Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects
Abstract
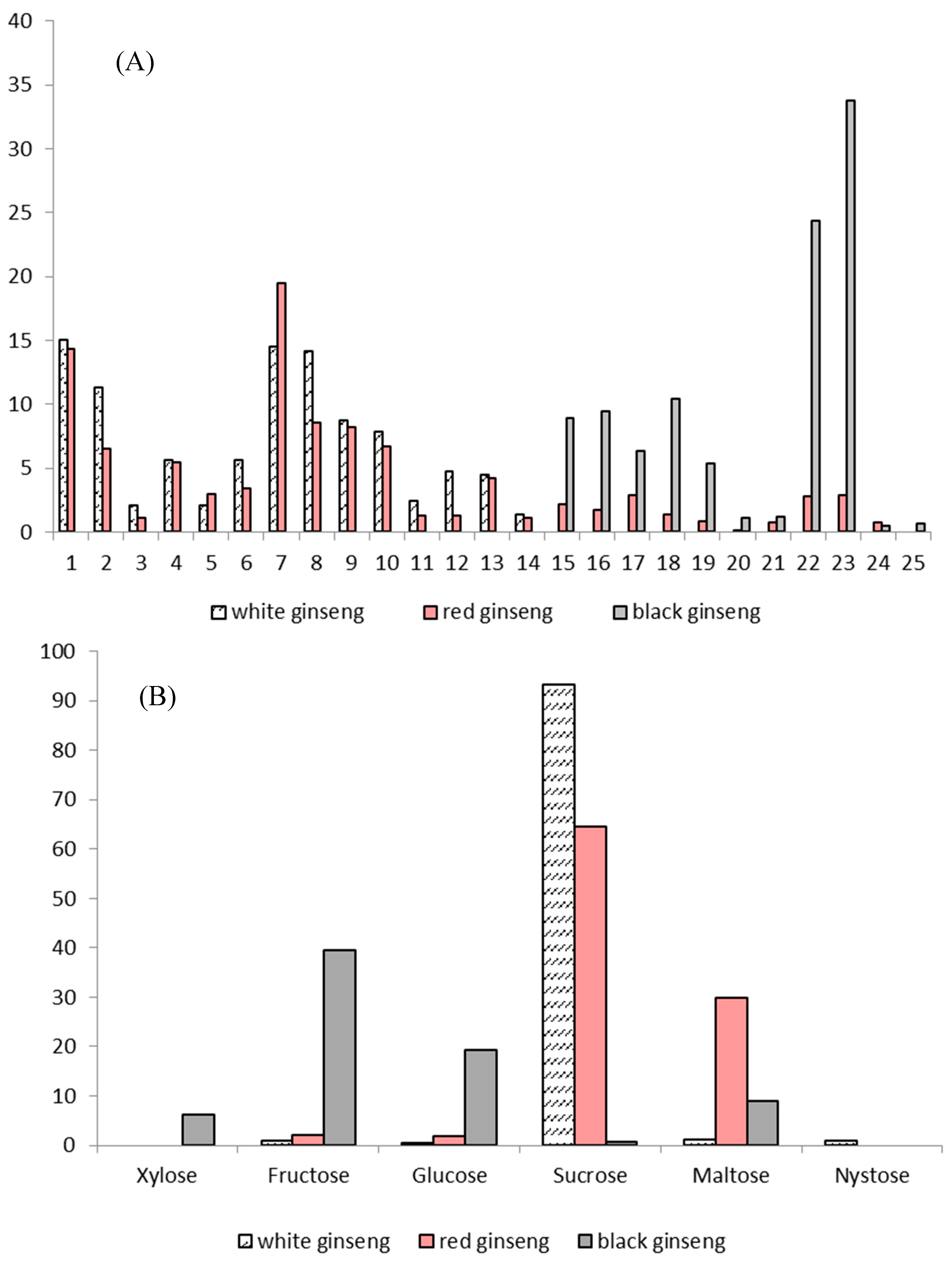
1. Introduction
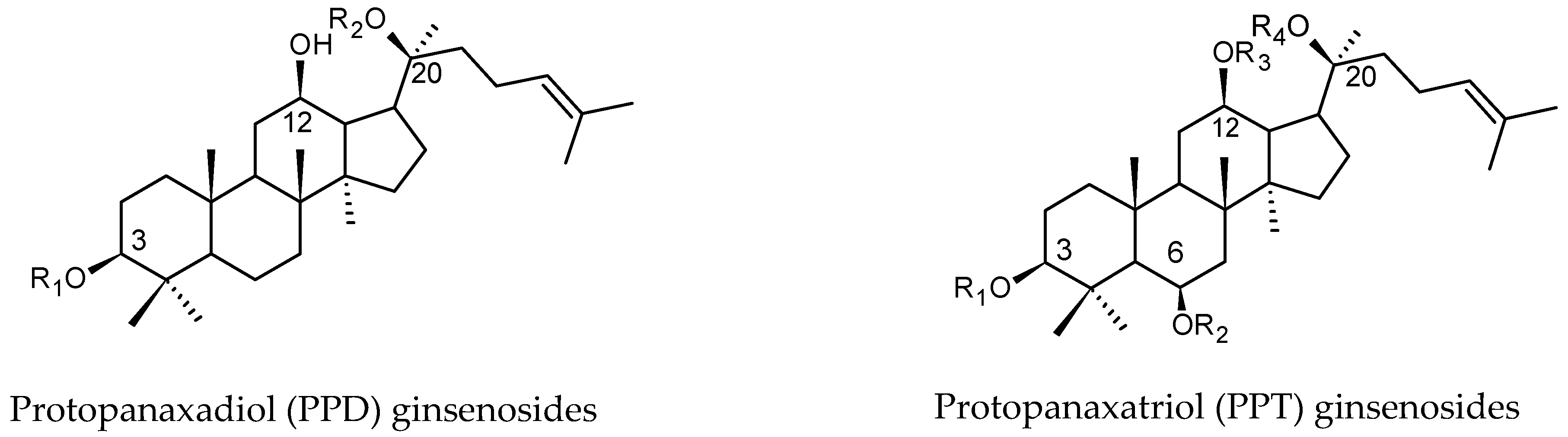
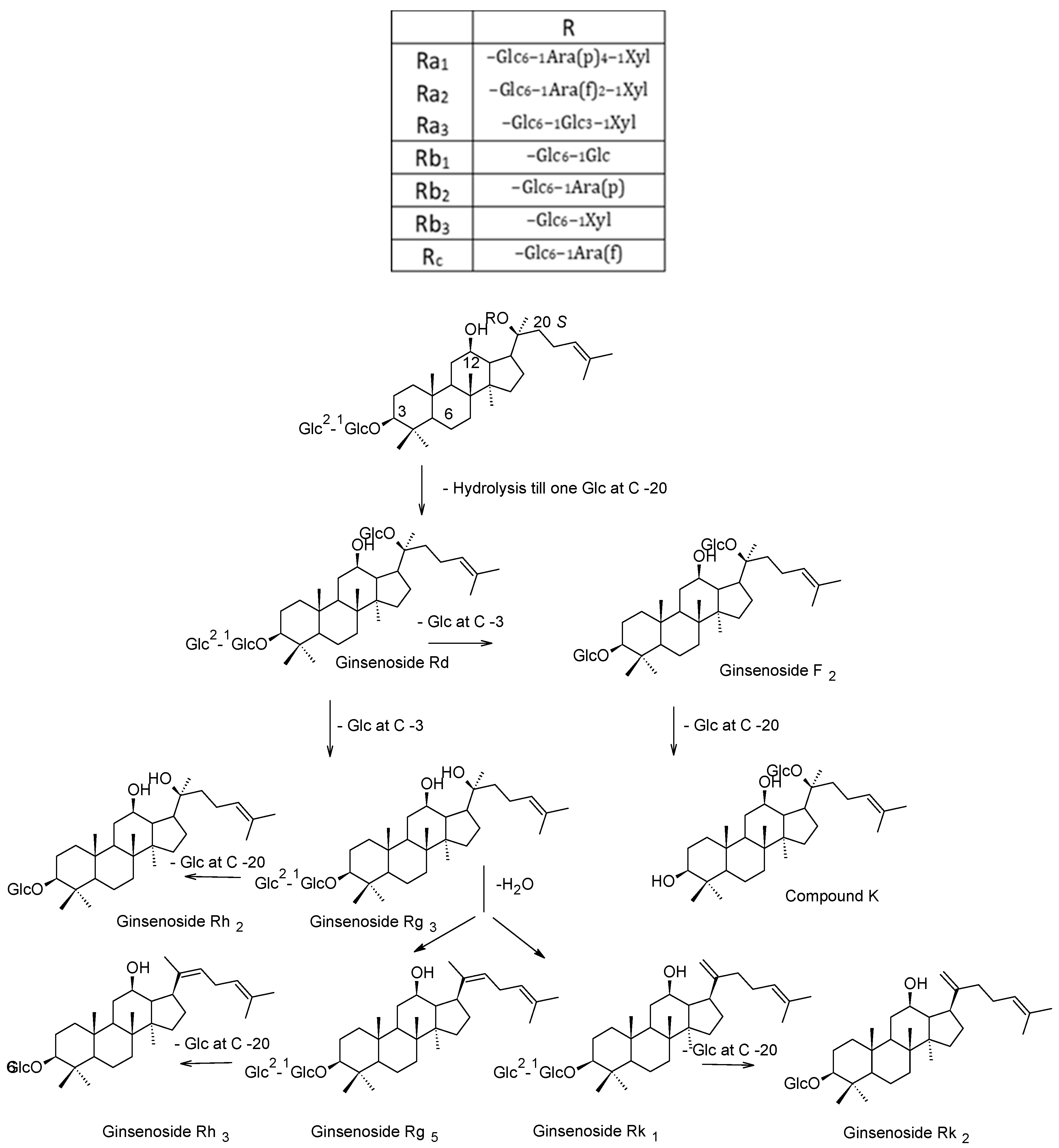
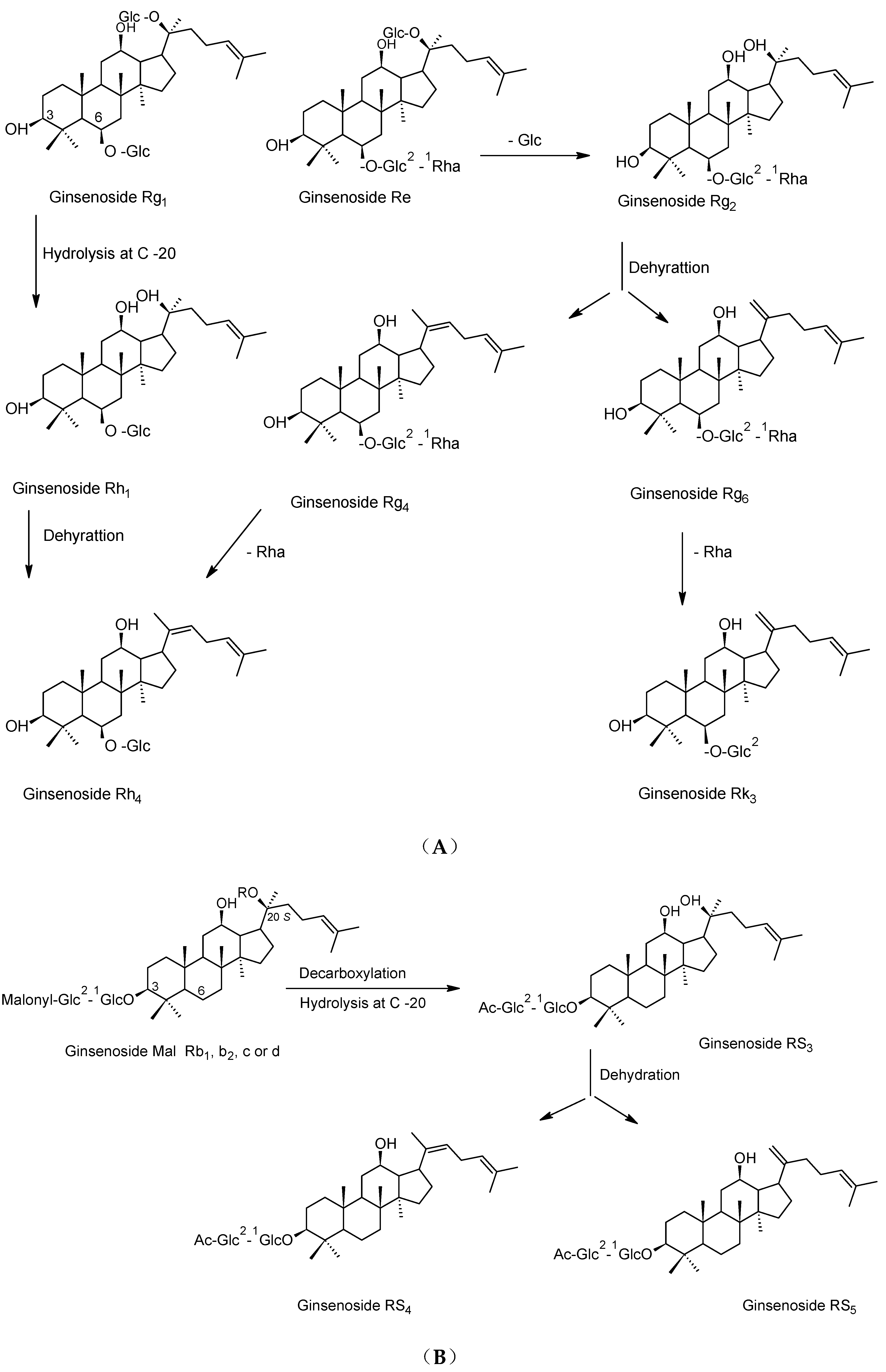
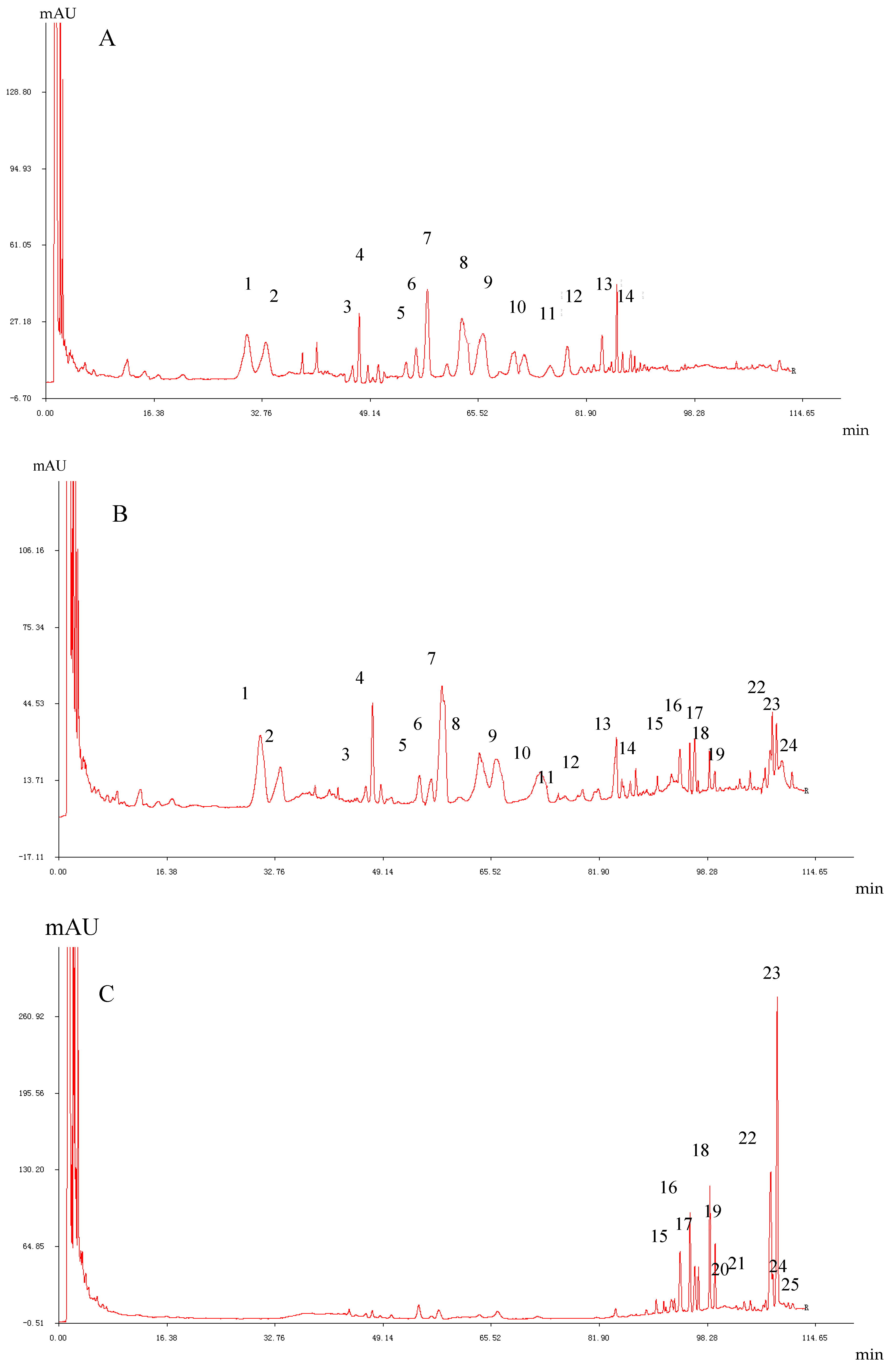
2. Preparation
3. Phytochemistry
4. Pharmacological Studies
4.1. Anticarcinogenic Effects
4.2. Immunomodulatory and Anti-Inflammatory Effects
4.3. Hepatoprotective Effect
4.4. Antidiabetic Effect
4.5. Anti-Obesity and Antihyperlipidemic Effects
4.6. Effects on the Central Nervous System
4.7. Antioxidant Effect
4.8. Tonic Effect
4.9. Topical Uses
4.10. Toxicity Studies
5. Methodology
Prospects
Funding
Conflicts of Interest
References
- Yun, T.K. Brief introduction of Panax ginseng CA Meyer. J. Korean Med. Sci. 2001, 16, S3. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, E.; Amato, M.; Izzo, A.A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000, 71, S1–S5. [Google Scholar] [CrossRef]
- Kang, S.; Min, H. Ginseng, the’immunity boost’: The effects of Panax ginseng on immune system. J. Ginseng Res. 2012, 36, 354. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Kim, J.Y.; Yun, T.K.; Morgan, G.; Vainio, H. The cancer-preventive potential of Panax ginseng: A review of human and experimental evidence. Cancer Causes Control. 2000, 11, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, Y.C.; Kyung Rhee, Y.; Lee, S.Y. Consumer acceptance of ginseng food products. J. Food Sci. 2011, 76, S516–S522. [Google Scholar] [CrossRef] [PubMed]
- Simoons, F.J. Food in China: A Cultural and Historical Inquiry; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Matsuura, H.; Kasai, R.; TANAKA, O.; SARUWATARI, Y.; KUNIHIRO, K.; FUWA, T. Further studies on dammarane-saponins of ginseng roots. Chem. Pharm. Bull. 1984, 32, 1188–1192. [Google Scholar] [CrossRef]
- Nag, S.; Qin, J.; Wang, W.; Wang, M.-H.; Wang, H.; Zhang, R. Ginsenosides as anticancer agents: In vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef]
- Christensen, L.P. Ginsenosides: Chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2008, 55, 1–99. [Google Scholar]
- Dou, D.-Q.; Chen, Y.-J.; Liang, L.-H.; PANG, F.-G.; Shimizu, N.; Takeda, T. Six new dammarane-type triterpene saponins from the leaves of Panax ginseng. Chem. Pharm. Bull. 2001, 49, 442–446. [Google Scholar] [CrossRef]
- Dou, D.-Q.; Hou, W.-B.; Chen, Y.-J. Studies of the characteristic constituents of Chinese ginseng and American ginseng. Planta Med. 1998, 64, 585–586. [Google Scholar] [CrossRef]
- Fuzzati, N. Analysis methods of ginsenosides. J. Chromatogr. B 2004, 812, 119–133. [Google Scholar] [CrossRef]
- Chung, A.-S.; Park, K.M. Anticancer and Antineurodegenerative effects of Ginsenosides. In Studies in Natural Products Chemistry; Elsevier: Pakistan, 2016; Volume 50, pp. 131–158. [Google Scholar]
- Lee, S.M.; Bae, B.-S.; Park, H.-W.; Ahn, N.-G.; Cho, B.-G.; Cho, Y.-L.; Kwak, Y.-S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, Y.-J.; Jeon, J.-N.; Wang, C.; Min, J.-W.; Noh, H.-Y.; Yang, D.-C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant. Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-Y.; Lee, N.-R.; Moon, B.-D.; Song, G.-Y.; Shin, H.-S.; Choi, J.-E. Changes of ginsenosides and color from black ginsengs prepared by steaming-drying cycles. Korean J. Med. Crop. Sci. 2012, 20, 27–35. [Google Scholar] [CrossRef]
- Wang, G.; Dong, C.; Shang, Y.; Sun, Y.-A.; Fu, D.; Zhao, J. Characterization of radix rehmanniae processing procedure using FT-IR spectroscopy through nonnegative independent component analysis. Anal. Bioanal. Chem. 2009, 394, 827–833. [Google Scholar] [CrossRef]
- Ban, Y.-J.; Yang, B.-W.; Baik, M.-Y.; Hahm, Y.-T.; Kim, B.-Y. Optimization of the manufacturing process for black ginseng. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 71–77. [Google Scholar] [CrossRef]
- Lee, M.-R.; Yun, B.-S.; Sun, B.-S.; Liu, L.; Zhang, D.-L.; Wang, C.-Y.; Wang, Z.; Ly, S.-Y.; Mo, E.-K.; Sung, C.-K. Change of ginsenoside Rg 3 and acetylcholinesterase inhibition of black ginseng manufactured by grape juice soaking. J. Ginseng Res. 2009, 33, 349–354. [Google Scholar] [CrossRef]
- Kim, E.-K.; Lee, J.-H.; Cho, S.-H.; Shen, G.-N.; Jin, L.-G.; Myung, C.-S.; Oh, H.-J.; Kim, D.-H.; Yun, J.-D.; Roh, S.-S. Preparation of black panax ginseng by new methods and its antitumor activity. Korea J. Herbol. 2008, 23, 85–92. [Google Scholar]
