BIH and 1.5 × 10−7 M Fe(phen)32+ after irradiation for 30 min. Inset: measured isotopic distribution.
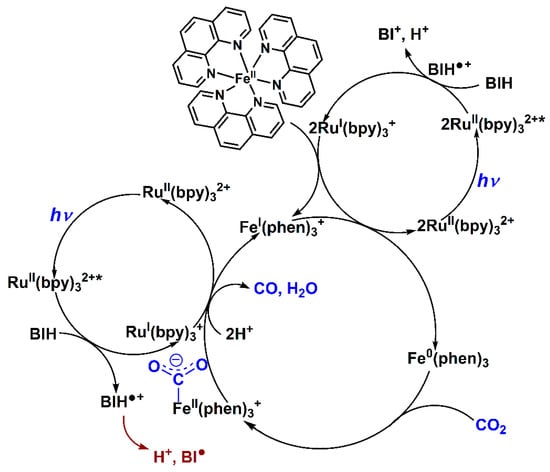
Scheme 2.
Proposed mechanism of the photocatalytic CO2-to-CO conversion for the presented system containing catalyst (1), [Ru(bpy)3]2+ and BIH.
3. Materials and Methods
3.1. Materials
Iron(II) chloride tetrhydrate (98%, Energy Chemical Ltd., Shanghai, China), 1,10-Phenanthroline (99%, J&K Scientific Ltd., Beijing, China), [Ru(bpy)3]Cl2·6H2O, 2-Phenylbenzimidazole (98%, J&K Scientific Ltd., Beijing, China), Iodomethane (97%, Ouhechem, Beijing, China), Sodium borohydride (99%, HuaDa, Guangdong, China). Sodium chloride, Sodium thiosulfate, Ethanol, Dichloromethane, Dithyl ether, N,N-Dimethylformamide, Triethanolamine come from Beijing Chemical Works (Beijing, China), and were used as purchased, without further purification.
3.2. Synthesis
The synthesis of Fe(phen)3Cl2 (1). Catalyst 1 was carried out as follows: FeCl2∙4H2O (1.00 g, 5.03 mmol) and phen (2.72 g, 15.09 mmol) were added to 300 mL of ethanol and stirred under Ar overnight. The solvent was then removed by evaporation under vacuum. The solid product was purified by recrystallization at least four times in ethanol and ether. Yield: 90% (3.02 g). 1H NMR (400 MHz, DMSO-d6): δ = 8.81 (d, 6H, J = 8.0 Hz), 8.40 (s, 6H), 7.66–7.81 (m, 12H); ESI-MS (MeOH): m/z = 298.0692 [M-2Cl]2+; elemental analysis calcd. for C36H24N6FeCl2·2H2O (703.40): C 61.47, H 4.01, N 11.95; found: C 62.34, H 4.57, N 11.17.
[Fe(phen)2(CH3CH2OH)Cl]Cl (2). Catalyst 2 was synthesized and purified by a procedure similar to that used for 1, except that 1.81 g (10.06 mmol) of phen was used. Yield: 74% (1.99 g). 1H NMR (400 MHz, DMSO-d6): δ = 8.81 (d, 4H, J = 8.0 Hz), 8.41 (s, 4H), 7.74 (dt, 8H J = 12.3, 5.0 Hz), 4.35 (t, 1H, J = 5.1 Hz), 3.38–3.50 (m, 2H), 1.06 (t, 3H, J = 7.0 Hz); ESI-MS (MeOH): m/z = 451.0422 [M-Cl-CH3CH2OH]+; elemental analysis calcd. for C26H22N4OFeCl2 (533.23): C 58.56, H 4.16, N 10.51; found: C 59.28, H 4.45, N 10.61.
