Steady Enhancement in Photovoltaic Properties of Fluorine Functionalized Quinoxaline-Based Narrow Bandgap Polymer
Abstract
:1. Introduction
2. Results and Discussion
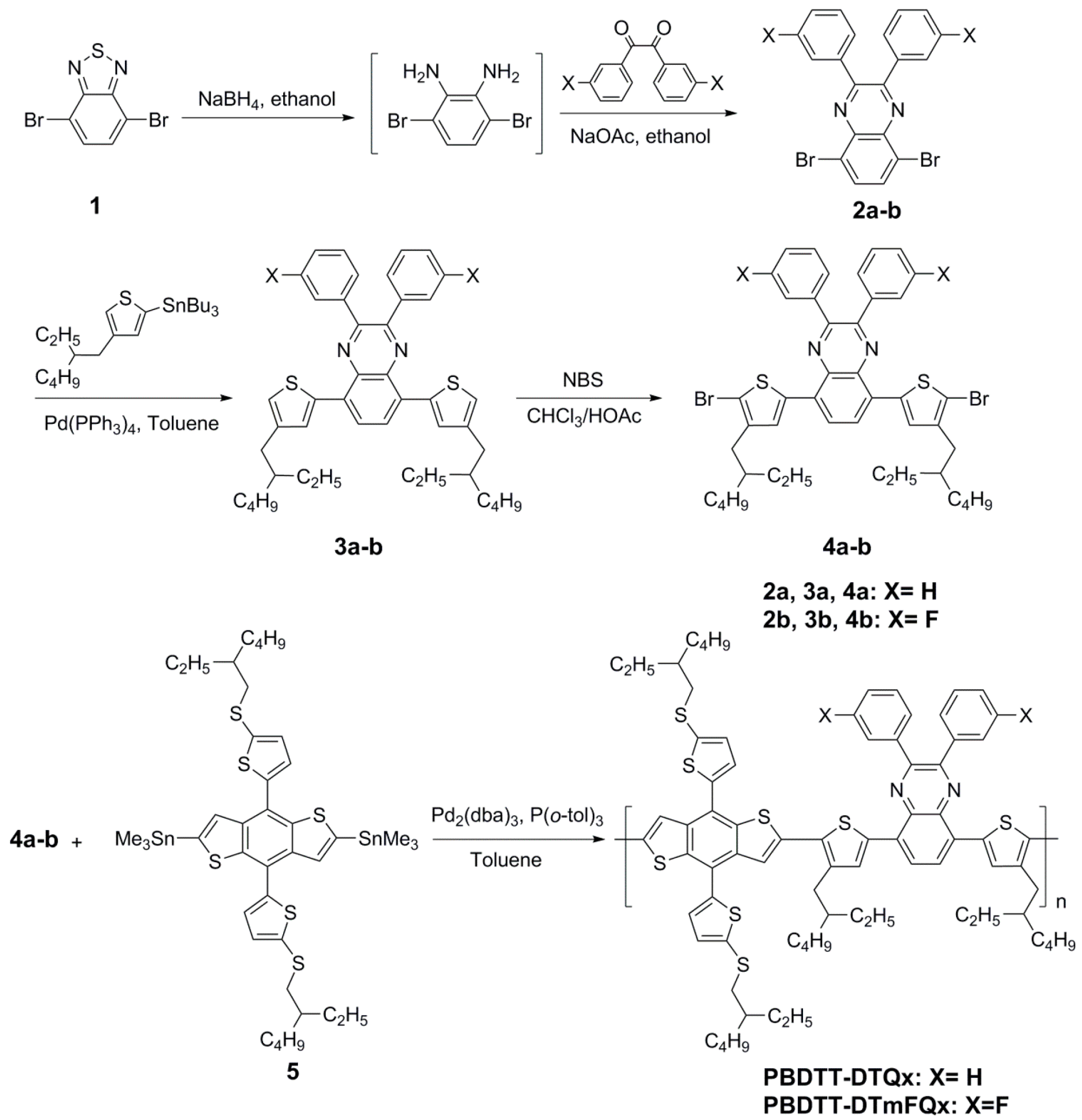
2.1. Synthesis and Thermal Stability
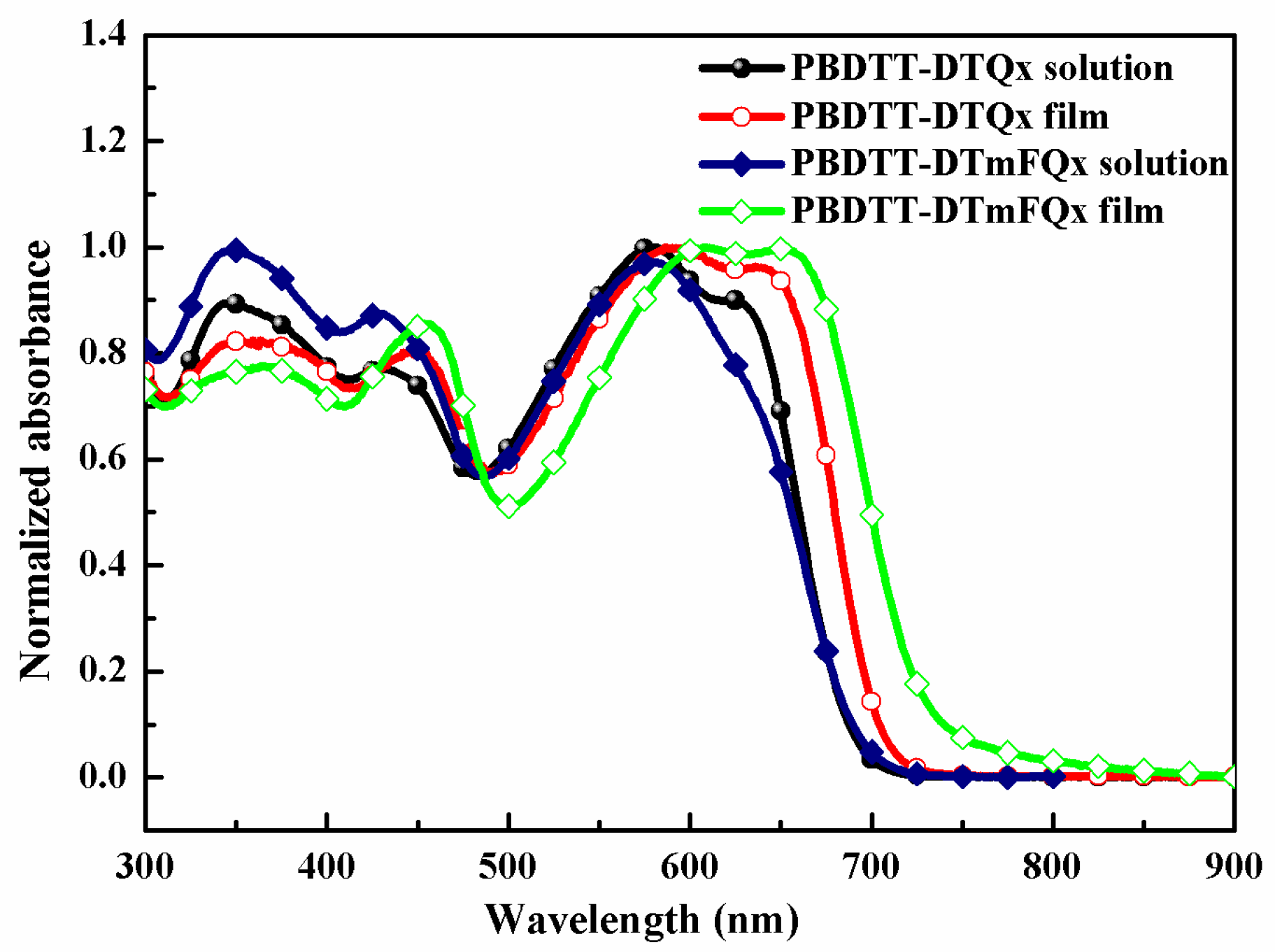
2.2. Optical Properties
2.3. Electrochemical Properties
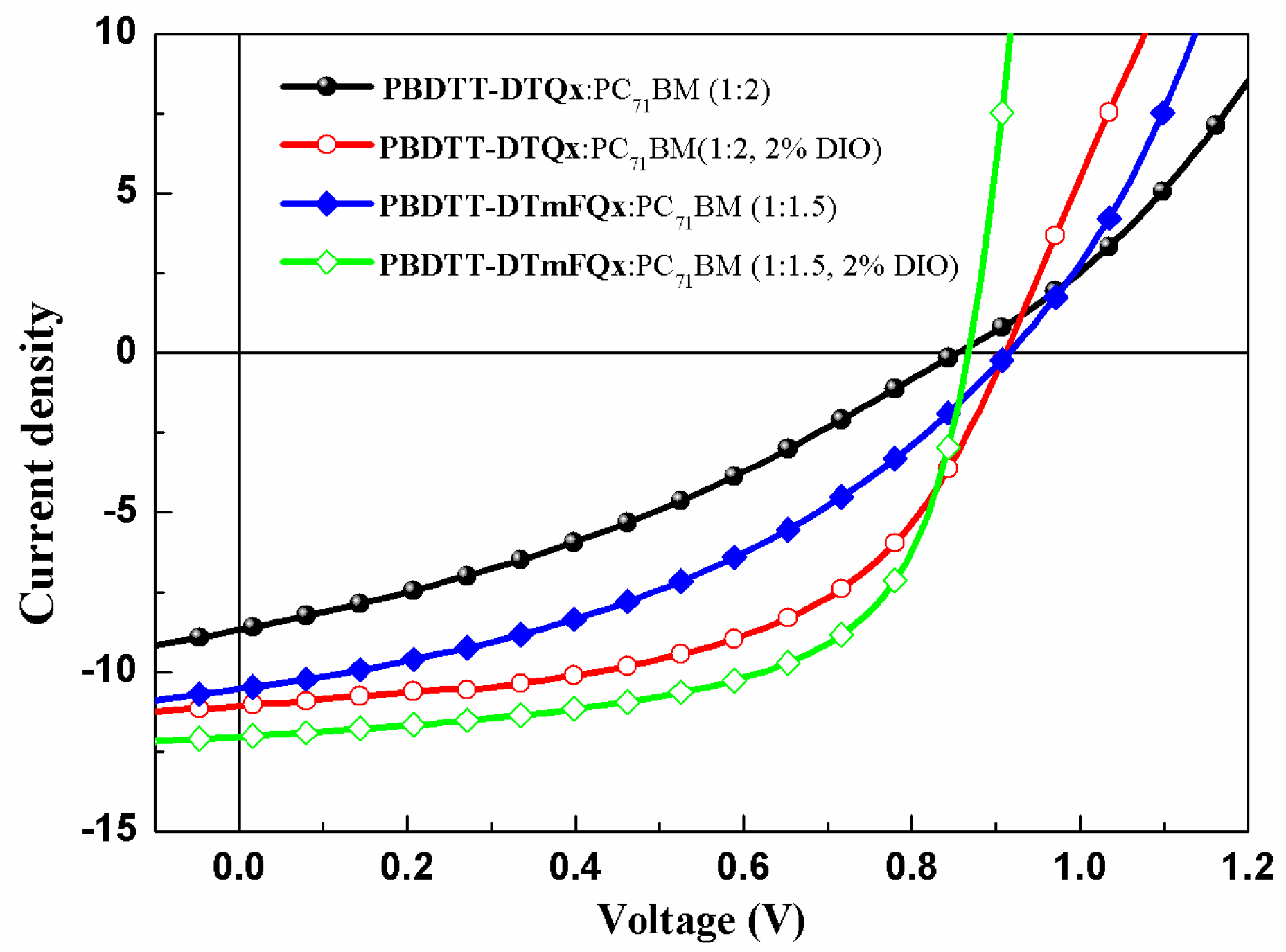
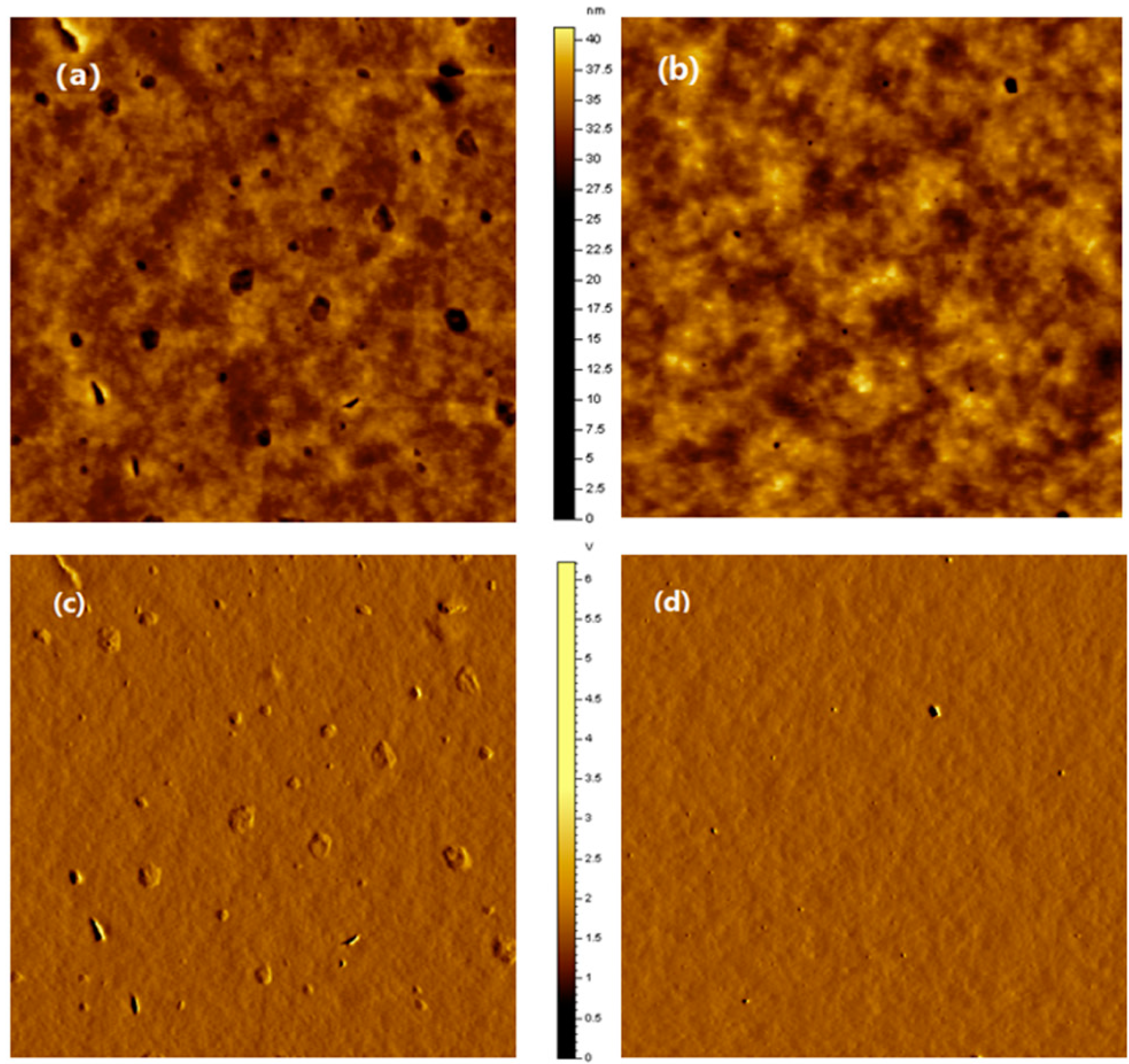
2.4. Photovoltaic Properties of the Copolymers
3. Experimental Section
3.1. Materials
3.2. Characterization
3.3. Fabrication and Characterization of PSCs
3.4. Synthesis of the Monomers and Copolymers
3.4.1. Synthesis of 5,8-dibromo-2,3-diphenylquinoxaline (2a)
3.4.2. Synthesis of 5,8-dibromo-2,3-bis(3-fluorophenyl)quinoxaline (2b)
3.4.3. Synthesis of 5,8-bis(4-(2-ethylhexyl)thiophen-2-yl)-2,3-diphenylquinoxaline (3a)
3.4.4. Synthesis of 5,8-bis(4-(2-ethylhexyl)thiophen-2-yl)-2,3-bis(3-fluorophenyl)quinoxaline (3b)
3.4.5. Synthesis of 5,8-bis(5-bromo-4-(2-ethylhexyl)thiophen-2-yl)-2,3-diphenylquinoxaline (4a)
3.4.6. Synthesis of 5,8-bis(5-bromo-4-(2-ethylhexyl)thiophen-2-yl)-2,3-bis-(3-fluoro-phenyl) quinoxaline (4b)
3.4.7. Synthesis of the Polymer PBDTT-DTQx
3.4.8. Synthesis of the Polymer PBDTT-DTmFQx