- Bak, M.; Jeong, W.; Kim, K. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int. J. Mol. Med. 2014, 34, 1516–1522. [Google Scholar] [CrossRef]
- Chen, G.; Li, H.; Gao, Y.; Zhang, L.; Zhao, Y. Flavored black ginseng exhibited antitumor activity via improving immune function and inducing apoptosis. Food Funct. 2017, 8, 1880–1889. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, C.Y.; Lee, S.J. Effects of Sun Ginseng on subjective quality of life in cancer patients: A double-blind, placebo-controlled pilot trial. J. Clin. Pharm. Ther. 2006, 31, 331–334. [Google Scholar] [CrossRef]
- Jo, E.-J.; Kang, S.-J.; Kim, A.-J. Effects of steam-and dry-processing temperatures on the benzo (a) pyrene content of black and red ginseng. Korean J. Food Nutr. 2009, 22, 199–204. [Google Scholar]
- Zhu, L.; Luan, X.; Dou, D.; Huang, L. Comparative Analysis of Ginsenosides and Oligosaccharides in White Ginseng (WG), red Ginseng (RG) and Black Ginseng (BG). J. Chromatogr. Sci. 2019. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.; Li, J.; Zhang, H.; Ding, L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007, 102, 664–668. [Google Scholar] [CrossRef]
- Sun, B.-S.; Xu, M.-Y.; Li, Z.; Wang, Y.-B.; Sung, C.-K. UPLC-Q-TOF-MS/MS analysis for steaming times-dependent profiling of steamed Panax quinquefolius and its ginsenosides transformations induced by repetitious steaming. J. Ginseng Res. 2012, 36, 277. [Google Scholar] [CrossRef]
- Sun, B.-S.; Pan, F.-Y.; Sung, C.-K. Repetitious steaming-induced chemical transformations and global quality of black ginseng derived from Panax ginseng by HPLC-ESI-MS/MS n based chemical profiling approach. Biotechnol. Bioprocess. Eng. 2011, 16, 956. [Google Scholar] [CrossRef]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. American ginseng: Potential structure–function relationship in cancer chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef]
- Wan, J.-Y.; Fan, Y.; Yu, Q.-T.; Ge, Y.-Z.; Yan, C.-P.; Alolga, R.N.; Li, P.; Ma, Z.-H.; Qi, L.-W. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J. Pharm. Biomed. Anal. 2015, 107, 89–97. [Google Scholar] [CrossRef]
- Lee, M.R.; Yun, B.S.; Sung, C.K. Comparative study of white and steamed black Panax ginseng, P. quinquefolium, and P. notoginseng on cholinesterase inhibitory and antioxidative activity. J. Ginseng Res. 2012, 36, 93. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, H.Y.; Pyo, J.S.; Yokozawa, T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol. Pharm. Bull. 2006, 29, 750–754. [Google Scholar] [CrossRef]
- Ellis, G. The maillard reaction. In Advances in Carbohydrate Chemistry; Elsevier, 1959; Volume 14, pp. 63–134. [Google Scholar]
- Lee, Y.-S.; Im, D.-H.; Yang, J.-C.; Noh, D.-S.; Kim, K.-I.; Oh, S.-K.; Choi, K.-C.; Cha, Y.-H. Study on the qualitative discrimination of white, red, and black ginseng extract. Korean J. Food Nutr. 2011, 24, 138–143. [Google Scholar] [CrossRef][Green Version]
- Jeong, H.C.; Hong, H.-D.; Kim, Y.-C.; Rhee, Y.K.; Choi, S.Y.; Kim, K.-T.; Kim, S.S.; Lee, Y.-C.; Cho, C.-W. Quantification of maltol in Korean ginseng (Panax ginseng) products by high-performance liquid chromatography-diode array detector. Pharmacogn. Mag. 2015, 11, 657. [Google Scholar]
- Cho, E.J.; Piao, X.L.; Jang, M.H.; Baek, S.H.; Kim, H.Y.; Kang, K.S.; Kwon, S.W.; Park, J.H. The effect of steaming on the free amino acid contents and antioxidant activity of Panax ginseng. Food Chem. 2008, 107, 876–882. [Google Scholar] [CrossRef]
- Guo, N.; Zhu, L.; Song, J.; Dou, D. A new simple and fast approach to analyze chemical composition on white, red, and black ginseng. Ind. Crop. Prod. 2019, 134, 185–194. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; World Health Organization. Smokeless Tobacco and Some Tobacco-Specific N-nitrosamines; World Health Organization: Geneva, Switzerland, 2007; Volume 89. [Google Scholar]
- Kim, A.-J.; Kang, S.-J.; Lee, K.-H.; Lee, M.; Ha, S.-D.; Cha, Y.-S.; Kim, S.Y. The chemopreventive potential and anti-inflammatory activities of Korean black ginseng in colon26-M3. 1 carcinoma cells and macrophages. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 101–105. [Google Scholar] [CrossRef]
- Song, G.Y.; Chung, K.J.; Shin, Y.J.; Lee, G.W.; Lee, S.Y.; Seo, Y.B. Study on Antiangiogenic Effect of Black Ginseng Radix. Kor. J. Herbol. 2011, 26, 83–90. [Google Scholar]
