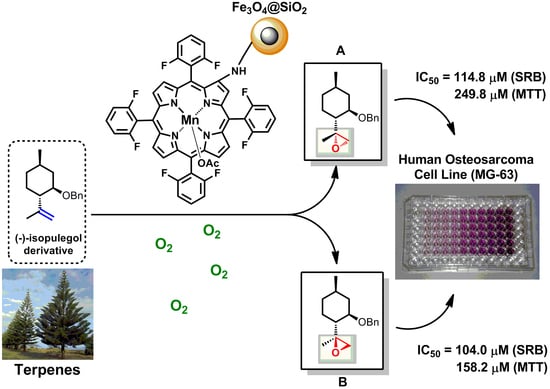
Bioinspired-Metalloporphyrin Magnetic Nanocomposite as a Reusable Catalyst for Synthesis of Diastereomeric (−)-Isopulegol Epoxide: Anticancer Activity Against Human Osteosarcoma Cells (MG-63)
Abstract
1. Introduction
2. Results and Discussion
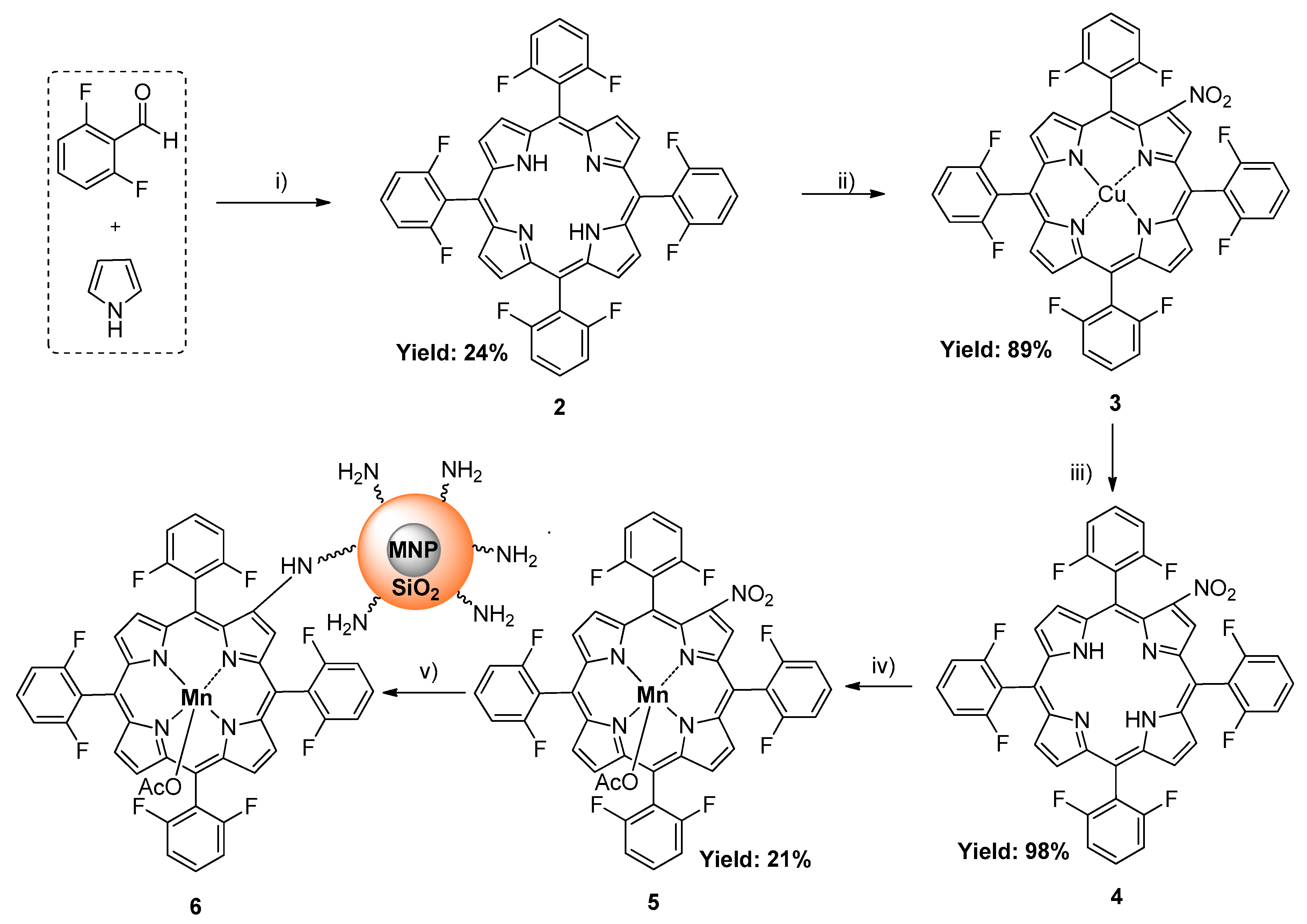
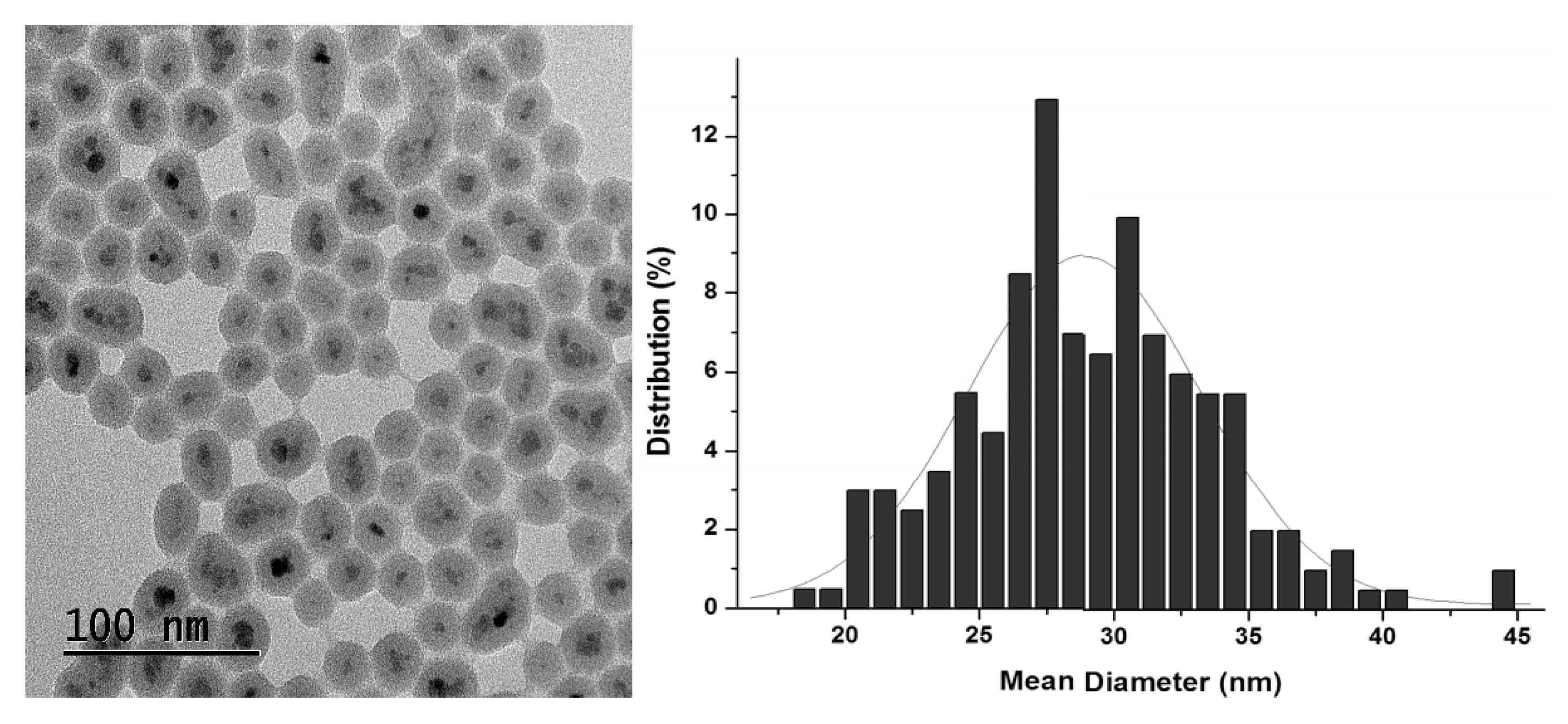
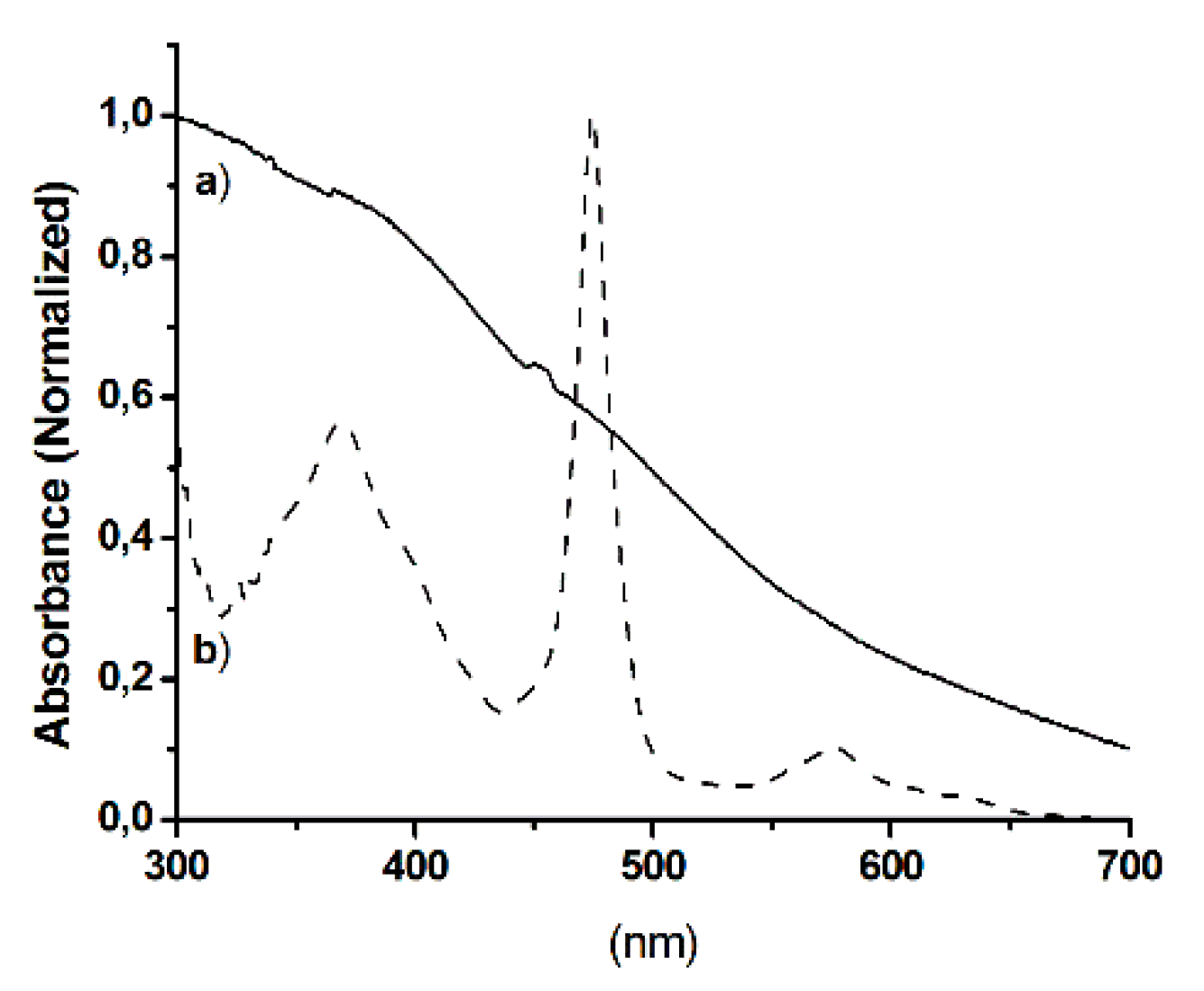
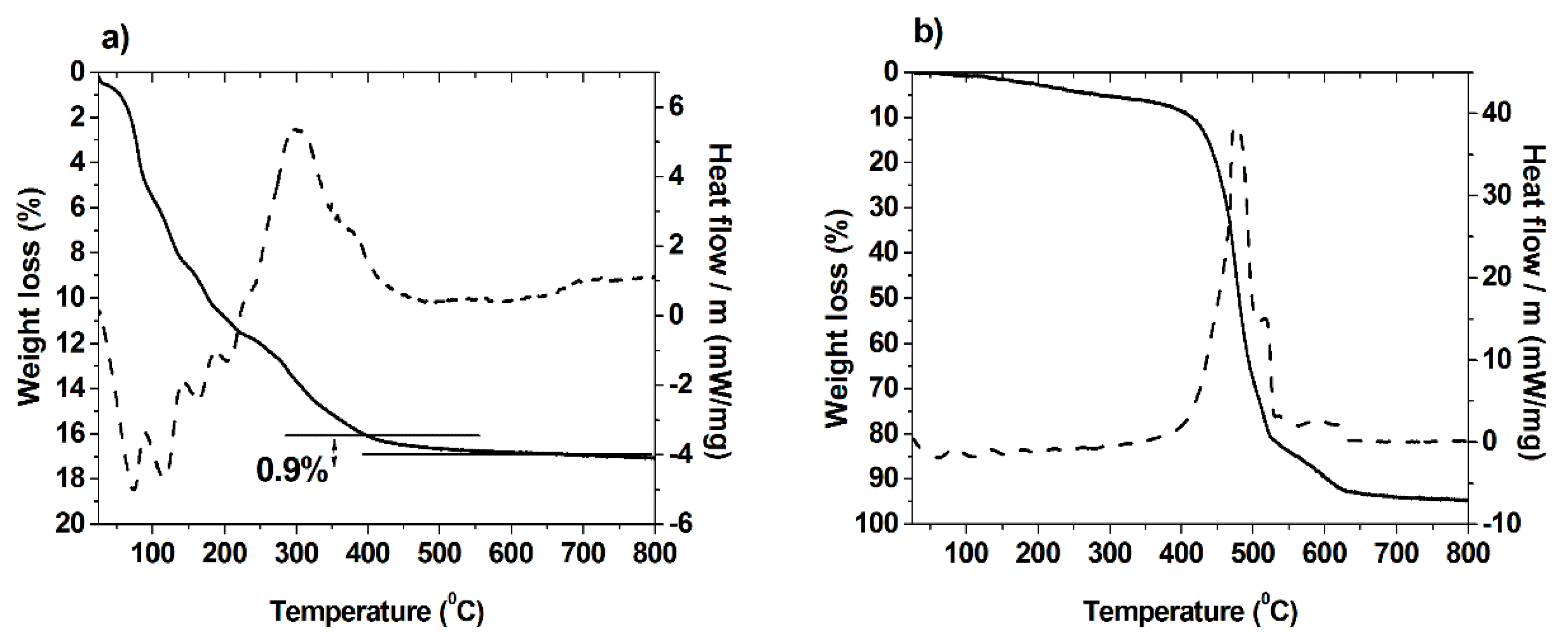
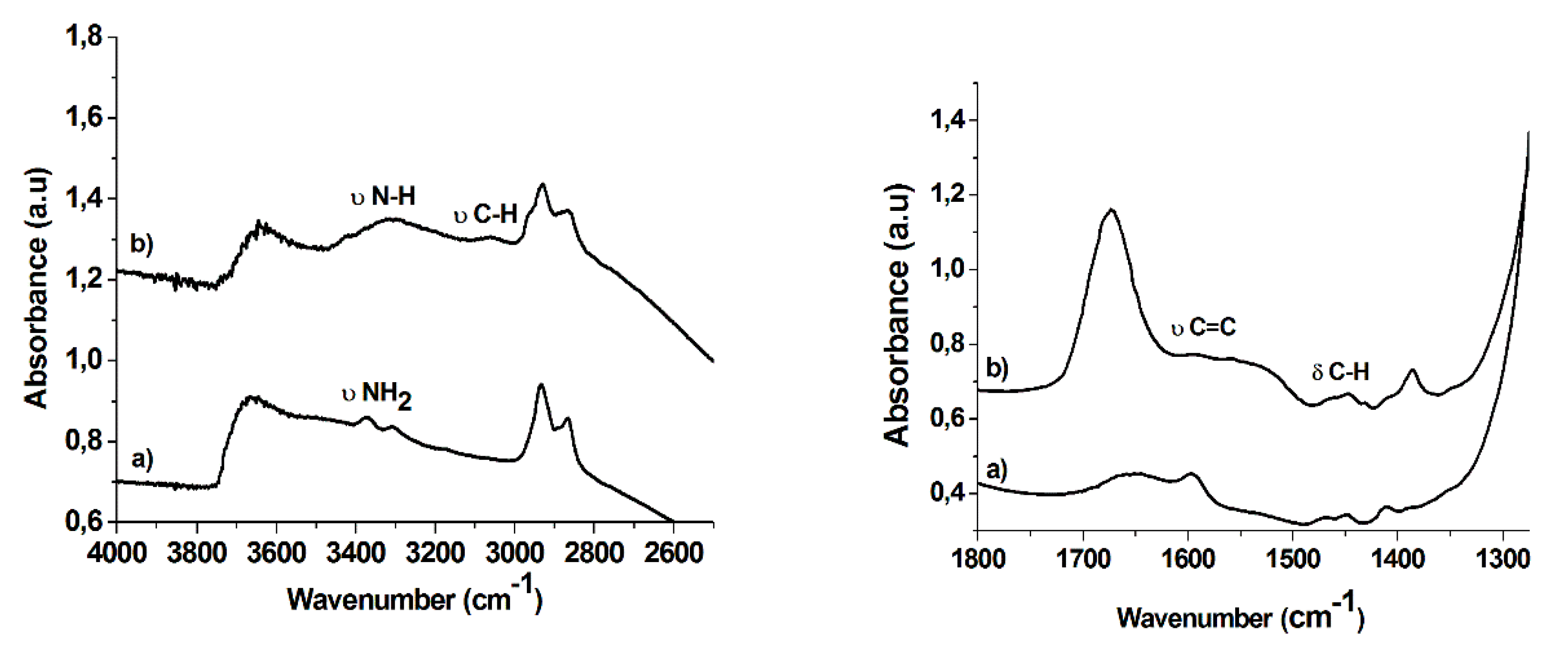
2.1. Synthesis and Characterization of Hybrid Manganese(III)-Porphyrin Magnetic Catalyst (6)
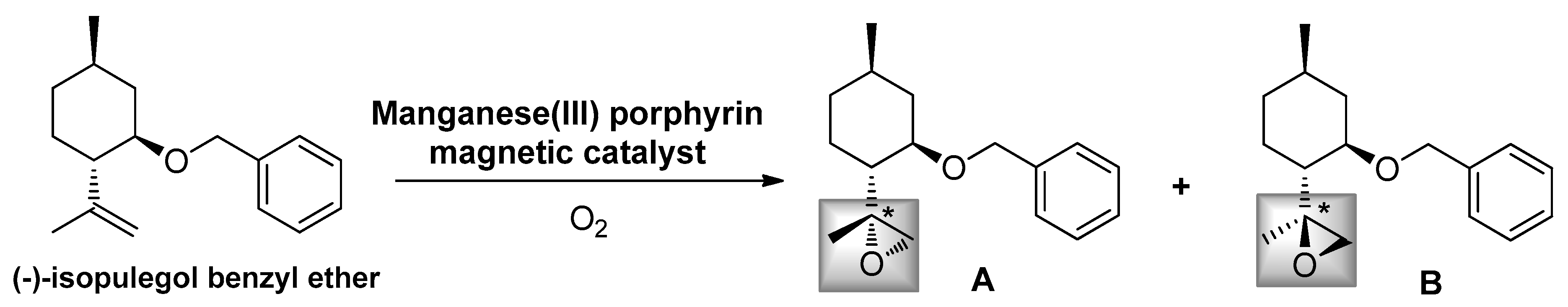
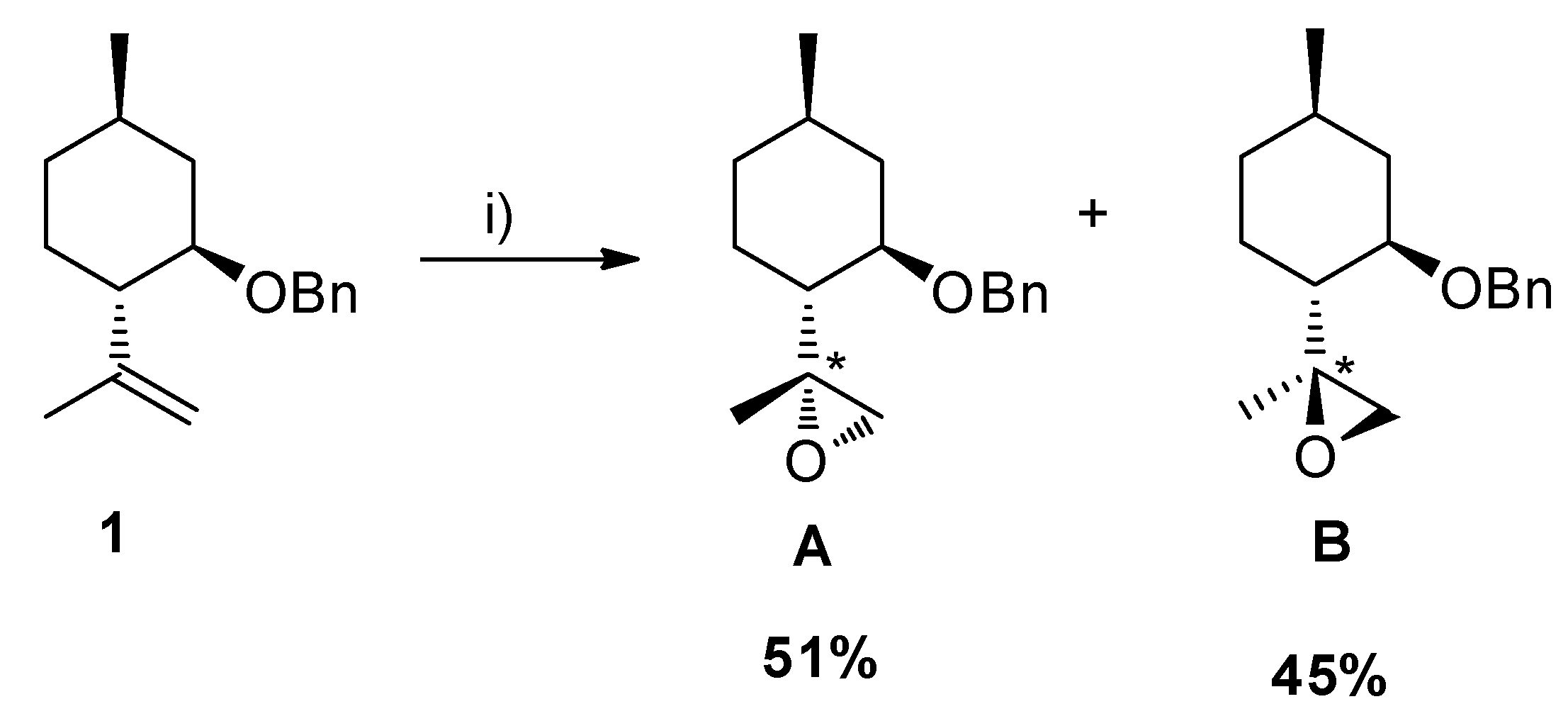
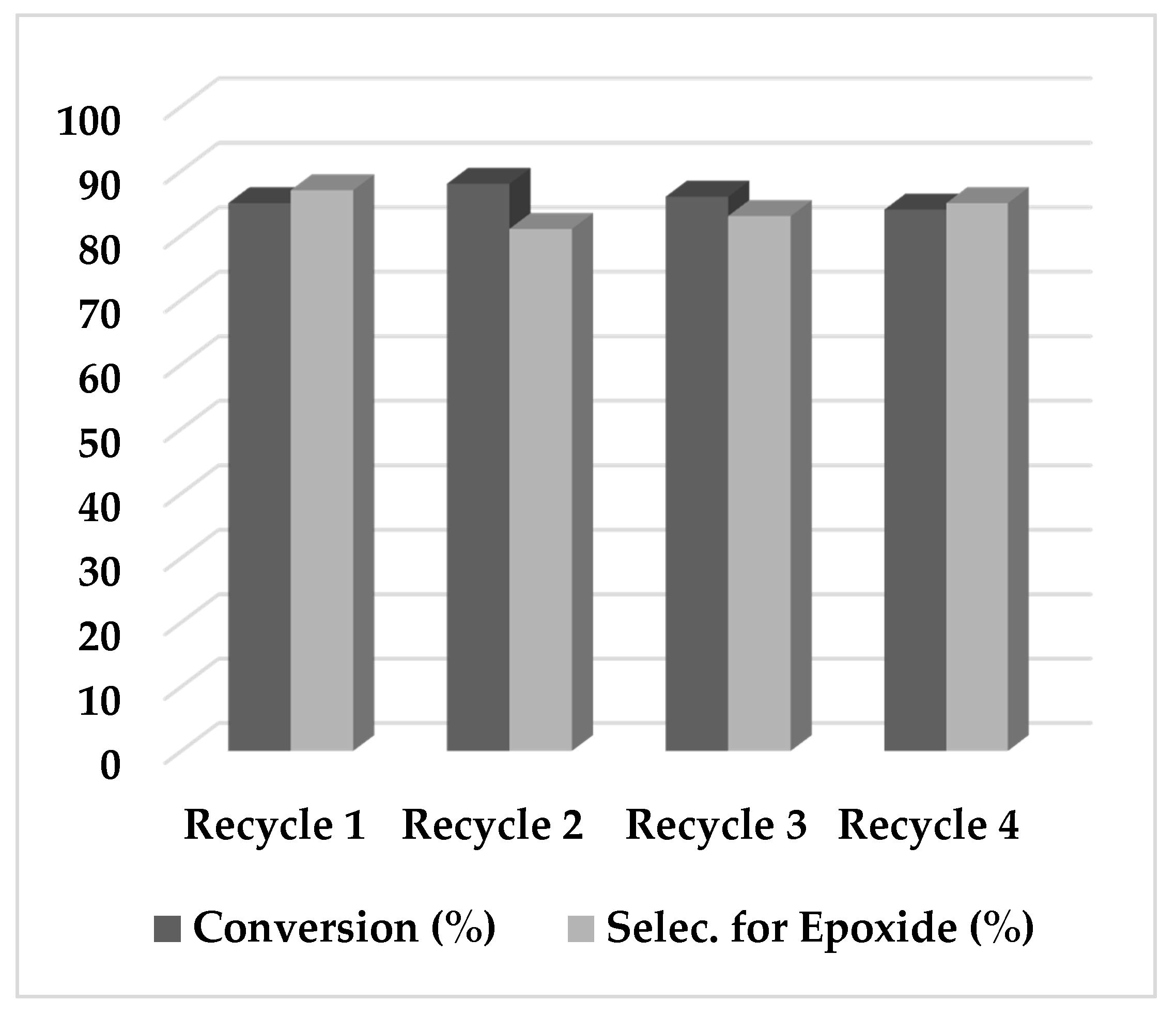
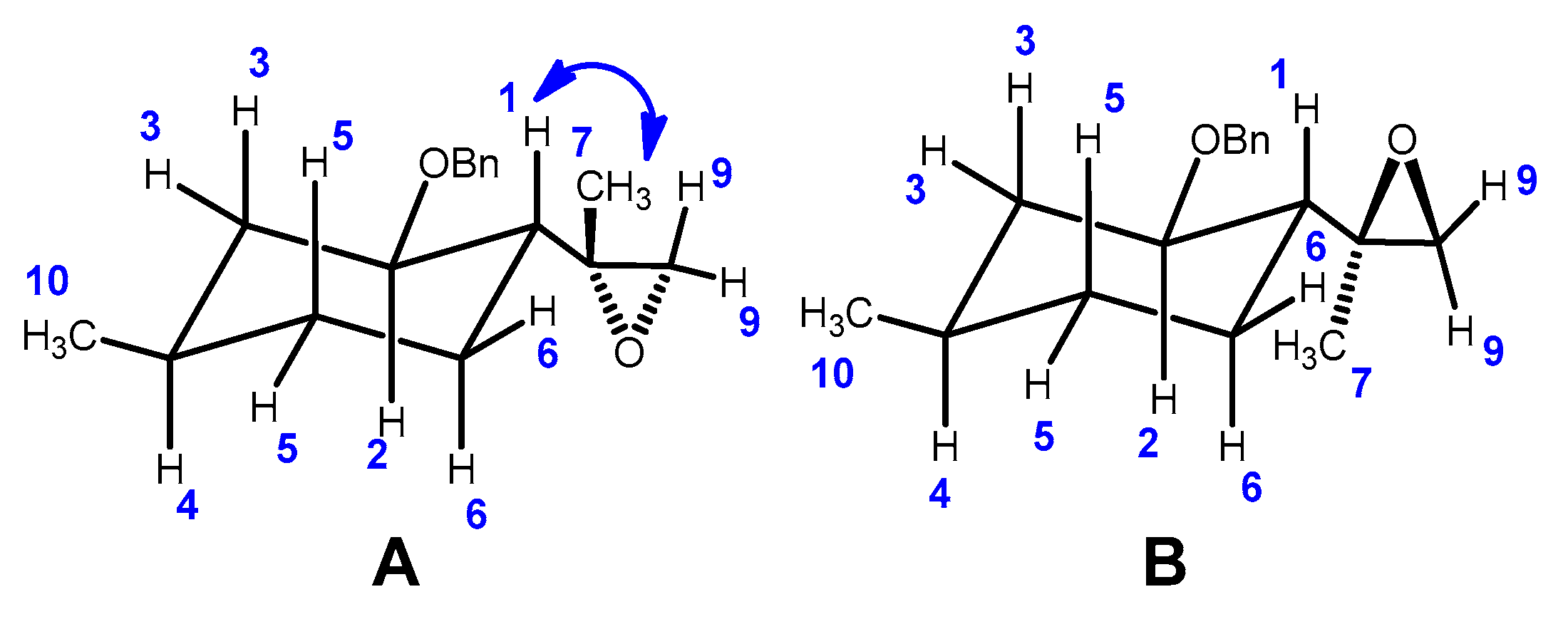
2.2. Epoxidation Reaction of (−)-Isopulegol Benzyl Ether (1)
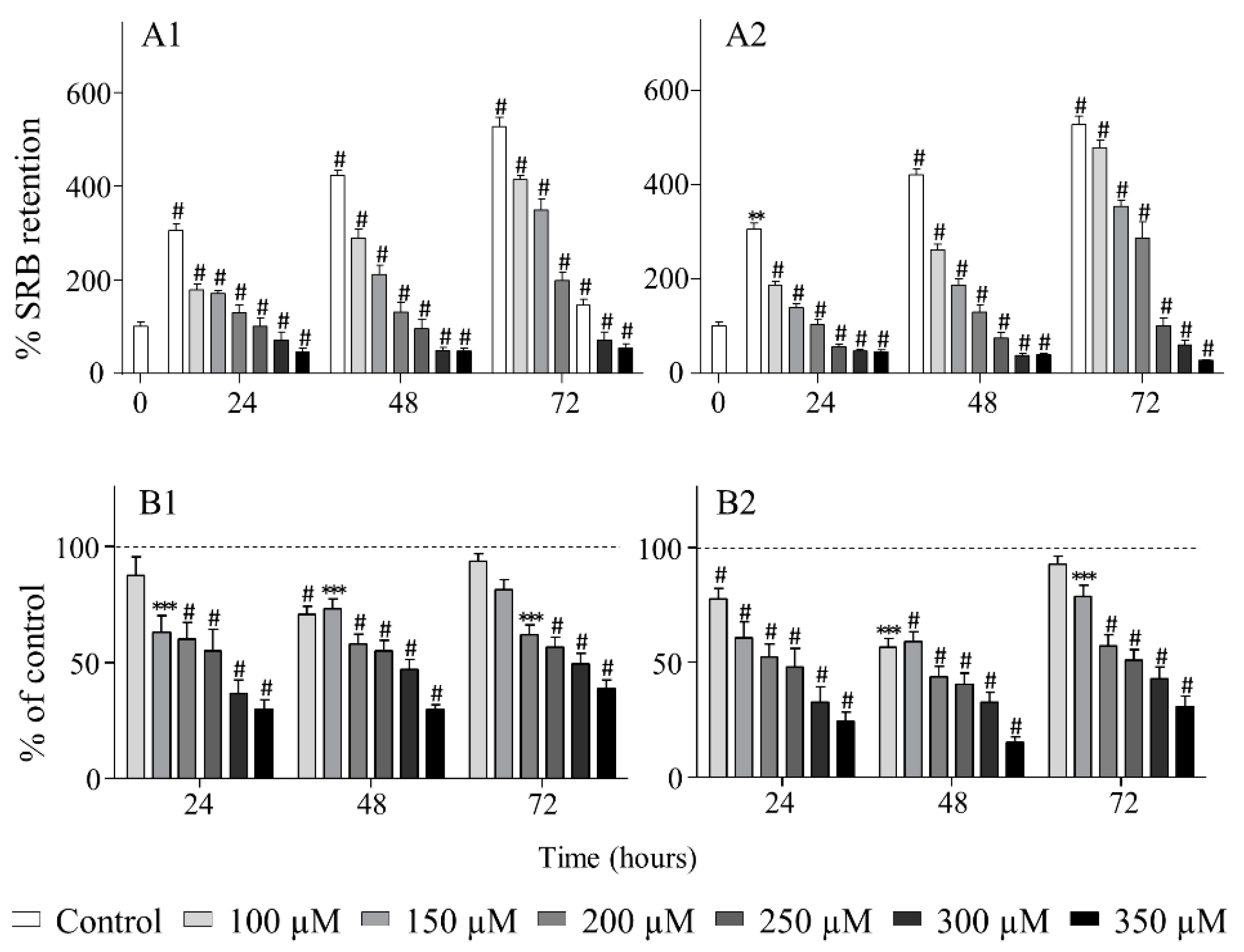
2.3. Biological Evaluation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of (−)-isopulegol benzyl ether (1)
3.3. Synthesis of the Catalyst
3.3.1. 5,10,15,20-tetrakis(2,6-difluorophenyl)porphyrin (2)
3.3.2. 2-Nitro-5,10,15,20-tetrakis(2,6-difluorophenyl)porphyrinatocopper(II) (3)
3.3.3. 2-Nitro-5,10,15,20-tetrakis(2,6-difluorophenyl)porphyrin (4)
3.3.4. 2-Nitro-5,10,15,20-tetrakis(2,6-difluorophenyl)porphyrinatomanganese(III) acetate (5)
3.3.5. Synthesis of Magnetic Nanoparticles (MNP)
3.3.6. Synthesis of Silica-coated Magnetic Nanoparticles (MNP@SiO2)
3.3.7. Synthesis of Amine Functionalized Silica-coated Nanoparticles (MNP@SiO2-NH2)
3.3.8. Synthesis of Hybrid Manganese(III)-porphyrin Magnetic Catalyst (6)
3.4. General Procedure for Catalytic Epoxidation of (−)-Isopulegol Benzyl with O2 and Scale-Up
3.5. Catalyst Recycling
3.6. Biological Evaluation
3.6.1. Cell Culture