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, C.; Liu, M.; Pschirer, N.G.; Baumgarten, M.; Müllen, K. Polyphenylene-Based Materials for Organic Photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ye, L.; Zhang, H.; Li, S.; Zhang, S.; Hou, J. Molecular Design of Benzodithiophene-based Organic Photovoltaic Materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef]
- Sun, C.; Pan, F.; Bin, H.; Zhang, J.; Xue, L.; Qiu, B.; Wei, Z.; Zhang, Z.-G.; Li, Y. A Low Cost and High Performance Polymer Donor Material for Polymer Solar Cell. Nat. Commun. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; et al. Fast Charge Separation in a Non-fullerene Organic Solar Cell with a Small Driving Force. Nat. Energy 2016, 1, 16089. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photon. 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Cheng, P.; Zhan, X. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer Solar Cells. Nat. Photon. 2012, 6, 153–161. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced Power-conversion Efficiency in Polymer Solar Cells Using an Inverted Device Structure. Nat. Photon. 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Wu, Z.; Fan, B.; Li, A.; Xue, F.; Ouyang, J. Low-band Gap Copolymers of Ethynylfluorene and 3,6-dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione Synthesized under Microwave Irradiation for Polymer Photovoltaic Cells. Org. Electron. 2011, 12, 993–1102. [Google Scholar] [CrossRef]