- Kim, S.-J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-J.; Han, J.-S.; Kim, A.-J. Ameliorate Effect of Black Ginseng on HepG2 Cell transplanted in BALB/c Nude Mice. Korean J. Food Nutr. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Wu, K.; Li, N.; Sun, H.; Xu, T.; Jin, F.; Nie, J. Endoplasmic reticulum stress activation mediates Ginseng Rg3-induced anti-gallbladder cancer cell activity. Biochem. Biophys. Res. Commun. 2015, 466, 369–375. [Google Scholar] [CrossRef]
- Shinkai, K.; Akedo, H.; Mukai, M.; Imamura, F.; Isoai, A.; Kobayashi, M.; Kitagawa, I. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J. Cancer Res. 1996, 87, 357–362. [Google Scholar] [CrossRef]
- Zheng, R.; Rao, Y.; Jiang, H.; Liu, X.; Zhu, X.; Li, J.; Xu, J. Therapeutic potential of Ginsenoside Rg3 via inhibiting Notch/HES1 pathway in lung cancer cells. Transl. Cancer Res. 2016, 5, 464–469. [Google Scholar] [CrossRef]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer effects of ginsenoside Rg3. Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.H.; Kim, H.; Hong, M.J.; Yang, M.H.; Kim, J.W.; Yoo, H.; Yang, H.; Park, J.H.; Sung, S.H.; Kim, H.P. Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: Disparity in cytotoxicity and autophagy-inducing effects due to 20 (S)-epimers. Biol. Pharm. Bull. 2015, 38, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Zhang, L.S.; Song, D.M.; Zhang, J.H.; Liu, Y.M. [Effect of gensenoside Rg3 on apoptosis of Hep-2 and expression of HIF-1alha in human laryngeal cancer cell line under anoxic conditions]. Zhong Yao Cai 2009, 32, 102–106. [Google Scholar]
- Liu, T.; Zhao, L.; Zhang, Y.; Chen, W.; Liu, D.; Hou, H.; Ding, L.; Li, X. Ginsenoside 20(S)-Rg3 targets HIF-1alpha to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS ONE 2014, 9. [Google Scholar]
- Wang, J.H.; Nao, J.F.; Zhang, M.; He, P. 20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol 2014, 35, 11985–11994. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.J.; Zhang, M.Z.; Wang, L.X. Gensenoside Rg3 Inhibits Hypoxia-induced VEGF Expression in Human Cancer Cells. Cell. Physiol. Biochem. 2010, 26, 849–858. [Google Scholar] [CrossRef]
- Kim, J.-W.; Jung, S.-Y.; Kwon, Y.-H.; Lee, J.-H.; Lee, Y.M.; Lee, B.-Y.; Kwon, S.-M. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol. Ther. 2012, 13, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Li, H.D.; Li, B.; Jiang, S.D.; Jiang, L.S. Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells. Oncol. Rep. 2014, 31, 919–925. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, E.H.; Ko, S.R.; Choi, K.J.; Park, J.H.; Im, D.S. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch. Pharm Res. 2004, 27, 429–435. [Google Scholar] [CrossRef]
- Sin, S.; Kim, S.Y.; Kim, S.S. Chronic treatment with ginsenoside Rg3 induces Akt-dependent senescence in human glioma cells. Int. J. Oncol. 2012, 41, 1669–1674. [Google Scholar] [CrossRef]
- Ng, W.Y.; Yang, M.S. Effects of ginsenosides Re and Rg3 on intracellular redox state and cell proliferation in C6 glioma cells. Chin. Med. 2008, 3, 8. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, G.M.; Su, J.; Shang, L.H.; Chen, G. Effects of ginsenoside Rg3 on the micro-lymphatic metastasis of colorectal neoplasms. Zhonghua Zhong Liu Za Zhi 2005, 27, 742. [Google Scholar]
- He, B.C.; Gao, J.L.; Luo, X.; Luo, J.; Shen, J.; Wang, L.; Zhou, Q.; Wang, Y.T.; Luu, H.H.; Haydon, R.C.; et al. Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/ss-catenin signaling. Int. J. Oncol. 2011, 38, 437–445. [Google Scholar] [CrossRef]
- Shan, X.; Fu, Y.S.; Aziz, F.; Wang, X.Q.; Yan, Q.; Liu, J.W. Ginsenoside Rg3 inhibits melanoma cell proliferation through down-regulation of histone deacetylase 3 (HDAC3) and increase of p53 acetylation. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Shan, X.; Aziz, F.; Tian, L.L.; Wang, X.Q.; Yan, Q.; Liu, J.W. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int J. Oncol 2015, 46, 1667–1676. [Google Scholar] [CrossRef]
- Tao, H.; Yao, M.; Zou, S.; Zhao, D.; Qiu, H. Effect of angiogenesis inhibitor Rg3 on the growth and metastasis of gastric cancer in SCID mice. Zhonghuawai Ke Za Zhi. 2002, 40, 606–608. [Google Scholar]
- Yun, T.K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003, 524, 63–74. [Google Scholar] [CrossRef]
- Liu, C.; Gong, Q.; Chen, T.; Lv, J.; Feng, Z.; Liu, P.; Deng, Z. Treatment with 20(S)-ginsenoside Rg3 reverses multidrug resistance in A549/DDP xenograft tumors. Oncol Lett. 2018, 15, 4376–4382. [Google Scholar] [CrossRef]