3.6.2. Evaluation of Cytotoxic and Antiproliferative Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.M.; Wang, F.L.; Zhang, Z.S.; Liu, X.Z.; Zhai, D.D.; Chen, W.D. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur. J. Pharmacol. 2016, 772, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Imran, M.; Butt, T.T.; Shah, S.W.A.; Sohail, M.; Malik, A.; Das, S.; Thu, H.E.; Adam, A.; Hussain, Z. Curcumin based nanomedicines as efficient nanoplatform for treatment of cancer: New developments in reversing cancer drug resistance, rapid internalization, and improved anticancer efficacy. Trends Food. Sci. Technol. 2018, 80, 8–22. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for Prevention and Treatment of Cancers. Nutrients 2016, 8. [Google Scholar] [CrossRef]
- Cho, S.C.; Lee, H.; Choi, B.Y. An updated review on molecular mechanisms underlying the anticancer effects of capsaicin. Food Sci. Biotechnol. 2017, 26, 1–13. [Google Scholar] [CrossRef]
- Li, H.M.; Krstin, S.; Wang, S.H.; Wink, M. Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules 2018, 23, 557. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Yu, C.; Zhao, G.S.; Zhou, J.; Zhang, G.; Li, M.; Jiang, D.M.; Quan, Z.X.; Zhang, Y. Synergistic inhibitory effects of capsaicin combined with cisplatin on human osteosarcoma in culture and in xenografts. J. Exp. Clin. Cancer Res. 2018, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of NF-kappa B signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef]
- Guesmi, F.; Prasad, S.; Tyagi, A.K.; Landoulsi, A. Antinflammatory and anticancer effects of terpenes from oily fractions of Teucruim alopecurus, blocker of I kappa B. alpha kinase, through downregulation of NF-kappa B. activation, potentiation of apoptosis and suppression of NF-kappa B-regulated gene expression. Biomed. Pharmacother. 2017, 95, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhaes, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Alternat. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, L.W. Inhibition of azoxymethane-induced neoplasia of the large-bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol). Carcinogenesis 1991, 12, 151–152. [Google Scholar] [CrossRef]