Synthesis of 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo-[d]-imidazole (BIH). NaOH (0.85 g, 21.3 mmol), 2-phenylbenzimidazole (2.9 g, 14.9 mmol) and CH3I (7.9 g, 55.7 mmol) were dissolved in 20 mL of methanol with stirring for 20 min. The resulting solution was then transferred into a 50 mL Teflon-sealed autoclave and heated directly to 120 °C in an air-blowing thermostatic oven at a ramping rate of 5 °C min−1; the temperature was maintained for 12 h. After cooling to room temperature, a white flake crystal (BI+I−) was obtained by filtration, and washing with deionized water and methanol. Thereafter, the solid sodium borohydride (567 mg, 15 mmol) was added slowly in batches to a solution containing 2.1 g of BI+I− (6 mmol) in methanol (80 mL) with stirring under an atmosphere of nitrogen at 273 K. After the reaction mixture was stirred for 2 h, reaction temperature was kept at 298 K, and processed for 1.5 h. Reaction solvent was then removed by distillation under reduced pressure, and 30 mL of deionized water was added. The resulting mixture was extracted with diethyl ether (3 × 30 mL), and the extractive was washed with the saturated aqueous solution of Na2S2O3 and NaCl, respectively. The organic phase was dried over anhydrous MgSO4 overnight. The filtration was concentrated to yield a white solid powder. 1H NMR spectrum (400 MHz, CDCl3): δ 7.84–7.35 (m, 5H), 6.82–6.33 (m, 4H), 4.93 (s, 1H), 2.61 (s, 6H). ESI-MS (MeOH): m/z = 223.1215 (M + H)+.
3.3. Characterization
Single-crystal X-ray diffraction. Single crystals of [Fe(phen)3]Cl2 suitable for X-ray diffraction analysis were grown by slow evaporation of dichloromethane solutions, which allowed their molecular structures and packing modes in the solid state to be studied. CCDC reference number 1,571,123 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Instrumentations. 1H NMR spectra were recorded on a Bruker Avance 400 spectrometer with chemical shifts (δ, ppm) relative to tetramethylsilane. The mass spectra were determined on a Finnigan LCQ quadrupole ion trap mass spectrometer and a Shimadzu LCMS-IT-TOF mass spectrometer. Elemental analyses were performed on a Vario EL III. The diffraction data of single crystal were collected on a Rigaku R-AXIS RAPID IP X-ray diffractometer using a graphite monochromator with Mo Kα radiation (λ = 0.071073 nm) at 113 K. Electrochemical measurements were carried out on a CHI660C electrochemical potentiostat. Dynamic light scattering measurement was carried out on a DynaPro NanoStar (Wyatt Technology Co., US).
3.4. Photocatalytic Experiments
The photocatalytic reduction of CO2 to CO was conducted under 1 atm of CO2 at 298 K. Typically, 4 mL of DMF/TEOA solution (v/v, 7:1) containing [Ru(bpy)3]Cl2, BIH, and Fe-based catalyst was added to a 18.5 mL cuvette sealed with a rubber septum placed on top of a magnetic stirrer. A stream of CO2 was then passed into the reaction system for 30 min. White light-emitting diodes (LEDs) (30 × 3 W, λ ≥ 420 nm) were used as the irradiation light source. The LEDs were positioned 3 cm away from the sample, which was kept under continuous stirring at room temperature. The generated gases were analyzed by a gas chromatography (GC-2014C, 5 Å molecular sieve column (3 m × 2 mm), DINJ 50 °C, DTCD 100 °C, column temperature 50 °C, carrier gas flow 30 mL/min). The amounts of products were determined using the external standard method as the basis for quantitative analysis. 13C-labelled CO2 experiments were performed following the same procedure and the gas products were analyzed by GC-TCD and GC-MS.