- Wu, R.; Ru, Q.; Chen, L.; Ma, B.; Li, C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J. Food Sci. 2014, 79, 1750–3841. [Google Scholar] [CrossRef]
- Yang, L.Q.; Wang, B.; Gan, H.; Fu, S.T.; Zhu, X.X.; Wu, Z.N.; Zhan, D.W.; Gu, R.L.; Dou, G.F.; Meng, Z.Y. Enhanced oral bioavailability and anti-tumour effect of paclitaxel by 20 (s)-ginsenoside Rg3 in vivo. Biopharm. Drug Dispos. 2012, 33, 425–436. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.Y.; Yuk, D.Y.; Moon, D.C.; Choi, S.S.; Kim, Y.; Han, S.B.; Oh, K.-W.; Hong, J.T. Inhibition of NF-κB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch. Pharmacal Res. 2009, 32, 755–765. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, G.T.; Roh, S.H.; Song, J.-S.; Kim, H.-J.; Hong, S.-S.; Kwon, S.W.; Park, J.H. Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Biosci. Biotechnol. Biochem. 2009, 0903051360. [Google Scholar]
- Kim, D.-G.; Jung, K.H.; Lee, D.-G.; Yoon, J.-H.; Choi, K.S.; Kwon, S.W.; Shen, H.-M.; Morgan, M.J.; Hong, S.-S.; Kim, Y.-S. 20 (S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget 2014, 5, 4438. [Google Scholar] [CrossRef]
- Che, J.-B.; Liu, Z.-H.; Ma, H.-B.; Li, Y.; Zhao, H.; Li, X.-H.; Liu, W.-C.; Shi, G.-N. Influence of As2O3 combined with ginsenosides Rg3 on inhibition of lung cancer NCI-H1299 cells and on subsistence of nude mice bearing hepatoma. Asian Pac. J. Trop. Med. 2014, 7, 772–775. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, X.; Yang, B.; Wang, J.; Yang, F. Antiangiogenic effect of capecitabine combined with ginsenoside Rg3 on breast cancer in mice. Cancer Biother. Radiopharm. 2008, 23, 647–654. [Google Scholar] [CrossRef]
- Lee, C.K.; Park, K.-K.; Chung, A.-S.; Chung, W.-Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD (P) H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem. Toxicol. 2012, 50, 2565–2574. [Google Scholar]
- Liu, T.-G.; Huang, Y.; Cui, D.-D.; Huang, X.-B.; Mao, S.-H.; Ji, L.-L.; Song, H.-B.; Yi, C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. Bmc Cancer 2009, 9, 250. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, J.; Huang, Y.; Han, S.; Liu, N.; Zhu, G.; Yao, J. Effect of adjuvant chemotherapy of ginsenoside Rg3 combined with mitomycin C and tegafur in advanced gastric cancer. Zhonghua Wei Chang. Wai Ke Za Zhi = Chin. J. Gastrointest. Surg. 2007, 10, 64–66. [Google Scholar]
- Shuqi, L.H.P.B.L. Department of Oncology, Guang An Men Hospital of China Academy of Traditional Chinese Medicine, Beijing; Phase II Clinical Trial Report of Treating Lung Cancer with Shen Yi Capsule. Chin. J. Clin. Oncol. 2002, 4. [Google Scholar]
- Oh, M.; Choi, Y.Y.; Choi, S.; Chung, H.Y.; Kim, K.; Kim, S.I.; Kim, D.K.; Kim, N.D. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int. J. Oncol. 1999, 14, 869–875. [Google Scholar] [CrossRef]
- An, I.; An, S.; Kwon, K.J.; Kim, Y.; Bae, S. Ginsenoside Rh2 mediates changes in the microRNA expression profile of human non-small cell lung cancer A549 cells. Oncol. Rep. 2013, 29, 523–528. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Wang, C.; Searle, J.S.; He, T.; Yuan, C.; Du, W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011, 301, 185–192. [Google Scholar] [CrossRef]
- Huang, J.; Peng, K.; Wang, L.; Wen, B.; Zhou, L.; Luo, T.; Su, M.; Li, J.; Luo, Z. Ginsenoside Rh2 inhibits proliferation and induces apoptosis in human leukemia cells via TNF-α signaling pathway. Acta Biochim. Et Biophys. Sin. 2016, 48, 750–755. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Li, Z.; Xie, S.; Zhou, J.; Guo, X.; Zhou, X.; Li, G.; Zhong, R.; Ma, A. Ginsenoside Rh2 suppresses growth of uterine leiomyoma in vitro and in vivo and may regulate ERα/c-Src/p38 MAPK activity. J. Funct. Foods 2015, 18, 73–82. [Google Scholar] [CrossRef]