- Hayes, A.J.; Leach, D.N.; Markham, J.L.; Markovic, B. In vitro Cytotoxicity of Australian Tea Tree Oil using Human Cell Lines. J. Essent. Oil Res. 1997, 9, 575–582. [Google Scholar] [CrossRef]
- Shi, W.G.; Gould, M.N. Induction of cytostasis in mammary carcinoma cells treated with the anticancer agent perillyl alcohol. Carcinogenesis 2002, 23, 131–142. [Google Scholar] [CrossRef]
- Holstein, S.A.; Hohl, R.J. Monoterpene regulation of Ras and Ras-related protein expression. J. Lipid Res. 2003, 44, 1209–1215. [Google Scholar] [CrossRef]
- Laxmi, Y.; Pierre, K.J.; Elegbede, A.; Wang, R.C.; Carper, S.W. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007, 257, 216–226. [Google Scholar] [CrossRef]
- Bailey, H.H.; Attia, S.; Love, R.R.; Fass, T.; Chappell, R.; Tutsch, K.; Harris, L.; Jumonville, A.; Hansen, R.; Shapiro, G.R.; et al. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother. Pharmacol. 2008, 62, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Manassero, C.A.; Girotti, J.R.; Mijailovsky, S.; de Bravo, M.G.; Polo, M. Invitro comparative analysis of antiproliferative activity of essential oil from mandarin peel and its principal component limonene. Nat. Prod. Res. 2013, 27, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Lima, T.C.; Amaral, R.G.; Pessoa, C.D.; de Moraes, M.O.; Soares, B.M.; do Nascimento, L.G.; Carvalho, A.A.; de Sousa, D.P. Evaluation of the Cytotoxicity of Structurally Correlated p-Menthane Derivatives. Molecules 2015, 20, 13264–13280. [Google Scholar] [CrossRef] [PubMed]
- Kydd, J.; Jadia, R.; Velpurisiva, P.; Gad, A.; Paliwal, S.; Rai, P. Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Yao, H.; Liu, J.K.; Xu, S.T.; Zhu, Z.Y.; Xu, J.Y. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef]
- Guo, Z.R. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef]
- Guidotti, M.; Moretti, G.; Psaro, R.; Ravasio, N. One-pot conversion of citronellal into isopulegol epoxide on mesoporous titanium silicate. Chem. Commun. 2000, 1789–1790. [Google Scholar] [CrossRef]