4. Conclusions
In summary, a highly efficient, visible-light-driven Fe-based catalytic system was established to reduce CO2 to CO. Catalyst 2, with its easily replaced ligands, displayed a catalytic activity twice as large as that of catalyst 1, suggesting that the generated empty coordinating sites in the catalytic reduction of CO2 play a crucial role. Generally, it is required that coordination-saturated Fe(II) complex catalysts proceed photoinduced reaction to generate active sites before forming Fe-CO2 adduct, and the overall results are notable because the photodriven initial periods were similar for catalysts 1 and 2. However, the results obtained from HRMS measurement indicate an interchange mechanism during CO2 photoreduction for complex 1 as catalyst. The interrelationships among the components in the catalytic system, especially the effect of electron donor BIH on the contribution of PS to CO and H2 generation, was therefore still further studied in detail. Undoubtedly, the higher selectivity and efficiency of the reported system for the visible-light-driven reduction of CO2 to CO offers significant potential for carbon-neutral artificial photosynthesis cycles.
Supplementary Materials
Supplementary Materials are available online. Figures S1–S9, Tables S1–S2.
Author Contributions
Z.-C.F., C.M., and W.-F.F. designed research; Z.-C.F., C.M., Y.S., Z.Y., and Q.-Q.X. performed research and analyzed the data; and Z.-C.F., C.M., and W.-F.F. wrote the paper.
Funding
This work was financially supported by the Ministry of Science and Technology (2012DFH40090). We thank the National Natural Science Foundation of China (21777136, 21471155, 21367026) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morris, A.J.; Meyer, G.J.; Fujita, E. Molecular approaches to the photocatalytic reduction of carbon dioxide for solar fuels. Acc. Chem. Res. 2009, 42, 1983–1994. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E., III; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-driven heterogeneous reduction of carbon dioxide: Photocatalysts and photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.R.; Dubois, D.L. Development of molecular electrocatalysts for CO2 reduction and H2 production/oxidation. Acc. Chem. Res. 2009, 42, 1972–1982. [Google Scholar]
- Mahammadunnisa, S.; Reddy, P.M.K.; Ramaraju, B.; Subrahmanyam, C. Catalytic nonthermal plasma reactor for dry reforming of methane. Energy Fuels 2013, 27, 4441–4447. [Google Scholar]
- Taheri, A.; Berben, L.A. Making C-H bonds with CO2: Production of formate by molecular electrocatalysts. Chem. Commun. 2016, 52, 1768–1777. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Rao, H.; Bonin, J.; Robert, M. Toward visible-light photochemical CO2-to-CH4 conversion in aqueous solutions using sensitized molecular catalysis. J. Phys. Chem. C 2018, 122, 13834–13839. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubickib, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Fu, Z.-C.; Xu, R.-C.; Moore, J.T.; Liang, F.; Nie, X.-C.; Mi, C.; Mo, J.; Xu, Y.; Xu, Q.-Q.; Yang, Z.; et al. Highly efficient photocatalytic system constructed from CoP/carbon nanotubes or graphene for visible-light-driven CO2 reduction. Chem. Eur. J. 2018, 24, 4273–4278. [Google Scholar] [CrossRef]
- Sen, P.; Mondal, B.; Saha, D.; Rana, A.; Dey, A. Role of 2nd sphere H-bonding residues in tuning the kinetics of CO2 reduction to CO by iron porphyrin complexes. Dalton. Trans. 2019, 48, 5965–5977. [Google Scholar] [CrossRef]