- Kim, Y.; Jin, S. Ginsenoside Rh2 induces apoptosisvia activation of caspase-1 and -3 and up-regulation of bax in human neuroblastoma. Arch. Pharmacal. Res. 2004, 27, 834–839. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Ma, W.; Cheng, T.; Liu, Y. Ginsenoside Rh2 inhibits invasiveness of glioblastoma through modulation of VEGF-A. Tumor Biol. 2016, 37, 15477–15482. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Jia, J.; Jin, X.; Yu, D.; Guo, C.; Xie, B.; Qian, L. Ginsenoside-Rh2 Inhibits Proliferation and Induces Apoptosis of Human Gastric Cancer SGC-7901 Side Population Cells. Asian Pac. J. Cancer Prev. 2016, 17, 1817–1821. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Liu, H.; Zhang, L. Ginsenoside Rh2 inhibits hepatocellular carcinoma through β-catenin and autophagy. Sci. Rep. 2016, 6, 19383. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Ginsenosides 20(S)-protopanaxadiol and Rh2 reduce cell proliferation and increase sub-G1 cells in two cultured intestinal cell lines, Int-407 and Caco-2. Can. J. Physiol. Pharmacol. 2004, 82, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Chen, L.; Ding, W.; Zhang, W.; Wang, H.L.; Wang, S.; Liu, W. Ginsenoside G-Rh2 synergizes with SMI-4a in anti-melanoma activity through autophagic cell death. Chin. Med. 2018, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yang, S.; Huang, C.F.; Chen, J.; Chang, W.; Hsu, S. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother. Pharmacol. 2005, 55, 531–540. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Ma, W.; Guo, W.; Zhou, G.; Cheng, T.; Liu, Y. EGFR signaling-dependent inhibition of glioblastoma growth by ginsenoside Rh2. Tumor Biol. 2014, 35, 5593–5598. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qiu, Y. Ginsenoside Rh2 Targets EGFR by Up-Regulation of miR-491 to Enhance Anti-tumor Activity in Hepatitis B Virus-Related Hepatocellular Carcinoma. Cell Biochem. Biophys. 2015, 72, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hong, B.; Wu, S.; Niu, T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumor Biol. 2015, 36, 2377–2381. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, Y.; Kim, S.; Park, J.H.; Lee, S. Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase activity via up-regulating p21Cip/WAF1 and down-regulating cyclin E in SK-HEP-1 cells. Anticancer Res. 1997, 17, 1067–1072. [Google Scholar]
- Liang, L.D.; He, T.; Du, T.W.; Fan, Y.G.; Chen, D.S.; Wang, Y. Ginsenoside-Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol. Med. Rep. 2015, 11, 940–946. [Google Scholar] [CrossRef]
- Feng, S.-L.; Luo, H.-B.; Cai, L.; Zhang, J.; Wang, D.; Chen, Y.-J.; Zhan, H.-X.; Jiang, Z.-H.; Xie, Y. Ginsenoside Rg5 overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter: In vitro and in vivo study. J. Ginseng Res. 2018. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Murakami, K.; Hasegawa, H.; Murata, J.; Saiki, I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem. Biophys. Res. Commun. 1998, 246, 725–730. [Google Scholar] [CrossRef]
- Zhou, W.; Feng, M.-Q.; Li, J.-Y.; Zhou, P. Studies on the preparation, crystal structure and bioactivity of ginsenoside compound K. 2006. [Google Scholar]
- Lee, S.-J.; Sung, J.-H.; Lee, S.-J.; Moon, C.-K.; Lee, B.-H. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999, 144, 39–43. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Zhu, Y.; Li, X. Anti-cancer effects of ginsenoside compound k on pediatric acute myeloid leukemia cells. Cancer Cell Int. 2013, 13, 24. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jiang, Y.; Liu, T.; Luo, Y.; Diao, E.; Cao, Y.; Chen, L.; Zhang, L.; Gu, Q. Enhanced cytotoxic and apoptotic potential in hepatic carcinoma cells of chitosan nanoparticles loaded with ginsenoside compound K. Carbohydr. Polym. 2018, 198, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, B.; Hu, X.; Zhang, H.; Jiang, B.; Spranger, M.I.; Zhao, Y. Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. J. Agric. Food Chem. 2007, 55, 9373–9379. [Google Scholar] [CrossRef]
- Ming, Y.L.; Song, G.; Chen, L.H.; Zheng, Z.Z.; Chen, Z.Y.; Ouyang, G.L.; Tong, Q.X. Anti-proliferation and apoptosis induced by a novel intestinal metabolite of ginseng saponin in human hepatocellular carcinoma cells. Cell Biol. Int. 2007, 31, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, G.-J.; Wang, C.-Z.; Wen, X.-D.; Calway, T.; Li, Z.; He, T.-C.; Du, W.; Bissonnette, M.; Musch, M. Compound K, a ginsenoside metabolite, inhibits colon cancer growth via multiple pathways including p53-p21 interactions. Int. J. Mol. Sci. 2013, 14, 2980–2995. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Song, G.; Zhang, B.; Liu, Z.; Chen, R.; Zhang, H.; Hu, T. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosis via Bid-mediated mitochondrial pathway. J. Cell. Mol. Med. 2012, 16, 96–106. [Google Scholar] [CrossRef]
- Kwak, C.W.; Son, Y.M.; Gu, M.J.; Kim, G.; Lee, I.K.; Kye, Y.C.; Kim, H.W.; Song, K.-D.; Chu, H.; Park, B.-C. A bacterial metabolite, compound K, induces programmed necrosis in MCF-7 cells via GSK3β. J. Microbiol Biotechnol 2015, 25, 1170–1176. [Google Scholar] [CrossRef]