- Avery, C.A.; Pease, R.J.; Smith, K.; Boothby, M.; Buckley, H.M.; Grant, P.J.; Fishwick, C.W.G. (+/−)cis-bisamido epoxides: A novel series of potent FXIII-A inhibitors. Eur. J. Med. Chem. 2015, 98, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, S.; Elgendy, E. Safranal epoxide—A potential source for diverse therapeutic applications. Saudi Pharm. J. 2018, 26, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Righi, G.; Pelagalli, R.; Isoni, V.; Tirotta, I.; Marini, M.; Palagri, M.; Dallocchio, R.; Dessi, A.; Macchi, B.; Frezza, C.; et al. Synthesis of potential HIV integrase inhibitors inspired by natural polyphenol structures. Nat. Prod. Res. 2018, 32, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.F.; Xu, X.L.; Ding, Y.H.; Hao, X.; Bai, Y.J.; Tang, Y.; Zhang, X.M.; Li, Q.Y.; Yang, Z.T.; Zhang, W.C.; et al. Synthesis and biological evaluation of nannocystin analogues toward understanding the binding role of the (2R,3S)-Epoxide in nannocystin A. Eur. J. Med. Chem. 2018, 150, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; Molina, M.T.; Anjum, S. Naturally occurring cyclohexane epoxides: Sources, biological activities, and synthesis. Chem. Rev. 2004, 104, 2857–2899. [Google Scholar] [CrossRef]
- Fingerhut, A.; Serdyuk, O.V.; Tsogoeva, S.B. Non-heme iron catalysts for epoxidation and aziridination reactions of challenging terminal alkenes: Towards sustainability. Green Chem. 2015, 17, 2042–2058. [Google Scholar] [CrossRef]
- Groves, J.T.; Stern, M.K. Olefin epoxidation by manganese(IV) porphyrins–evidence for 2 reaction pathways. J. Am. Chem. Soc. 1987, 109, 3812–3814. [Google Scholar] [CrossRef]
- Groves, J.T.; Nemo, T.E. Epoxidation reactions catalyzed by iron porphyrins–oxygen-transfer from iodosylbenzene. J. Am. Chem. Soc. 1983, 105, 5786–5791. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Silva, A.M.N.; Medforth, C.J.; Freire, C. Iron(III) Fluorinated Porphyrins: Greener Chemistry from Synthesis to Oxidative Catalysis Reactions. Molecules 2016, 21, 481. [Google Scholar] [CrossRef]