- Li, S.; Ye, L.; Zhao, W.; Yan, H.; Yang, B.; Liu, D.; Li, W.; Ade, H.; Hou, J. A Wide Band Gap Polymer with a Deep Highest Occupied Molecular Orbital Level Enables 14.2% Efficiency in Polymer Solar Cells. J. Am. Chem. Soc. 2018, 140, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhang, K.; Jiang, X.-F.; Ying, L.; Huang, F.; Cao, Y. High-performance Nonfullerene Polymer Solar Cells Based on Imide-functionalized Wide-bandgap Polymers. Adv. Mater. 2017, 29, 1606396. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Z.; Wang, Z.; Li, K.; Feng, K.; Peng, Q. 10.20% Efficiency Polymer Solar Cells via Employing Bilaterally Hole-cascade Diazaphenanthrobisthiadiazole Polymer Donors and Electron-cascade Indene-C70 Bisadduct Acceptor. Nano Energy 2016, 25, 170–183. [Google Scholar] [CrossRef]
- Fan, Q.; Su, W.; Wang, Y.; Guo, B.; Jiang, Y.; Guo, X.; Liu, F.; Russell, T.P.; Zhang, M.; Li, Y. Synergistic Effect of Fluorination on Both Donor and Acceptor Materials for High Performance Non-fullerene Polymer Solar Cells with 13.5% Efficiency. Sci. China Chem. 2018, 61, 531–537. [Google Scholar] [CrossRef]
- Gedefaw, D.; Prosa, M.; Bolognesi, M.; Seri, M.; Andersson, M.R. Recent Development of Quinoxaline Based Polymers/Small Molecules for Organic Photovoltaics. Adv. Energy Mater. 2017, 7, 1700575. [Google Scholar] [CrossRef]
- Liu, M.; Gao, Y.; Zhang, Y.; Liu, Z.; Zhao, L. Quinoxaline-based Conjugated Polymers for Polymer Solar Cells. Polym. Chem. 2017, 8, 4613–4636. [Google Scholar] [CrossRef]