- Law, C.K.-M.; Kwok, H.-H.; Poon, P.-Y.; Lau, C.-C.; Jiang, Z.-H.; Tai, W.C.-S.; Hsiao, W.W.-L.; Mak, N.-K.; Yue, P.Y.-K.; Wong, R.N.-S. Ginsenoside compound K induces apoptosis in nasopharyngeal carcinoma cells via activation of apoptosis-inducing factor. Chin. Med. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Woo, M.S.; Kim, S.Y.; Kim, W.K.; Hyun, J.W.; Kim, E.J.; Kim, D.H.; Kim, H.S. Ginseng saponin metabolite suppresses phorbol ester–induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathways in human astroglioma cells. Int. J. Cancer 2006, 118, 490–497. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, M.C.; Jang, J.-P.; Sohng, J.K.; Jung, H.J. The ginsenoside metabolite compound K inhibits growth, migration and stemness of glioblastoma cells. Int. J. Oncol. 2017, 51, 414–424. [Google Scholar] [CrossRef]
- Yang, X.-D.; Yang, Y.-Y.; Ouyang, D.-S.; Yang, G.-P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, Y.-S.; Jung, I.-S.; Park, S.-Y.; Chung, H.-Y.; Lee, I.-R.; Yun, Y.-S. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998, 64, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Z.-N.; Cai, J.-P.; Chen, K.-X.; Zhang, B.; Feng, M.-Y.; Shi, Q.-T.; Li, R.; Qin, Y.; Geng, J.-S. Panax ginseng polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1 pathway in human gastric cancer. Carbohydr. Polym. 2014, 102, 103–109. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Saba, E.; Irfan, M.; Kim, M.; Yi-Le Chan, J.; Jeon, B.S.; Choi, S.K.; Rhee, M.H. The anti-inflammatory and anti-nociceptive effects of Korean black ginseng. Phytomedicine 2019, 54, 169–181. [Google Scholar] [CrossRef]
- Han, M.-S.; Han, I.-H.; Lee, D.; An, J.M.; Kim, S.-N.; Shin, M.-S.; Yamabe, N.; Hwang, G.S.; Yoo, H.H.; Choi, S.-J. Beneficial effects of fermented black ginseng and its ginsenoside 20 (S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 2016, 40, 135–140. [Google Scholar] [CrossRef]
- Shin, Y.J.; Jang, H.H.; Song, G.Y. Study on Anti-atopic Effects of Black Ginseng. Kor. J. Aesthet. Cosmetol 2012, 10, 91–97. [Google Scholar]
- Kang, J.A.; Song, H.; Byun, E.; Ahn, N.; Kim, H.; Nam, Y.R.; Lee, G.H.; Jang, B.; Choi, D.S.; Lee, D. Gamma-irradiated black ginseng extract inhibits mast cell degranulation and suppresses atopic dermatitis-like skin lesions in mice. Food Chem. Toxicol. 2018, 111, 133–143. [Google Scholar] [CrossRef]
- Shin, Y.-M.; Jung, H.-J.; Choi, W.-Y.; Lim, C.-J. Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20 (S)-ginsenoside Rg3 in cultured mammalian cell lines. Mol. Biol. Rep. 2013, 40, 269–279. [Google Scholar] [CrossRef]
- Bae, E.-A.; Kim, E.-J.; Park, J.-S.; Kim, H.-S.; Ryu, J.H.; Kim, D.-H. Ginsenosides Rg3 and Rh2 Inhibit the Activation of AP-1 and Protein Kinase A Pathway in Lipopolysaccharide/Interferon-γ-Stimulated BV-2 Microglial Cells. Planta Med. 2006, 72, 627–633. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, Y.S.; Jeong, T.C.; Joe, C.O.; Shin, H.J.; Lee, Y.H.; Nam, K.Y.; Park, J.D. Nitric oxide is involved in the immunomodulating activities of acidic polysaccharide from Panax ginseng. Planta Med. 2001, 67, 122–126. [Google Scholar] [CrossRef]
- Du, X.F.; Jiang, C.Z.; Wu, C.F.; Won, E.K.; Choung, S.Y. Synergistic immunostimulatory effect of pidotimod and red ginseng acidic polysaccharide on humoral immunity of immunosuppressed mice. Die Pharm. -Int. J. Pharm. Sci. 2008, 63, 904–908. [Google Scholar]
- Kong, Y.-H.; Lee, Y.-C.; Choi, S.-Y. Neuroprotective and anti-inflammatory effects of phenolic compounds in Panax ginseng CA Meyer. J. Ginseng Res. 2009, 33, 111–114. [Google Scholar]
- Hu, J.-N.; Liu, Z.; Wang, Z.; Li, X.-D.; Zhang, L.-X.; Li, W.; Wang, Y.-P. Ameliorative effects and possible molecular mechanism of action of black ginseng (Panax ginseng) on acetaminophen-mediated liver injury. Molecules 2017, 22, 664. [Google Scholar]
- Kang, O.-H.; Shon, M.-Y.; Kong, R.; Seo, Y.-S.; Zhou, T.; Kim, D.-Y.; Kim, Y.-S.; Kwon, D.-Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. Bmc Complementary Altern. Med. 2017, 17, 341. [Google Scholar] [CrossRef]
- Lee, H.-U.; Bae, E.-A.; Han, M.J.; Kim, D.-H. Hepatoprotective effect of 20 (S)-ginsenosides Rg3 and its metabolite 20 (S)-ginsenoside Rh2 on tert-butyl hydroperoxide-induced liver injury. Biol. Pharm. Bull. 2005, 28, 1992–1994. [Google Scholar] [CrossRef]