- Castro, K.; de Lima, F.H.C.; Simoes, M.M.Q.; Neves, M.; Paz, F.A.A.; Mendes, R.F.; Nakagaki, S.; Cavaleiro, J.A.S. Synthesis, characterization and catalytic activity under homogeneous conditions of ethylene glycol substituted porphyrin manganese(III) complexes. Inorganica Chim. Acta 2017, 455, 575–583. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Pineiro, M.; Dias, L.D.; Pereira, M.M. Hydrogen Peroxide and Metalloporphyrins in Oxidation Catalysis: Old Dogs with Some New Tricks. ChemCatChem 2018, 10, 3615–3635. [Google Scholar] [CrossRef]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 11, 10784–10808. [Google Scholar] [CrossRef]
- Dias, L.D.; Carrilho, R.M.B.; Henriques, C.A.; Piccirillo, G.; Fernandes, A.; Rossi, L.M.; Ribeiro, M.F.; Calvete, M.J.F.; Pereira, M.M. A recyclable hybrid manganese(III) porphyrin magnetic catalyst for selective olefin epoxidation using molecular oxygen. J. Porphyr. Phthalocyanines 2018, 22, 331–341. [Google Scholar] [CrossRef]
- Groves, J.T.; Watanabe, Y. On the mechanism of olefin epoxidation by oxo-iron porphyrins–direct observation of an intermediate. J. Am. Chem. Soc. 1986, 108, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Bhyrappa, P.; Young, J.K.; Moore, J.S.; Suslick, K.S. Shape selective epoxidation of alkenes by metalloporphyrin-dendrimers. J. Mol. Catal. A Chem. 1996, 113, 109–116. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhou, H.B.; Huang, J.S.; Che, C.M. Dendritic ruthenium porphyrins: A new class of highly selective catalysts for alkene epoxidation and cyclopropanation. Chem. Eur. J. 2002, 8, 1554–1562. [Google Scholar] [CrossRef]
- Zhang, W.J.; Jiang, P.P.; Wang, Y.; Zhang, J.; Zhang, P.B. Directing two azo-bridged covalent metalloporphyrinic polymers as highly efficient catalysts for selective oxidation. Appl. Catal. A Gen. 2015, 489, 117–122. [Google Scholar] [CrossRef]