- Ha, Y.H.; Hong, J.; An, T.K.; Yun, J.-H.; Kim, K.; Park, C.E.; Kim, Y.-H.; Kwon, S.-K. Low-band Gap Copolymers Based on Diketopyrrolopyrrole and Dibenzosilole and Their Application in Organic Photovoltaics. Dyes Pigments 2017, 146, 73–81. [Google Scholar] [CrossRef]
- Meng, B.; Song, H.; Chen, X.; Xie, Z.; Liu, J.; Wang, L. Replacing Alkyl with Oligo(ethylene glycol) as Side Chains of Conjugated Polymers for Close π–π Stacking. Macromolecules 2015, 48, 4357–4363. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A Low-bandgap Poly(2,7-carbazole) Derivative for Use in High-performance Solar Cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Feng, D.; Tsai, S.-T.; Son, H.-J.; Li, G.; Yu, L. Development of New Semiconducting Polymers for High Performance Solar Cells. J. Am. Chem. Soc. 2009, 131, 56–57. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly Efficient Solar Cell Polymers Developed via Fine-tuning of Structural and Electronic Properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wong, W.-Y.; Li, Y. Improvement of Open-circuit Voltage and Photovoltaic Properties of 2D-conjugated Polymers by Alkylthio Substitution. Energy Environ. Sci. 2014, 7, 2276–2284. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Huo, L.; Zhang, M.; Hou, J. Molecular Design toward Highly Efficient Photovoltaic Polymers Based on Two-dimensional Conjugated Benzodithiophene. Acc. Chem. Res. 