- Liu, X.; Inagaki, S.; Gong, L. Heterogeneous molecular systems for photocatalytic CO2 reduction with water oxidation. Angew. Chem. Int. Ed. 2016, 55, 14924–14950. [Google Scholar] [CrossRef]
- Sato, S.; Morikawa, T.; Kajino, T.; Ishitani, O. A highly efficient mononuclear iridium complex photocatalyst for CO2 reduction under visible light. Angew. Chem. Int. Ed. 2013, 52, 988–992. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.M.; Ziessel, R. Photochemical and electrochemical reduction of carbon dioxide to carbon monoxide mediated by (2,2′-bipyridine)tricarbonylchlororhenium(I) and related complexes as homogeneous catalysts. Helv. Chim. Acta. 1986, 69, 1990–2012. [Google Scholar] [CrossRef]
- Gholamkhass, B.; Mametsuka, H.; Koike, K.; Tanabe, T.; Furue, M.; Ishitani, O. Architecture of Supramolecular Metal Complexes for Photocatalytic CO2 Reduction: Ruthenium−Rhenium Bi- and Tetranuclear Complexes. Inorg. Chem. 2005, 44, 2326–2336. [Google Scholar] [CrossRef]
- Tamaki, Y.; Ishitani, O. Supramolecular photocatalysts for the reduction of CO2. ACS Catal. 2017, 7, 3394–3409. [Google Scholar] [CrossRef]
- Bonin, J.; Robert, M.; Routier, M. Selective and efficient photocatalytic CO2 reduction to CO using visible light and an iron-based homogeneous catalyst. J. Am. Chem. Soc. 2014, 136, 16768–16771. [Google Scholar] [CrossRef]
- Chen, L.J.; Guo, Z.G.; Wei, X.G.; Gallenkamp, C.; Bonin, J.; Mallart, E.A.; Lau, K.C.; Lau, T.C.; Robert, M. Molecular catalysis of the electrochemical and photochemical reduction of CO2 with earth-abundant metal complexes. Selective production of CO vs. HCOOH by switching of the metal center. J. Am. Chem. Soc. 2015, 137, 10918–10927. [Google Scholar] [CrossRef]
- Ouyang, T.; Hou, C.; Wang, J.W.; Liu, W.J.; Zhong, D.C.; Ke, Z.F.; Lu, T.B. A highly selective and robust Co(II)-based homogeneous catalyst for reduction of CO2 to CO in CH3CN/H2O solution driven by visible light. Inorg. Chem. 2017, 56, 7307–7311. [Google Scholar] [CrossRef]
- Bonin, J.; Chaussemier, M.; Robert, M.; Routier, M. Homogeneous photocatalytic reduction of CO2 to CO using Iron(0) porphyrin catalysts: Mechanism and intrinsic limitations. ChemCatChem 2014, 6, 3200–3207. [Google Scholar] [CrossRef]
- Hernández, A.R.; Steinlechner, C.; Junge, H.; Beller, M. Earth-abundant photocatalytic systems for the visible-light-driven reduction of CO2 to CO. Green Chem. 2017, 19, 2356–2360. [Google Scholar]
- Rao, H.; Schmidt, L.C.; Bonin, J.; Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 2017, 548, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, T.; Grodkowski, J.; Neta, P.; Hambright, P.; Fujita, E. p-Terphenyl-sensitized photoreduction of CO2 with cobalt and iron porphyrins. Interaction between CO and reduced metalloporphyrins. J. Phys. Chem. A 1999, 103, 7742–7748. [Google Scholar] [CrossRef]
- Chan, S.L.F.; Lam, T.L.; Yang, C.; Yan, S.C.; Cheng, N.M. A robust and efficient cobalt molecular catalyst for CO2 reduction. Chem. Commun. 2015, 51, 7799–7801. [Google Scholar] [CrossRef]
- Chen, L.J.; Qin, Y.F.; Chen, G.; Li, M.Y.; Cai, L.R.; Qiu, Y.F.; Fan, H.B.; Robert, M.; Lau, T.C. A molecular noble metal-free system for efficient visible light-driven reduction of CO2 to CO. Dalton. Trans. 2019. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Orchard, K.L.; Dalle, K.E.; Reisner, E. Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals. J. Am. Chem. Soc. 2017, 139, 7217–7223. [Google Scholar] [CrossRef]