- Zhou, X.T.; Ji, H.B. Manganese porphyrin immobilized on montmorillonite: A highly efficient and reusable catalyst for the aerobic epoxidation of olefins under ambient conditions. J. Porphyr. Phthalocyanines 2012, 16, 1032–1039. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Goncalves, A.R.; Pereira, M.M.; Simoes, M.M.Q.; Neves, M.; Cavaleiro, J.A.S. Epoxidation reactions with hydrogen peroxide activated by a novel heterogeneous metalloporphyrin catalyst. J. Mol. Catal. A Chem. 2006, 256, 321–323. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Gomes, A.T.P.C.; Moura, N.M.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Lodeiro, C.; Veríssimo, M.I.S.; Fernandes, T.; Daniel-da-Silva, A.L.; Gomes, M.T.S.R.; et al. N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy. Molecules 2018, 23, 867. [Google Scholar] [CrossRef]
- Santos, E.H.; Carvalho, C.; Terzi, C.M.; Nakagaki, S. Recent Advances in Catalyzed Sequential Reactions and the Potencial Use of Tetrapyrrolic Macrocycles as Catalysts. Molecules 2018, 23, 2796. [Google Scholar] [CrossRef]
- Berijani, K.; Farokhi, A.; Hosseini-Monfared, H.; Janiak, C. Enhanced enantioselective oxidation of olefins catalyzed by Mn-porphyrin immobilized on graphene oxide. Tetrahedron 2018, 74, 2202–2210. [Google Scholar] [CrossRef]
- Farokhi, A.; Hosseini-Monfared, H. A recyclable Mn-porphyrin catalyst for enantioselective epoxidation of unfunctionalized olefins using molecular dioxygen. New J. Chem. 2016, 40, 5032–5043. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Mortazavi-Manesh, A. Nanoparticle supported, magnetically separable manganese porphyrin as an efficient retrievable nanocatalyst in hydrocarbon oxidation reactions. RSC Adv. 2016, 6, 41551–41560. [Google Scholar] [CrossRef]