2014, 47, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Liu, Y.; Xiao, M.; Su, W.; Gao, H.; Chen, J.; Tan, H.; Wang, Y.; Yang, R.; Zhu, W. Enhancing the Photovoltaic Properties of Terpolymers Containing Benzo[1,2-b:4,5-b′]dithiophene, Phenanthro[4,5-abc]phenazine and Benzo[c][1,2,5]thiadiazole by Changing the Substituents. J. Mater. Chem. C 2015, 3, 6240–6248. [Google Scholar] [CrossRef]
- Fan, Q.; Jiang, H.; Liu, Y.; Su, W.; Tan, H.; Wang, Y.; Yang, R.; Zhu, W. Efficient Polymer Solar Cells Based on a New Quinoxaline Derivative with Fluorinated Phenyl Side Chain. J. Mater. Chem. C 2016, 4, 2606–2613. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Zhang, S.; Ye, L.; Zheng, Z.; Cui, Y.; Chen, Y.; Hou, J. Highly Efficient Photovoltaic Polymers Based on Benzodithiophene and Quinoxaline with Deeper HOMO Levels. Macromolecules 2015, 48, 5172–5178. [Google Scholar] [CrossRef]
- Hu, T.; Han, L.; Xiao, M.; Bao, X.; Wang, T.; Sun, M.; Yang, R. Enhancement of Photovoltaic Performance by Increasing Conjugation of the Acceptor Unit in Benzodithiophene and Quinoxaline Copolymers. J. Mater. Chem. C 2014, 2, 8047–8053. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Shin, W.-S.; Hawa, J.-R.; Moon, D.-K. Low Band-gap Polymers Based on Quinoxaline Derivatives and Fused Thiophene as Donor Materials for High Efficiency Bulk-heterojunction Photovoltaic Cells. J. Mater. Chem. 2009, 19, 4938–4945. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chen, Y.-H.; Liu, C.-C.; Chien, Y.-C.; Chou, S.-W.; Chou, P.-T. Prominent Short-circuit Currents of Fluorinated Quinoxaline-based Copolymer Solar Cells with a Power Conversion Efficiency of 8.0%. Chem. Mater. 2012, 24, 4766–4772. [Google Scholar]
- Xu, S.; Feng, L.; Yuan, J.; Zhang, Z.-G.; Li, Y.; Peng, H.; Zou, Y. Hexafluoroquinoxaline Based Polymer for Nonfullerene Solar Cells Reaching 9.4% Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 18816–18825. [Google Scholar] [CrossRef]
- Yuan, J.; Qiu, L.; Zhang, Z.-G.; Li, Y.; Chen, Y.; Zou, Y. Tetrafluoroquinoxaline Based Polymers for Non-fullerene Polymer Solar Cells with Efficiency over 9%. Nano Energy 2016, 30, 312–320. [Google Scholar] [CrossRef]
- Zhang, Q.; Kelly, M.A.; Bauer, N.; You, W. The Curious Case of Fluorination of Conjugated Polymers for Solar Cells. Acc. Chem. Res. 2017, 50, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.D.; Aguirre, A.; Shahid, M.; Gevaerts, V.S.; Meskers, S.C.; Janssen, R.A.J. Improved Film Morphology Reduces Charge Carrier Recombination into the Triplet Excited State in a Small Bandgap Polymer-Fullerene Photovoltaic Cell. Adv. Mater. 2010, 22, 4321–4324. [Google Scholar]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device Physics of Polymer:fullerene Bulk Heterojunction Solar Cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Zhao, W.; Yao, H.; Hou, J. Highly Efficient 2D-conjugated Benzodithiophene-based Photovoltaic Polymer with Linear Alkylthio Side Chain. Chem. Mater. 2014, 26, 3603–3817. [Google Scholar] [CrossRef]