- Lee, H.U.; Bae, E.A.; Han, M.J.; Kim, N.J.; Kim, D.H. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005, 25, 1069–1073. [Google Scholar] [CrossRef]
- Kim, J.H.; Pan, J.H.; Cho, H.T.; Kim, Y.J. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLoS ONE 2016, 11, e0146843. [Google Scholar] [CrossRef]
- Kim, S.-N.; Kang, S.-J. Effects of black ginseng (9 times-steaming ginseng) on hypoglycemic action and changes in the composition of ginsenosides on the steaming process. Korean J. Food Sci. Technol. 2009, 41, 77–81. [Google Scholar]
- Seo, Y.-S.; Shon, M.-Y.; Kong, R.; Kang, O.-H.; Zhou, T.; Kim, D.-Y.; Kwon, D.-Y. Black ginseng extract exerts anti-hyperglycemic effect via modulation of glucose metabolism in liver and muscle. J. Ethnopharmacol. 2016, 190, 231–240. [Google Scholar] [CrossRef]
- Lee, W.K.; Kao, S.T.; Liu, I.M.; Cheng, J.T. Increase of insulin secretion by ginsenoside Rh2 to lower plasma glucose in Wistar rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 27–32. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, Y.; Li, C.; Qi, P.; Bao, J. Antihyperglycemic effect of ginsenoside Rh2 by inducing islet β-cell regeneration in mice. Horm. Metab. Res. 2012, 44, 33–40. [Google Scholar] [CrossRef]
- Kim, S.; Jang, H.; Oh, M.; Eom, D.; Kang, K.; Kim, Y.; Lee, J.; Ham, J.; Choi, S.; Wee, Y. Ginsenoside Rg3 enhances islet cell function and attenuates apoptosis in mouse islets. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2014; Volume 46, pp. 1150–1155. [Google Scholar]
- Lee, M.R.; Kim, B.C.; Kim, R.; Oh, H.I.; Kim, H.K.; Choi, K.J.; Sung, C.K. Anti-obesity effects of black ginseng extract in high fat diet-fed mice. J. Ginseng Res. 2013, 37, 308. [Google Scholar] [CrossRef]
- Saba, E.; Jeon, B.R.; Jeong, D.-H.; Lee, K.; Goo, Y.-K.; Kim, S.-H.; Sung, C.-K.; Roh, S.-S.; Kim, S.D.; Kim, H.-K. Black ginseng extract ameliorates hypercholesterolemia in rats. J. Ginseng Res. 2016, 40, 160–168. [Google Scholar] [CrossRef]
- Kim, A.-J.; Yoo, H.-S.; Kang, S.-J. Ameliorative effect of black ginseng on diabetic complications in C57BLKS/J-db/db mice. Korean J. Food Nutr. 2012, 25, 99–104. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, A.-J.; Cheon, Y.-P.; Lee, M. Anti-obesity effects of water and ethanol extracts of black ginseng. J. Korean Soc. Food Sci. Nutr. 2015, 44, 314–323. [Google Scholar] [CrossRef]
- Lee, M.-R.; Yun, B.-S.; Liu, L.; Zhang, D.-L.; Wang, Z.; Wang, C.-L.; Gu, L.-J.; Wang, C.-Y.; Mo, E.-K.; Sung, C.-k. Effect of black ginseng on memory improvement in the amnesic mice induced by scopolamine. J. Ginseng Res. 2010, 34, 51–58. [Google Scholar] [CrossRef]
- Saba, E.; Jeong, D.-H.; Roh, S.-S.; Kim, S.-H.; Kim, S.-D.; Kim, H.-K.; Rhee, M.-H. Black ginseng-enriched Chong-Myung-Tang extracts improve spatial learning behavior in rats and elicit anti-inflammatory effects in vitro. J. Ginseng Res. 2017, 41, 151–158. [Google Scholar] [CrossRef]
- Park, H.-J.; Shim, H.S.; Kim, K.S.; Shim, I. The protective effect of black ginseng against transient focal ischemia-induced neuronal damage in rats. Korean J. Physiol. Pharmacol. 2011, 15, 333–338. [Google Scholar] [CrossRef]
- Lee, M.R.; Begum, S.; Sung, C.K. Effect of red and black ginseng on cholinergic markers, presynaptic markers, and neurotrophins in the brain of aged mice. Food Sci. Biotechnol. 2017, 26, 1743–1747. [Google Scholar] [CrossRef]
- Lee, E.; Kim, S.; Chung, K.C.; Choo, M.-K.; Kim, D.-H.; Nam, G.; Rhim, H. 20 (S)-ginsenoside Rh2, a newly identified active ingredient of ginseng, inhibits NMDA receptors in cultured rat hippocampal neurons. Eur. J. Pharmacol. 2006, 536, 69–77. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Lv, J.; Jiang, N.; Fan, B.; Qu, L.; Li, Y.; Chen, S.; Wang, F.; Liu, X. Ginsenoside Rh2 reverses sleep deprivation-induced cognitive deficit in mice. Behav. Brain Res. 2018, 349, 109–115. [Google Scholar] [CrossRef]
- Tian, J.; Fu, F.; Geng, M.; Jiang, Y.; Yang, J.; Jiang, W.; Wang, C.; Liu, K. Neuroprotective effect of 20 (S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci. Lett. 2005, 374, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Gu, J.; Feng, L.; Liu, J.; Zhang, M.; Jia, X.; Liu, M.; Yao, D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 2014, 19, 317–326. [Google Scholar] [CrossRef]