- Silva, M.; Fernandes, A.; Bebiano, S.S.; Calvete, M.J.F.; Ribeiro, M.F.; Burrows, H.D.; Pereira, M.M. Size and ability do matter! Influence of acidity and pore size on the synthesis of hindered halogenated meso-phenyl porphyrins catalysed by porous solid oxides. Chem. Comm. 2014, 50, 6571–6573. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, S.; Corni, S.; Mennucci, B. Fluorescence Enhancement of Chromophores Close to Metal Nanoparticles. Optimal Setup Revealed by the Polarizable Continuum Model. J. Phys. Chem. 2009, 113, 121–133. [Google Scholar] [CrossRef]
- Sun, C.G.; Hu, B.C.; Liu, Z.L. Efficient and ecofriendly options for the chemoselective oxidation of alkenes using manganese porphyrin and dioxygen. Chem. Eng. J. 2013, 232, 96–103. [Google Scholar] [CrossRef]
- Shankar, S.P.; Jagodzinska, M.; Malpezzi, L.; Lazzari, P.; Manca, I.; Greig, I.R.; Sani, M.; Zanda, M. Synthesis and structure-activity relationship studies of novel tubulysin U analogues–effect on cytotoxicity of structural variations in the tubuvaline fragment. Org. Biomol. Chem. 2013, 11, 2273–2287. [Google Scholar] [CrossRef]
- Garnier-Amblard, E.C.; Mays, S.G.; Arrendale, R.F.; Baillie, M.T.; Bushnev, A.S.; Culver, D.G.; Evers, T.J.; Holt, J.J.; Howard, R.B.; Liebeskind, L.S.; et al. Novel Synthesis and Biological Evaluation of Enigmols as Therapeutic Agents for Treating Prostate Cancer. ACS Med. Chem. Lett. 2011, 2, 438–443. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Dias, L.D.; Henriques, C.A.; Pinto, S.M.A.; Carrilho, R.M.B.; Pereira, M.M. A Cost-Efficient Method for Unsymmetrical Meso-Aryl Porphyrin Synthesis Using NaY Zeolite as an Inorganic Acid Catalyst. Molecules 2017, 22, 741. [Google Scholar] [CrossRef]
- Grancho, J.C.P.; Pereira, M.M.; Miguel, M.D.; Gonsalves, A.M.R.; Burrows, H.D. Synthesis, spectra and photophysics of some free base tetrafluoroalkyl and tetrafluoroaryl porphyrins with potential applications in imaging. Photochem. Photobiol. 2002, 75, 249–256. [Google Scholar] [CrossRef]