- Hicks, L.D.; Hyatt, J.L.; Moak, T.; Edwards, C.C.; Tsurkan, L.; Wierdl, M.; Ferreirac, A.M.; Wadkins, R.M.; Potter, P.M. Analysis of the Inhibition of Mammalian Carboxylesterases by Novel Fluorobenzoins and Fluorobenzils. Bioorgan. Med. Chem. 2007, 15, 3801–3817. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, E.; Krebs, F.C. Low-Band-Gap Conjugated Polymers Based on Thiophene, Benzothiadiazole, and Benzobis(thiadiazole). Macromolecules 2006, 39, 2823–2831. [Google Scholar] [CrossRef]
Sample Availability: Samples of the polymers PBDTT-DTQx and PBDTT-DTmFQx are available from the authors. |
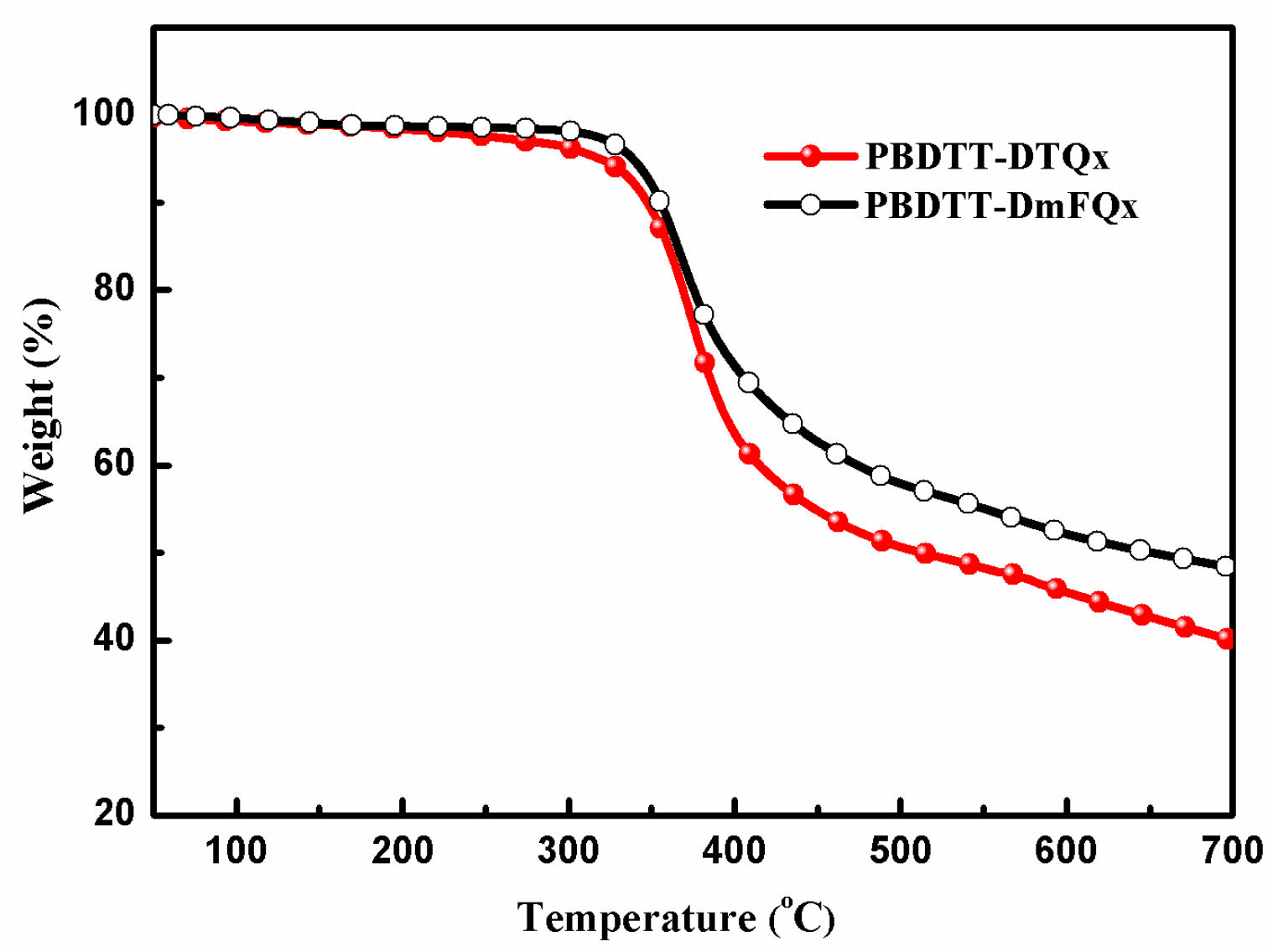


| Polymer | Mn (kDa) | Mw (kDa) | PDI | Td (°C) |
|---|---|---|---|---|
| PBDTT-DTQx | 18.5 | 44.5 | 2.4 | 316 |
| PBDTT-DmFQx | 21.2 | 53.2 | 2.5 | 338 |
| polymer | λmax, Solution (nm) | λmax, Film (nm) | λonset, Film (nm) | a (eV) | HOMO (eV) | LUMO (eV) | b (eV) |
|---|---|---|---|---|---|---|---|
| PBDTT-DTQx | 581 | 586 | 717 | 1.73 | −5.23 | −3.49 | 1.74 |
| PBDTT-DTmFQx | 578 | 652 | 737 | 1.68 | −5.33 | −3.63 | 1.70 |
| Polymer | Ratio a | Voc [V] | Jsc [mA cm−2] | FF [%] | PCE [%] | μhb [cm2 V−1 s−1] |
|---|---|---|---|---|---|---|