- Hong, D.C.; Tsukakoshi, Y.; Kotani, H.; Ishizuka, T.; Kojima, T. Visible-light-driven photocatalytic CO2 reduction by a Ni(II) complex bearing a bioinspired tetradentate ligand for selective CO production. J. Am. Chem. Soc. 2017, 139, 6538–6541. [Google Scholar] [CrossRef]
- Lin, J.L.; Qin, B.; Fang, Z.X. Nickel bipyridine (Ni(bpy)3Cl2) complex used as molecular catalyst for photocatalytic CO2 reduction. Catal. Leet. 2019, 149, 25–33. [Google Scholar] [CrossRef]
- Angamuthu, R.; Byers, P.; Lutz, M.; Spek, A.L.; Bouwman, E. Electrocatalytic CO2 conversion to oxalate by a copper complex. Science 2010, 327, 313–315. [Google Scholar] [CrossRef]
- Guo, Z.G.; Yu, F.; Yang, Y.; Leung, C.-F.; Ng, S.M.; Ko, C.-C.; Cometto, C.; Lau, T.-C.; Robert, M. Photocatalytic Conversion of CO2 to CO by a Copper(II) Quaterpyridine Complex. ChemSusChem 2017, 10, 4009–4013. [Google Scholar] [CrossRef]
- Liu, W.-J.; Huang, H.-H.; Ouyang, T.; Jiang, L.; Zhong, D.-C.; Zhang, W.; Lu, T.-B. A Copper(II) Molecular Catalyst for Efficient and Selective Photochemical Reduction of CO2 to CO in a Water-Containing System. Chem. Eur. J. 2018, 24, 4503–4508. [Google Scholar] [CrossRef]
- Takeda, H.; Koizumi, H.; Okamoto, K.; Ishitani, O. Photocatalytic CO2 reduction using a Mn complex as a catalyst. Chem. Commun. 2014, 50, 1491–1493. [Google Scholar] [CrossRef]
- Torralba-Peñalver, E.; Luo, Y.; Compain, J.D.; Noblat, S.C.; Fabre, B. Selective catalytic electroreduction of CO2 at silicon nanowires (SiNWs) photocathodes using non-noble metal-based manganese carbonyl bipyridyl molecular catalysts in solution and grafted onto SiNWs. ACS. Catal. 2015, 5, 6138–6147. [Google Scholar] [CrossRef]
- Cheung, P.L.; Machan, C.W.; Malkhasian, A.Y.S.; Agarwal, J.; Kubiak, C.P. Photocatalytic reduction of carbon dioxide to CO and HCO2H using fac-Mn(CN)(bpy)(CO)3. Inorg. Chem. 2016, 55, 3192–3198. [Google Scholar] [CrossRef]
- Thoi, V.S.; Kornienko, N.; Margarit, C.G.; Yang, P.D.; Chang, C.J. Visible-light photoredox catalysis: Selective reduction of carbon dioxide to carbon monoxide by a nickel N-heterocyclic carbene-isoquinoline complex. J. Am. Chem. Soc. 2013, 135, 14413–14424. [Google Scholar] [CrossRef]
- Guo, Z.G.; Cheng, S.W.; Cometto, C.; Anxolabéhère-Mallart, E.; Ng, S.M.; Ko, C.C.; Liu, G.J.; Chen, L.J.; Robert, M.; Lau, T.C. Highly efficient and selective photocatalytic CO2 reduction by iron and cobalt quaterpyridine complexes. J. Am. Chem. Soc. 2016, 138, 9413–9416. [Google Scholar] [CrossRef]
- Takeda, H.; Ohashi, K.; Sekine, A.; Ishitani, O. Photocatalytic CO2 reduction using Cu(I) photosensitizers with a Fe(II) catalyst. J. Am. Chem. Soc. 2016, 138, 4354–4357. [Google Scholar] [CrossRef]
- Ouyang, T.; Huang, H.H.; Wang, J.W.; Zhong, D.C.; Lu, T.B. A dinuclear cobalt cryptate as a homogeneous photocatalyst for highly selective and efficient visible-light driven CO2 reduction to CO in CH3CN/H2O Solution. Angew. Chem. Int. Ed. 2017, 56, 738–743. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Zhang, M.T.; Yu, A.; Wang, C.H.; Cheng, J.P. Hydride, hydrogen atom, proton, and electron transfer driving forces of various five-membered heterocyclic organic hydrides and their reaction intermediates in acetonitrile. J. Am. Chem. Soc. 2008, 130, 2501–2516. [Google Scholar] [CrossRef]