- Rossi, L.M.; Vono, L.L.R.; Silva, F.P.; Kiyohara, P.K.; Duarte, E.L.; Matos, J.R. Magnetically recoverable scavenger for palladium based on thiol-modified magnetite nanoparticles. Appl. Catal. A Gen. 2007, 330, 139–144. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Kiyohara, P.K.; Masunaga, S.H.; Jardim, R.F.; Rossi, L.M. Recoverable rhodium nanoparticles: Synthesis, characterization and catalytic performance in hydrogenation reactions. Appl. Catal. A Gen. 2008, 338, 52–57. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds 6, epoxide A and B are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, L.D.; Batista de Carvalho, A.L.M.; Pinto, S.M.A.; Aquino, G.L.B.; Calvete, M.J.F.; Rossi, L.M.; Marques, M.P.M.; Pereira, M.M. Bioinspired-Metalloporphyrin Magnetic Nanocomposite as a Reusable Catalyst for Synthesis of Diastereomeric (−)-Isopulegol Epoxide: Anticancer Activity Against Human Osteosarcoma Cells (MG-63). Molecules 2019, 24, 52. https://doi.org/10.3390/molecules24010052
Dias LD, Batista de Carvalho ALM, Pinto SMA, Aquino GLB, Calvete MJF, Rossi LM, Marques MPM, Pereira MM. Bioinspired-Metalloporphyrin Magnetic Nanocomposite as a Reusable Catalyst for Synthesis of Diastereomeric (−)-Isopulegol Epoxide: Anticancer Activity Against Human Osteosarcoma Cells (MG-63). Molecules. 2019; 24(1):52. https://doi.org/10.3390/molecules24010052
Chicago/Turabian StyleDias, Lucas D., Ana L. M. Batista de Carvalho, Sara M. A. Pinto, Gilberto L. B. Aquino, Mário J. F. Calvete, Liane M. Rossi, M. P. M. Marques, and Mariette M. Pereira. 2019. "Bioinspired-Metalloporphyrin Magnetic Nanocomposite as a Reusable Catalyst for Synthesis of Diastereomeric (−)-Isopulegol Epoxide: Anticancer Activity Against Human Osteosarcoma Cells (MG-63)" Molecules 24, no. 1: 52. https://doi.org/10.3390/molecules24010052
APA StyleDias, L. D., Batista de Carvalho, A. L. M., Pinto, S. M. A., Aquino, G. L. B., Calvete, M. J. F., Rossi, L. M., Marques, M. P. M., & Pereira, M. M. (2019). Bioinspired-Metalloporphyrin Magnetic Nanocomposite as a Reusable Catalyst for Synthesis of Diastereomeric (−)-Isopulegol Epoxide: Anticancer Activity Against Human Osteosarcoma Cells (MG-63). Molecules, 24(1), 52. https://doi.org/10.3390/molecules24010052