- Oh, C.-H.; Kim, G.-N.; Lee, S.-H.; Lee, J.-S.; Jang, H.-D. Effects of heat processing time on total phenolic content and antioxidant capacity of ginseng Jung Kwa. J. Ginseng Res. 2010, 34, 198–204. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-Y.; You, B.-R.; Kim, H.-R.; Choi, J.-E.; Nam, K.-Y.; Moon, B.-D.; Kim, M.-R. Antioxidant activities of ethanol extracts from black ginseng prepared by steaming-drying cycles. J. Korean Soc. Food Sci. Nutr. 2011, 40, 156–162. [Google Scholar] [CrossRef]
- Lee, S.-R.; Kim, M.-R.; Yon, J.-M.; Baek, I.-J.; Park, C.G.; Lee, B.J.; Yun, Y.W.; Nam, S.-Y. Black ginseng inhibits ethanol-induced teratogenesis in cultured mouse embryos through its effects on antioxidant activity. Toxicol. Vitro 2009, 23, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Lee, Y.Y.; Kim, M.; Kim, S.-H.; Hong, S.-B.; Rhee, M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J. Ginseng Res. 2018, 42, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.-Y.; Han, Y.N.; Han, B.H. Maltol, an antioxidant component of Korean red ginseng, shows little prooxidant activity. Arch. Pharmacal Res. 1996, 19, 112–115. [Google Scholar] [CrossRef]
- Gong, L.-H.; Lei, T.; Zhang, Z.-L.; Liang, Q.-C.; Zhai, F.-G.; Wu, Y.-Y.; Zhang, X.-P.; Liu, J.-Q.; Liu, J.-W. Purification, compositional analysis and antioxidant properties of polysaccharides from black ginseng. Trop. J. Pharm. Res. 2018, 17, 1317–1324. [Google Scholar] [CrossRef]
- Jo, G.S.; Chai, H.-Y.; Ji, H.J.; Kang, M.H.; Kang, S.-J.; Ji, J.-G.; Kim, D.J.; Lee, B.J. Enhancement of exercise capacity by black ginseng extract in rats. Lab. Anim. Res. 2010, 26, 279–286. [Google Scholar] [CrossRef][Green Version]
- Lee, S.-Y.; Go, G.-Y.; Vuong, T.A.; Kim, J.W.; Lee, S.; Jo, A.; An, J.M.; Kim, S.-N.; Seo, D.-W.; Kim, J.-S. Black ginseng activates Akt signaling, thereby enhancing myoblast differentiation and myotube growth. J. Ginseng Res. 2018, 42, 116–121. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.Y.; Kim, S.S.; Kim, J.; Boo, S.J.; Hur, J. Function of Korean black ginseng: Improvement of andropause symptoms by a complex extract of black ginseng and fenugreek in TM3 Leydig cells and aged rats. J. Ethn. Foods 2016, 3, 228–234. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Fan, Y.; Chen, Y.; Liu, D.; Cheng, H.; Gao, X.; Zhou, Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. J. Ethnopharmacol. 2010, 130, 421–423. [Google Scholar] [CrossRef]
- Pham, Q.L.; Jang, H.J.; Kim, K.B. Anti-wrinkle effect of fermented black ginseng on human fibroblasts. Int. J. Mol. Med. 2017, 39, 681–686. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, D.S.; Kim, C.; Shin, M.; Seo, C.; Shin, H.; Hwang, G.S.; An, J.M.; Kim, S.; Kang, K.S. Effects of fermented black ginseng on wound healing mediated by angiogenesis through the mitogen-activated protein kinase pathway in human umbilical vein endothelial cells. J. Ginseng Res. 2017, 42, 524–531. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, J.H.; Hong, H.-D.; Kwon, J.; Lee, E.J.; Jang, M.; Lee, S.-Y.; Han, A.-R.; Nam, T.G.; Hong, S.K. Ginsenosides Rg5 and Rk1, the skin-whitening agents in black ginseng. J. Funct. Foods 2018, 45, 67–74. [Google Scholar] [CrossRef]
- Lee, M.; Oh, C.; Li, Z.; Li, J.; Wang, C.; Wang, Z.; Gu, L.; Lee, S.; Lee, J.; Lim, B. Evaluation of the Oral Acute Toxicity of Black Ginseng in Rats. J. Ginseng Res. 2011, 35, 39–44. [Google Scholar] [CrossRef][Green Version]
- Jung, K.; An, J.M.; Eom, D.; Kang, K.S.; Kim, S. Preventive effect of fermented black ginseng against cisplatin-induced nephrotoxicity in rats. J. Ginseng Res. 2017, 41, 188–194. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, C.; Cha, B.; Choi, S.; Kwon, K. Component analysis of cultivated ginseng, cultivated wild ginseng, and wild ginseng and the change of ginsenoside components in the process of red ginseng. J. Pharmacopunct. 2010, 13, 63–77. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules 2019, 24, 1856. https://doi.org/10.3390/molecules24101856
Metwaly AM, Lianlian Z, Luqi H, Deqiang D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules. 2019; 24(10):1856. https://doi.org/10.3390/molecules24101856
Chicago/Turabian StyleMetwaly, Ahmed M., Zhu Lianlian, Huang Luqi, and Dou Deqiang. 2019. "Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects" Molecules 24, no. 10: 1856. https://doi.org/10.3390/molecules24101856
APA StyleMetwaly, A. M., Lianlian, Z., Luqi, H., & Deqiang, D. (2019). Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules, 24(10), 1856. https://doi.org/10.3390/molecules24101856