- Saji, T.; Fukai, T.; Aoyagui, S. Polarography of tris(4,7-diphenyl-1,10-phenanthroline)-iron(II) complex. J. Electroanal. Chem. 1975, 66, 81–84. [Google Scholar] [CrossRef]
- Simpson, T.C.; Durand, R.R., Jr. Ligand participation in the reduction of CO2 catalyzed by complexes of 1,10 o-phenanthroline. Electrochim. Acta 1988, 33, 581–583. [Google Scholar] [CrossRef]
- Azcarate, I.; Costentin, C.; Robert, M.; Savéant, J.M. Through-space charge interaction substituent effects in molecular catalysis leading to the design of the most efficient catalyst of CO2-to-CO electrochemical conversion. J. Am. Chem. Soc. 2016, 138, 16639–16644. [Google Scholar] [CrossRef]
- Lehn, J.M.; Ziessel, R. Photochemical generation of carbon monoxide and hydrogen by reduction of carbon dioxide and water under visible light irradiation. PNAS 1982, 79, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.H.; White, R.P.; Rillema, D.P.; Meyer, T.J. Synthetic control of excited-state properties. tris-chelate complexes containing the ligands 2,2′-bipyrazine, 2,2′-bipyridine, and 2,2′-bipyrimidine. J. Am. Chem. Soc. 1984, 106, 2613–2620. [Google Scholar] [CrossRef]
- Tamaki, Y.; Morimoto, T.; Koike, K.; Ishitani, O. Photocatalytic CO2 reduction with high turnover frequency and selectivity of formic acid formation using Ru(II) multinuclear complexes. PNAS 2012, 109, 15673–15678. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.X.; Wang, H.; Chen, L.J.; Bian, Z.Y. Photocatalytic and electrocatalytic reduction of CO2 to methanol by the homogeneous pyridine-based systems. Appl. Catal. A Gen. 2016, 520, 1–6. [Google Scholar] [CrossRef]
- Wang, C.-J.; Cao, S.; Qin, B.; Zhang, C.; Li, T.-T.; Fu, W.-F. Photoreduction of iron(III) to iron(0) nanoparticles for simultaneous hydrogen evolution in aqueous solution. ChemSusChem 2014, 7, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Cao, S.; Fu, W.-F. A stable dual-functional system of visible-light-driven Ni(II) reduction to a nickel nanoparticle catalyst and robust in situ hydrogen production. Chem. Commun. 2013, 49, 11251–11253. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Koike, K.; Morimoto, T.; Ishitani, O. Substantial improvement in the efficiency and durability of a photocatalyst for carbon dioxide reduction using a benzoimidazole derivative as an electron donor. J. Catal. 2013, 304, 22–28. [Google Scholar] [CrossRef]
- Dong, J.; Wang, M.; Li, X.; Chen, L.; He, Y.; Sun, L. Simple nickel-based catalyst systems combined with graphitic carbon nitride for stable photocatalytic hydrogen production in water. ChemSusChem 2012, 5, 2133–2138. [Google Scholar] [CrossRef]
- Behar, D.; Dhanasekaran, T.; Neta, P.; Hosten, C.M.; Ejeh, D.; Hambright, P.; Fujita, E. Cobalt porphyrin catalyzed reduction of CO2. Radiation chemical, photochemical, and electrochemical studies. J. Phys. Chem. A 1998, 102, 2870–2877. [Google Scholar] [CrossRef]
- Schneider, T.W.; Ertem, M.Z.; Muckerman, J.T.; Boza, A.M.A. Mechanism of photocatalytic reduction of CO2 by Re(bpy)(CO)3Cl from differences in carbon isotope discrimination. ACS Catal. 2016, 6, 5473–5481. [Google Scholar] [CrossRef]
- Liu, G.G.; Meng, X.G.; Zhang, H.B.; Zhao, G.X.; Pang, H.; Wang, T.; Li, P.; Kako, T.; Ye, J.H. Elemental boron for efficient carbon dioxide reduction under light irradiation. Angew. Chem. Int. Ed. 2017, 56, 5570–5574. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).