| PBDTT-DTQx | 1:2 | 0.85 | 8.67 | 33.27 | 2.46 | |
| 1:2 (2% DIO) | 0.91 | 11.05 | 53.95 | 5.43 | 8.38 × 10−5 | |
| PBDTT-DTmFQx | 1:1.5 | 0.91 | 10.52 | 39.44 | 3.79 | |
| 1:1.5 (2% DIO) | 0.87 | 12.00 | 61.45 | 6.40 | 2.34 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Jiang, H.; Wang, X.; Yan, L.; Zeng, W.; Wu, X.-G.; Zhuang, H.; Zhu, W.; Yang, R. Steady Enhancement in Photovoltaic Properties of Fluorine Functionalized Quinoxaline-Based Narrow Bandgap Polymer. Molecules 2019, 24, 54. https://doi.org/10.3390/molecules24010054
Wu Z, Jiang H, Wang X, Yan L, Zeng W, Wu X-G, Zhuang H, Zhu W, Yang R. Steady Enhancement in Photovoltaic Properties of Fluorine Functionalized Quinoxaline-Based Narrow Bandgap Polymer. Molecules. 2019; 24(1):54. https://doi.org/10.3390/molecules24010054
Chicago/Turabian StyleWu, Zhonglian, Huanxiang Jiang, Xingzhu Wang, Lei Yan, Wei Zeng, Xiu-Gang Wu, Haiyu Zhuang, Wen Zhu, and Renqiang Yang. 2019. "Steady Enhancement in Photovoltaic Properties of Fluorine Functionalized Quinoxaline-Based Narrow Bandgap Polymer" Molecules 24, no. 1: 54. https://doi.org/10.3390/molecules24010054
APA StyleWu, Z., Jiang, H., Wang, X., Yan, L., Zeng, W., Wu, X.-G., Zhuang, H., Zhu, W., & Yang, R. (2019). Steady Enhancement in Photovoltaic Properties of Fluorine Functionalized Quinoxaline-Based Narrow Bandgap Polymer. Molecules, 24(1), 54. https://doi.org/10.3390/molecules24010054





